1. Introduction
The human aorta is defined as the main blood vessel that starts from the upper part of the heart and is responsible for transporting blood pumped from the left ventricle into the rest of the circulatory system [
1]. Since aortic diseases are often life-threatening, understanding aortic pathophysiology through research is crucial. Critical objects of the field are the compositional structure of the aortic wall and hemodynamics, such as blood flow type, flow velocity, blood pressure, and pulse rate [
2]. Aneurysms are one of the primary pathologies impacting the aorta. Based on existing literature [
3], aortic aneurysms are defined as the swelling and expansion of the surface (wall) of the blood vessel, specifically greater than 50% of the normal values of its diameter. This dilation is progressively manifested in parts of the aorta with a high risk of rupture, causing internal bleeding and death [
4]. The aorta is divided into two sections: the thoracic aorta and the abdominal aorta. However, the abdominal aorta has a higher risk of aneurysm formation [
5]. Therefore, by understanding the pathophysiology of the aorta, patient-specific clinical cases of abdominal aortic aneurysms can be modeled through specific parameterization.
The causes of aneurysms are not predetermined, but factors such as heredity, atherosclerosis, diabetes, hypertension, or injuries from previous vascular surgery may contribute to their occurrence [
6]. Usually, patients do not show symptoms as aneurysms are often incidental findings during tests. However, some of the most common symptoms include abdominal, chest, back, or groin pain, as well as palpitations in the abdomen in synchrony with the heartbeat. In rare cases, areas of blue color and pain may appear in the feet and toes due to the dispersal of debris from the aneurysm downward [
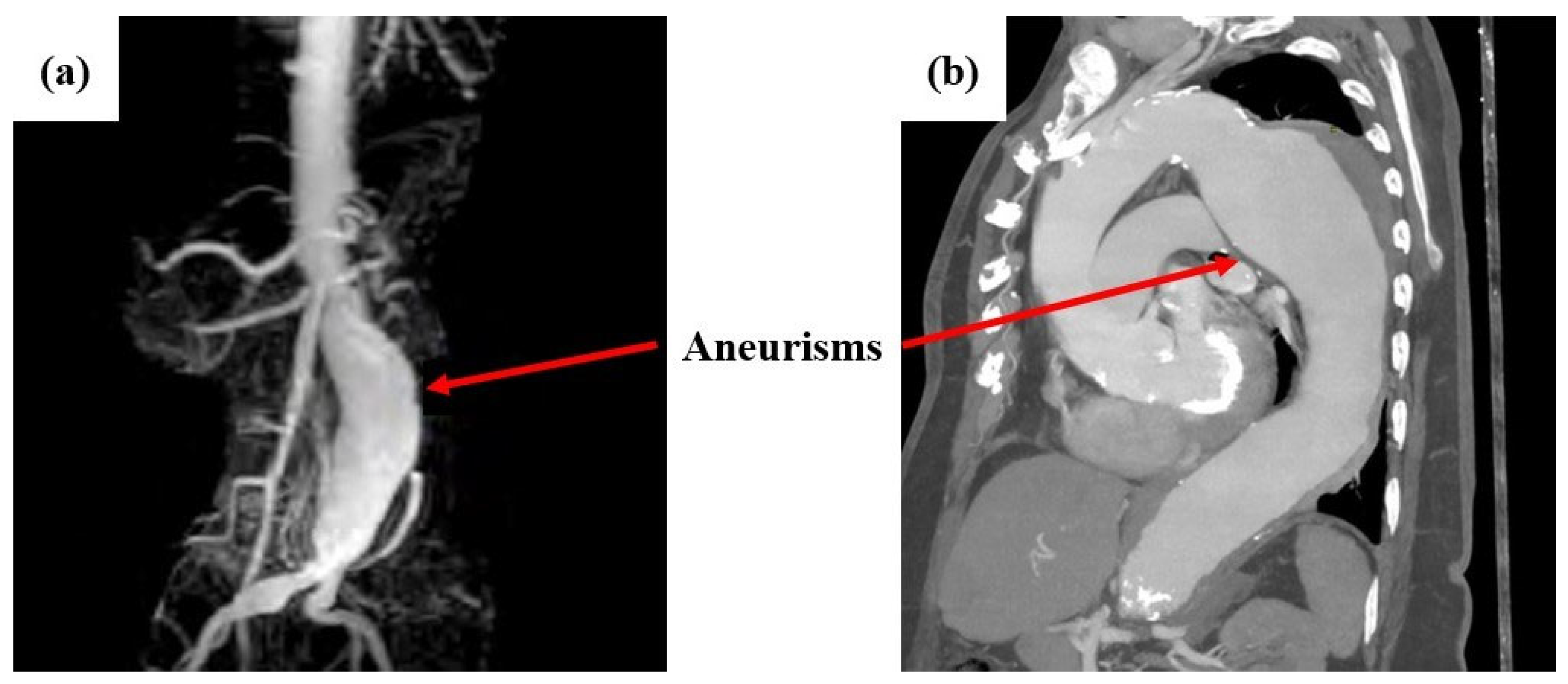
7]. Aneurysms are usually diagnosed as incidental findings because patients are asymptomatic. They are typically detected through, computed tomography (CT) or angiography (CTA), triplex ultrasound, magnetic resonance imaging (MRI), or angiography (MRA). In very rare cases, when imaging tests are negative, but an aneurysm is strongly suspected, more specialized tests may be necessary (Positron Emission Tomography (PET) Scan, etc.) [
8]. Indicatively,
Figure 1 shows cases of abdominal aortic aneurysms captured with MRA (left) and MRI (right) imaging [
9,
10].
Over the last decade, simulation studies have been performed on aneurysmal aortic regions focusing both on hemodynamics and structural integrity of the examined aortas. In detail, the vast majority of published studies have investigated the flow stream inside the aorta and how it is influenced by wall abnormalities [
11,
12,
13,
14]. Thus, this aspect of computational analysis for aortic arteries has reached a sufficient technology readiness level. Therefore, nowadays, more and more studies are focusing on the structural integrity simulation of aortic wall behavior and assessment of the risk of rupture. This problem has increased complexity due to the exotic nature of the aortic tissue, which possesses dynamic characteristics depending on the patient’s age, gender, weight, etc. [
15,
16,
17]. Towards this direction, fluid–structure interaction within realistic three-dimensional models of the aneurysmatic aortic section has been employed as guidance to assess the risk of rupture of the aneurysm [
18,
19]. In addition, Humphrey and Holzapfel [
20] have summarized in a comprehensive review the mechanics, mechanobiology, and modeling of human abdominal aortic arteries and aneurysms, showing the need for patient-specific computational models. All these studies are capable of evaluating the mechanical behavior of the aortic wall and assessing the risk of potential rupture. However, the complexity of the aforementioned studies increased the execution time and the required computational power for each analysis. Therefore, there is a need to develop patient-specific computational tools utilizing finite element models that can produce reliable and sufficiently accurate results assisting the in-situ medical decision-making for the examined cases.
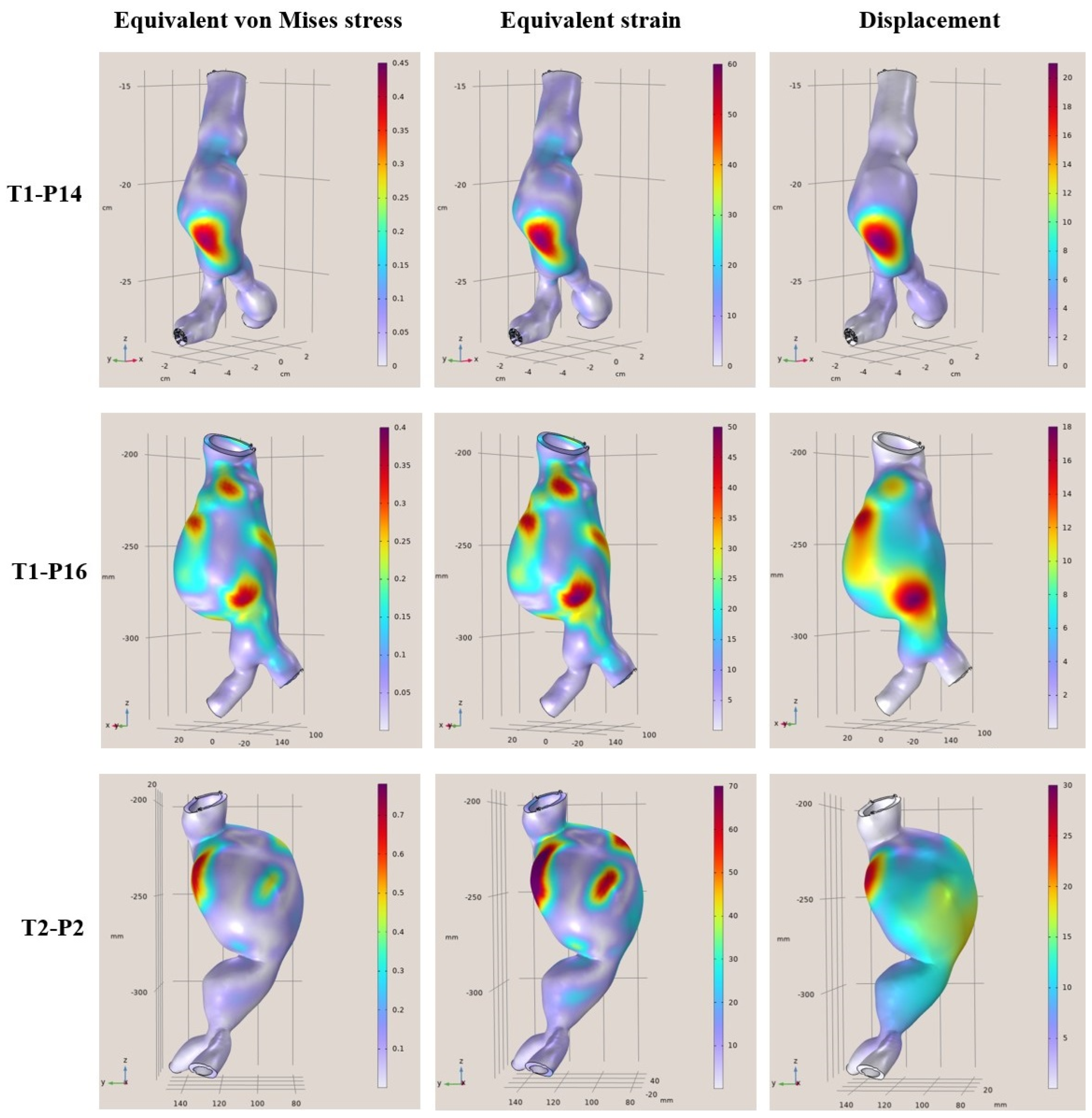
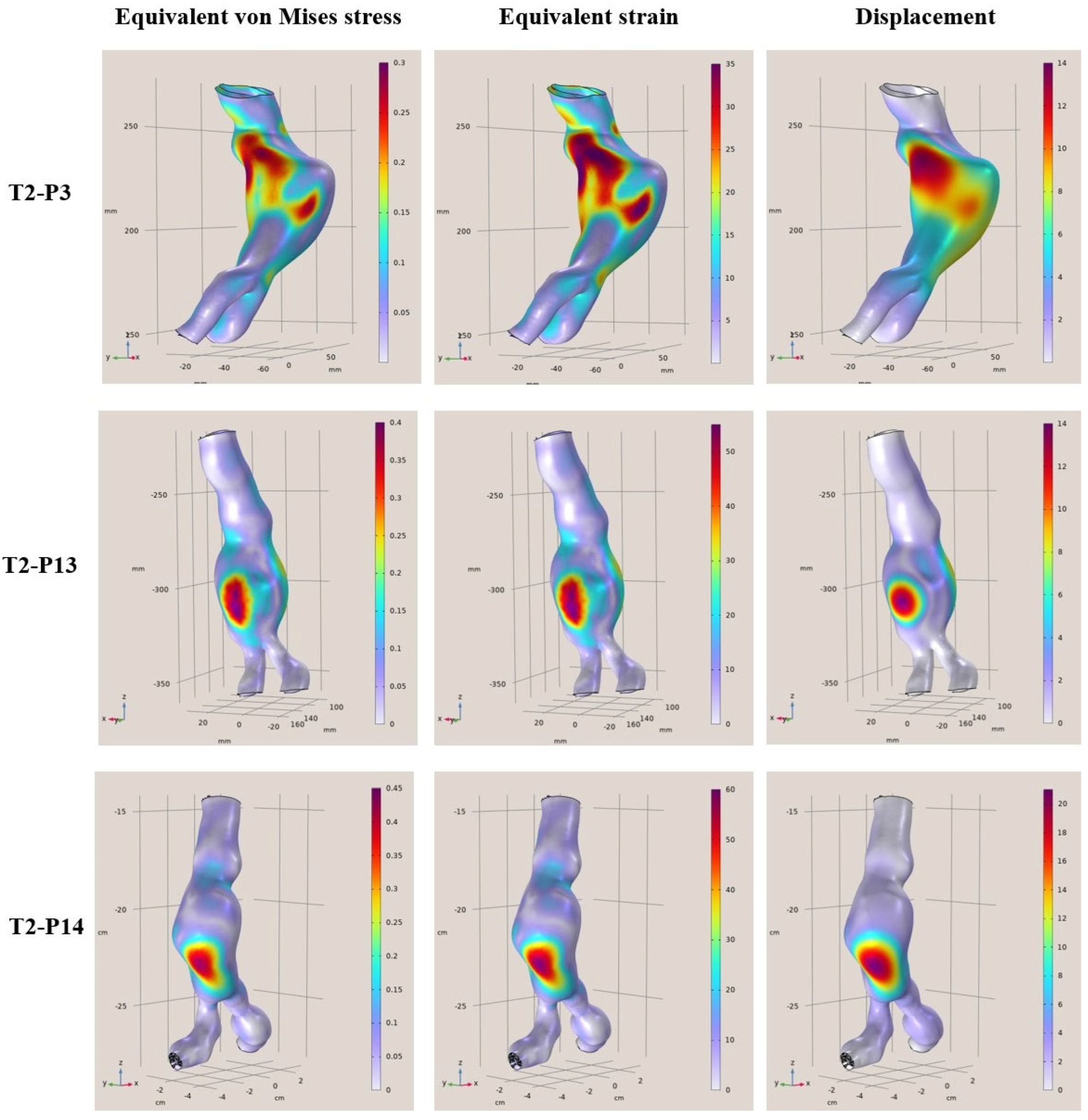
In this context, the present study performed a numerical evaluation of aortas with abdominal aneurysm employing FEMs in order to rapidly extract reliable and accurate results assessing the risk of rupture. More specifically, geometrical data were acquired from CT scans of 12 abdominal aortas previously diagnosed with aneurysms and the analysis of the mechanical response under specific assumptions was performed using computational mechanics. The objective of this work is to develop and study aneurysmal aortas in a simulation environment using finite elements models employing static linear analysis of the aortic wall and then rapidly assess the rupture risk. The novelty of this paper is the provision of how the aneurysmal abdominal aortic region behaves under constant pressure to find and highlight the areas of maximum stresses, deformations, and displacements on the surfaces. Then, these acquired data were used in order to rapidly assess the risk of potential rupture, providing healthcare professionals with a reliable data-driven decision-making tool. It must be noted that predictions of stresses and strains in parts of the aortic artery that are not accessible can be provided, like a virtual sensor. The developed finite models were built and evaluated utilizing existing geometric data from the CT scans supplemented with assumptions on the values of the unknowns (material properties, arterial thickness, and arterial pressure) to demonstrate the capabilities of the mechanical computational models created. The results can be extended in several directions. This will pave the way for further study both in a theoretical setting and in clinical application.
Figure 2 shows the flowchart of the current study.
4. Conclusions
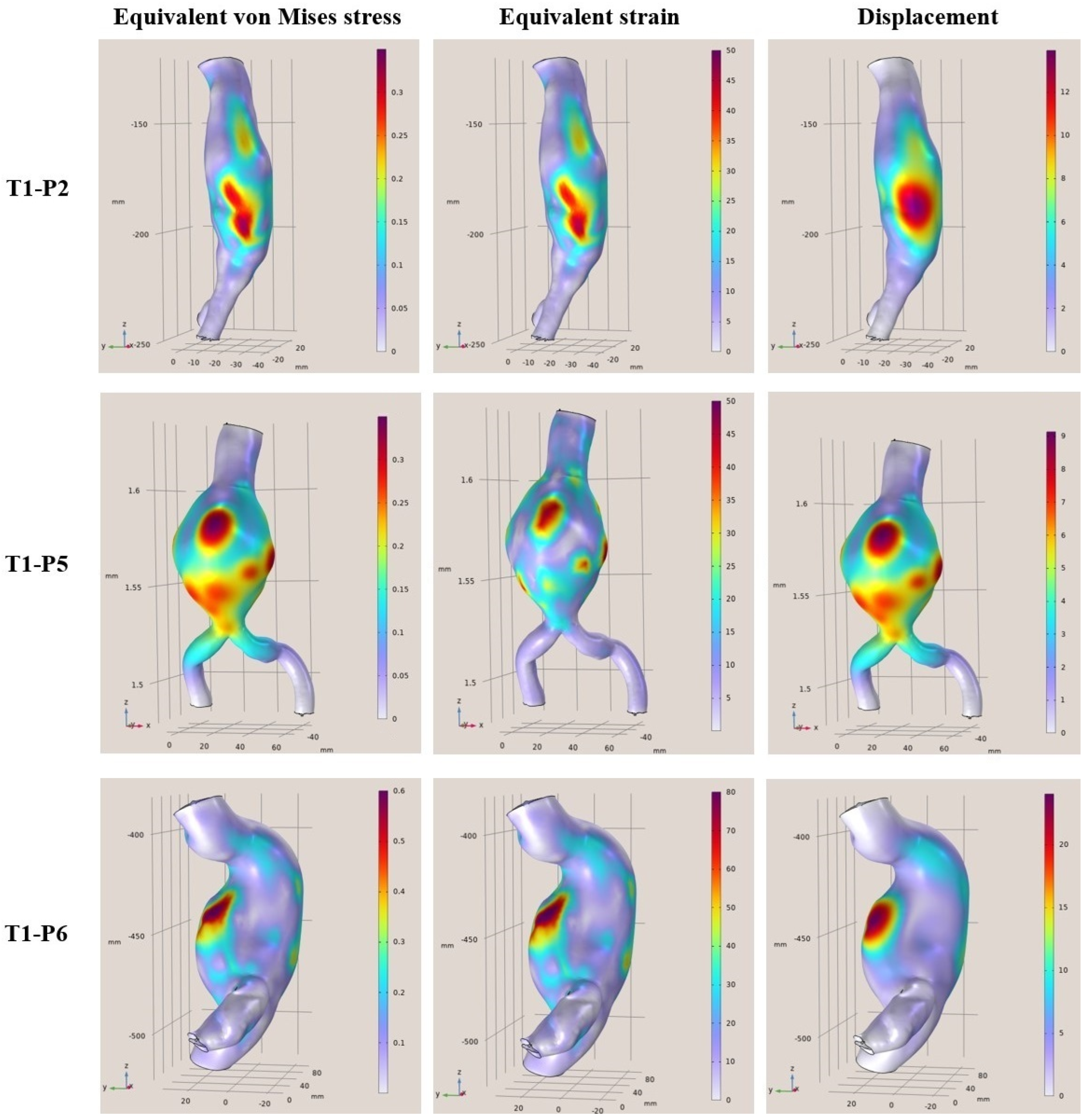
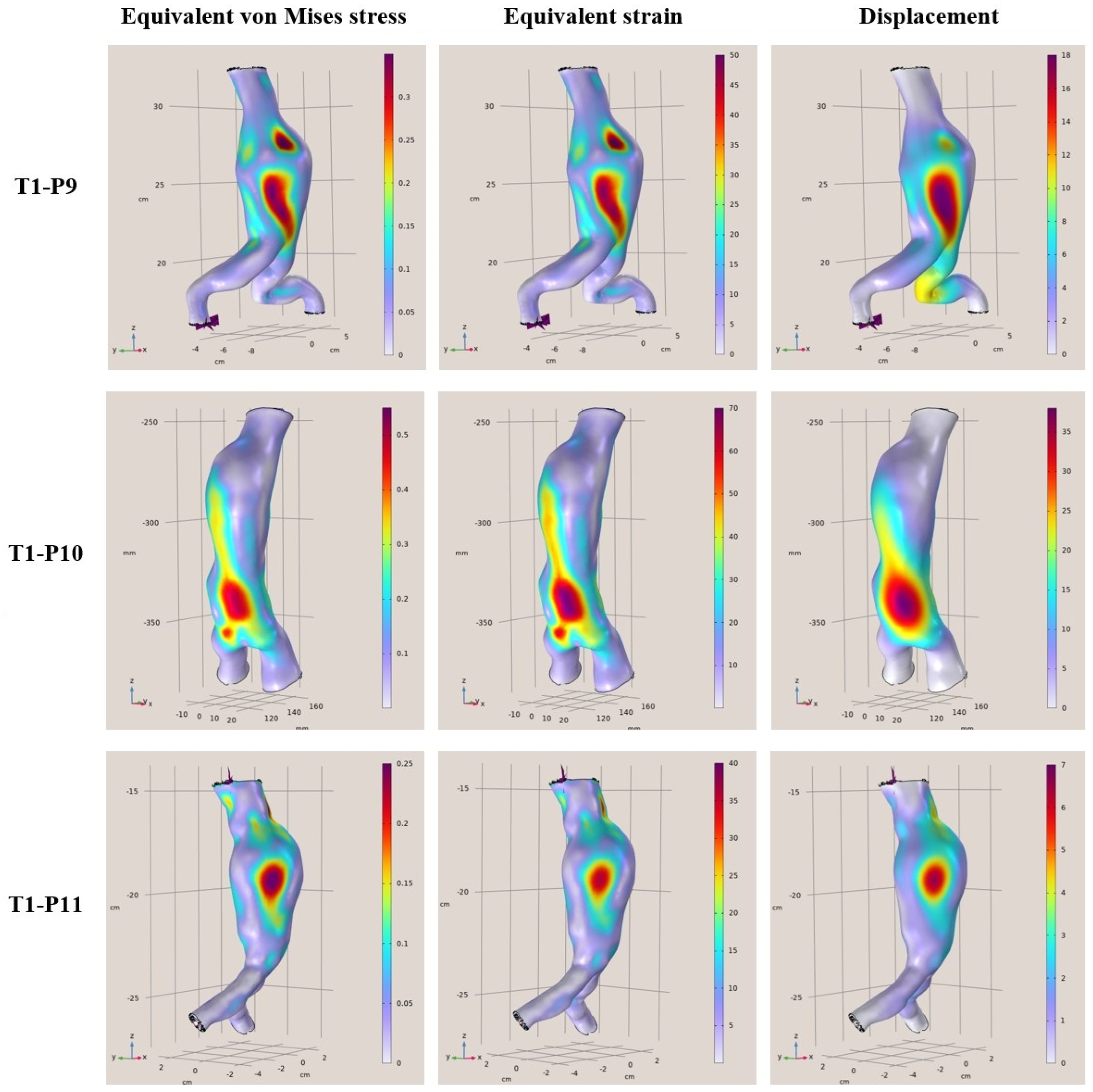
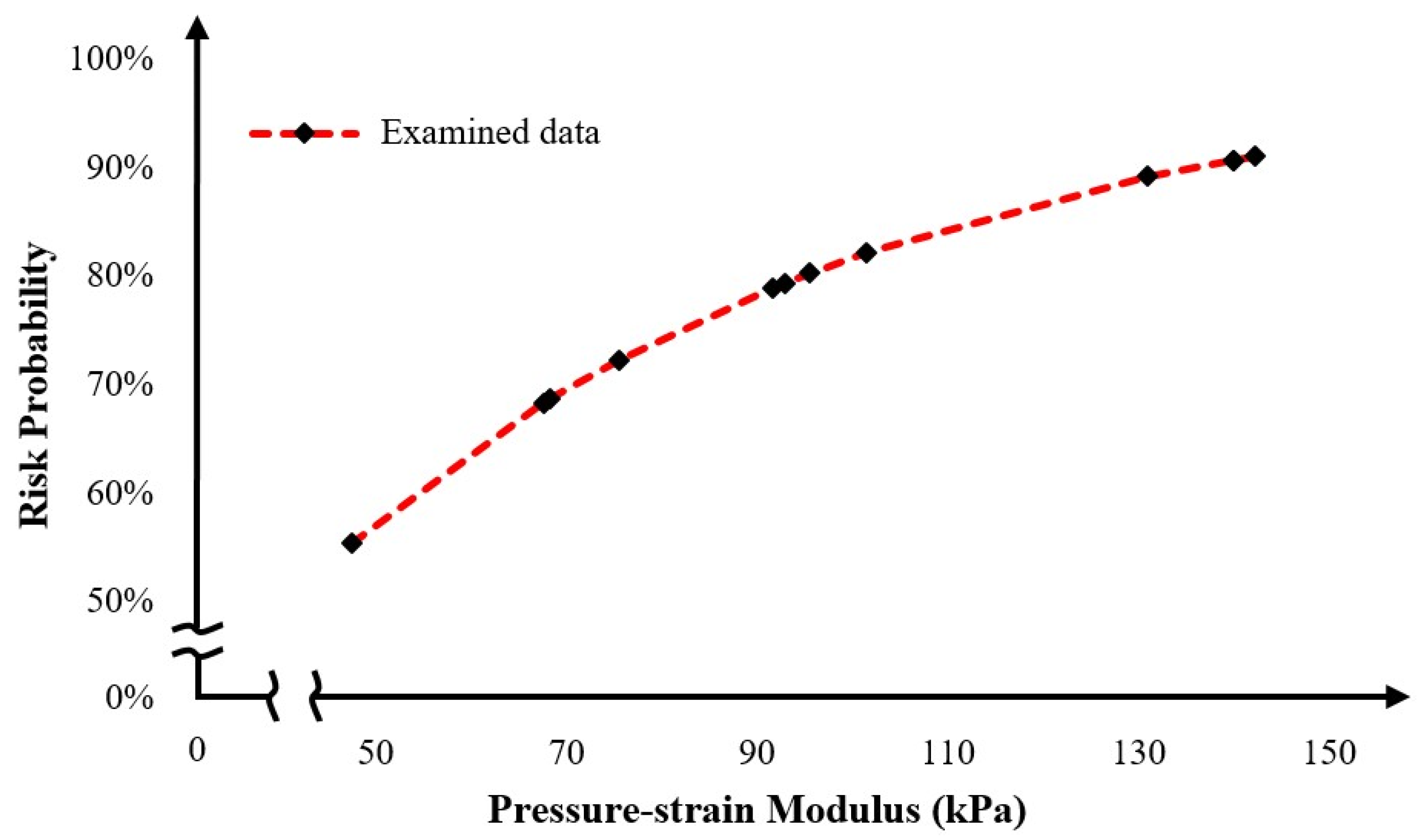
To conclude, clinical cases from patients that are middle-aged to elderly from 57 to 81 years of age with a diagnostic diameter between 4.8 cm and 6.8 cm, were examined. Based on certain simplified assumptions like linearly elastic behavior, known support boundary conditions, and uniform blood pressure, it was derived that the areas where the greatest strains and displacements occur are considered to be areas of risk. In addition, the maximum stresses and strains differed from patient to patient, and it was concluded that each patient is different, the modeling was unique, and the maximum values changed for each patient case. Furthermore, based on the presented results, the maximum deviations ranged from 35% to 80% in some models. This means that the points where the maximum values occur on the shell surfaces are at a higher risk of aortic rupture under the pulsatile function. In terms of displacements, the maximum displacements from the surfaces appeared to range from 9 mm to 40 mm with the highest displacements occurring in the range 18 mm to 40 mm. Finally, regarding the rupture risk, larger aneurysmatic regions significantly increase the risk of aortic rupture, with some samples reaching a 90% risk, highlighting the effectiveness of comprehensive and fast FEAs in providing reliable rupture probability indicators. Through this process, the current paper provides a novel computational tool for rupture risk assessment that can assist in a more efficient and reliable decision-making process.
The correlation of aneurysm shape parameters with the arising stresses and strains—and the subsequent prediction of rupture risk—represents a promising proof-of-concept based on existing patient case studies. However, this initial study is subject to several limitations. Notably, the linear static analysis applied to a simplified shell structure does not fully capture the complex biomechanical behavior of the aortic wall. In particular, the model lacks the incorporation of non-linear material properties, the pulsatile dynamics of blood flow, and the detailed geometric features associated with thrombus formation and calcifications. These omissions/simplifications can significantly affect the accuracy of stress and strain estimations. A more robust approach would involve developing a parameterized aortic model—potentially leveraging statistical shape models—to automate the generation of diverse and realistic aortic geometries [
24,
25]. Such models should integrate advanced non-linear simulation techniques, dynamic pulsatile loading conditions, and enhanced anatomical detail to better mimic the anisotropic and layered structure of the aortic wall [
26]. Addressing these aspects will be crucial for refining finite element analyses (FEAs) and advancing toward a fully automated, digital twin framework for personalized aortic rupture risk assessment.