Prebiopsy Magnetic Resonance Imaging Followed by Combination Biopsy for Prostate Cancer Diagnosis Is Associated with a Lower Risk of Biochemical Failure After Treatment Compared to Systematic Biopsy Alone
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MRI Details
2.3. Treatment and Follow-Up
2.4. Outcomes
2.5. Statistical Analysis
3. Results
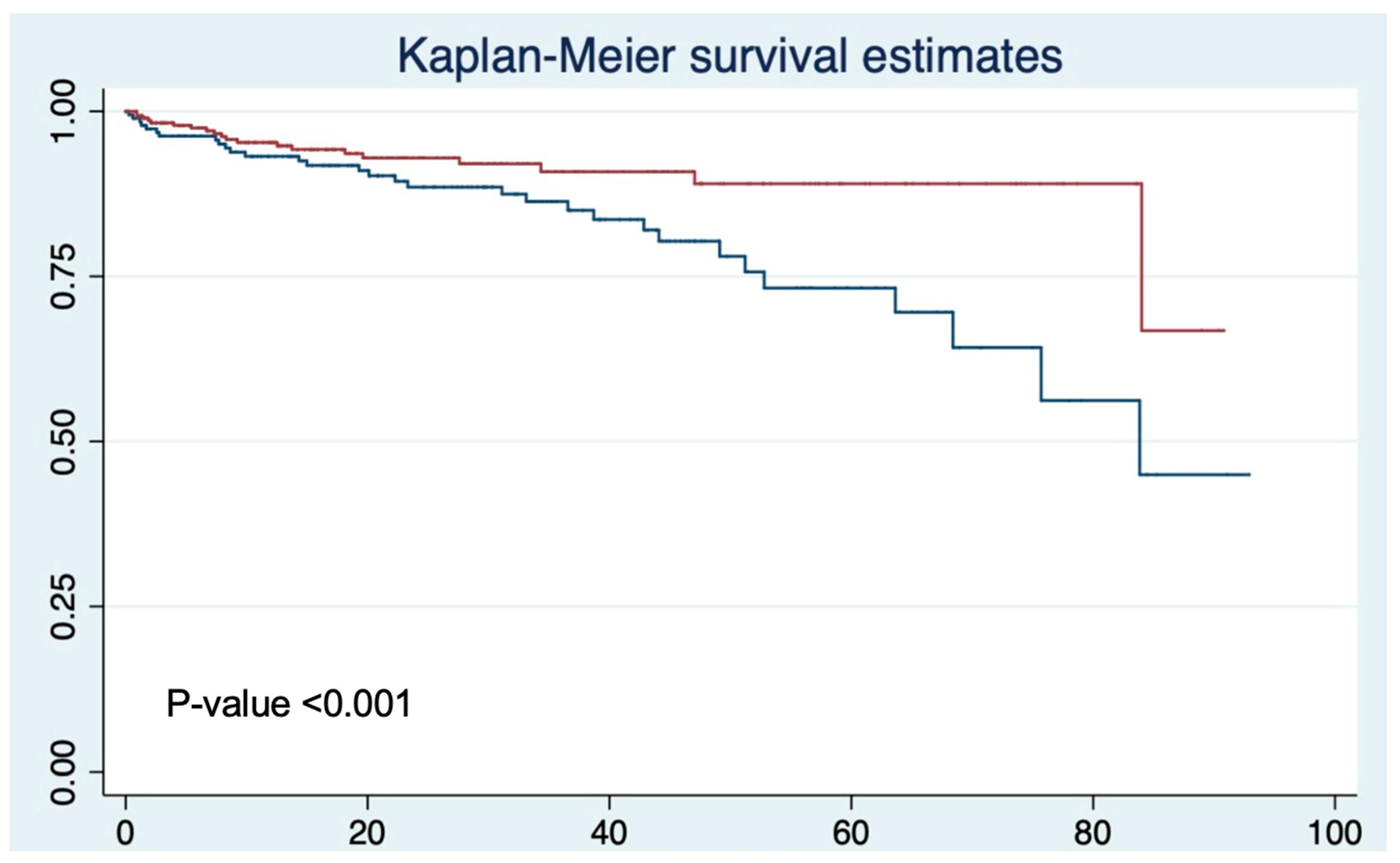
Biochemical Recurrence Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, S.; Debnath, N.; Sidana, A. A Review of Energy Modalities Used for Focal Therapy of Prostate Cancer. Curr. Surg. Rep. 2023, 11, 331–346. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of inci-dence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Devi, V.; Chaudhary, V.; Sharma, M.; Kumari, S.; Murti, K.; Meenakshi, S.; Pal, B. Serum levels of heavy metals in patients with prostate cancer: A systematic review and meta-analysis. Biol. Trace Elem. Res. 2025, 1–10. [Google Scholar] [CrossRef]
- Sidana, A.; Tayebi, S.; Blank, F.; Lama, D.J.; Meyer, M.; Saeed, Y.; Tobler, J.; Hsu, W.W.; Verma, S. Magnetic resonance imaging-ultrasound fusion guided focal cryoabla-tion for men with intermediate-risk prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2024, 42, 158.e1–158.e10. [Google Scholar]
- Fernandes, M.C.; Yildirim, O.; Woo, S.; Vargas, H.A.; Hricak, H. The role of MRI in prostate cancer: Current and future directions. Magn. Reson. Mater. Phys. Biol. Med. 2022, 35, 503–521. [Google Scholar] [CrossRef]
- Drost, F.H.; Osses, D.; Nieboer, D.; Bangma, C.H.; Steyerberg, E.W.; Roobol, M.J.; Schoots, I.G. Prostate magnetic resonance imaging, with or without magnetic resonance imaging-targeted biopsy, and systematic biopsy for detecting prostate cancer: A Cochrane sys-tematic review and meta-analysis. Eur. Urol. 2020, 77, 78–94. [Google Scholar] [CrossRef]
- Shariat, S.F.; Roehrborn, C.G. Using biopsy to detect prostate cancer. Rev. Urol. 2008, 10, 262–280. [Google Scholar]
- Sidana, A.; Watson, M.J.; George, A.K.; Rastinehad, A.R.; Vourganti, S.; Rais-Bahrami, S.; Muthigi, A.; Maruf, M.; Gordetsky, J.B.; Nix, J.W.; et al. Fusion prostate biopsy outperforms 12-core systematic prostate biopsy in patients with prior negative systematic biopsy: A multi-institutional analysis. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 341.e1–341.e7. [Google Scholar] [CrossRef]
- Sussman, J.; Haj-Hamed, M.; Talarek, J.; Verma, S.; Sidana, A. How does a prebiopsy mri approach for prostate cancer diagnosis affect prostatectomy upgrade rates? Urol. Oncol. Semin. Orig. Investig. 2021, 39, 784.e11–784.e16. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Stabile, A.; Neves, J.B.; Giganti, F.; Valerio, M.; Shanmugabavan, Y.; Clement, K.D.; Sarkar, D.; Philippou, Y.; Thurtle, D.; et al. Magnetic resonance imaging-targeted biopsy versus systematic biopsy in the detection of prostate cancer: A systematic review and meta-analysis. Eur. Urol. 2019, 76, 284–303. [Google Scholar] [CrossRef] [PubMed]
- Ahdoot, M.; Wilbur, A.R.; Reese, S.E.; Lebastchi, A.H.; Mehralivand, S.; Gomella, P.T.; Bloom, J.; Gurram, S.; Siddiqui, M.; Pinsky, P.; et al. MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. N. Engl. J. Med. 2020, 382, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.Y.; Koh, D.H.; Goh, H.J.; Kim, M.S.; Lee, J.S.; Jang, W.S.; Choi, Y.D. Clinical significance and predictors of oncologic outcome after radical prostatectomy for invisible prostate cancer on multiparametric MRI. BMC Cancer 2018, 18, 1057. [Google Scholar] [CrossRef]
- He, J.; Albertsen, P.C.; Moore, D.; Rotter, D.; Demissie, K.; Lu-Yao, G. Validation of a contemporary five-tiered Gleason grade grouping using population-based data. Eur. Urol. 2017, 71, 760–763. [Google Scholar] [CrossRef]
- Siddiqui, M.M.; Rais-Bahrami, S.; Turkbey, B.; George, A.K.; Rothwax, J.; Shakir, N.; Okoro, C.; Raskolnikov, D.; Parnes, H.L.; Linehan, W.M.; et al. Comparison of MR/ultrasound fusion–guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015, 313, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef]
- Rouvière, O.; Puech, P.; Renard-Penna, R.; Claudon, M.; Roy, C.; Mège-Lechevallier, F.; Decaussin-Petrucci, M.; Dubreuil-Chambardel, M.; Magaud, L.; Remontet, L.; et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): A prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019, 20, 100–109. [Google Scholar] [CrossRef]
- Eklund, M.; Jäderling, F.; Discacciati, A.; Bergman, M.; Annerstedt, M.; Aly, M.; Glaessgen, A.; Carlsson, S.; Grönberg, H.; Nordström, T. MRI-targeted or standard biopsy in prostate cancer screening. N. Engl. J. Med. 2021, 385, 908–920. [Google Scholar] [CrossRef]
- Tayebi, S.; Verma, S.; Sidana, A. Real-time and delayed imaging of tissue and effects of prostate tissue ablation. Curr. Urol. Rep. 2023, 24, 477–489. [Google Scholar] [CrossRef]
- Diamand, R.; Peltier, A.; Roche, J.; Lievore, E.; Lacetera, V.; Chiacchio, G.; Beatrici, V.; Mastroianni, R.; Simone, G.; Windisch, O.; et al. Risk stratification for early biochemical recurrence of prostate cancer in the era of multiparametric magnetic resonance imagining-targeted biopsy. Prostate 2023, 83, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Godtman, R.A.; Schoots, I.G. Key learning on the promise and limitations of MRI in prostate cancer screening. Eur. Radiol. 2024, 34, 6168–6174. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.C.; Lebastchi, A.H.; Hadaschik, B.A.; Emberton, M.; Moore, C.; Laguna, P.; Fütterer, J.J.; George, A.K. Role of MRI for the detection of prostate cancer. World J. Urol. 2021, 39, 637–649. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer—2024 update. Part I: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef]
- van der Leest, M.; Cornel, E.; Israel, B.; Hendriks, R.; Padhani, A.R.; Hoogenboom, M.; Zamecnik, P.; Bakker, D.; Setiasti, A.Y.; Veltman, J.; et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic reso-nance-guided biopsy in biopsy-naïve men with elevated prostate-specific antigen: A large prospective multicenter clinical study. Eur. Urol. 2019, 75, 570–578. [Google Scholar] [PubMed]
- Buyyounouski, M.K.; Choyke, P.L.; McKenney, J.K.; Sartor, O.; Sandler, H.M.; Amin, M.B.; Kattan, M.W.; Lin, D.W. Prostate cancer—Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA A Cancer J. Clin. 2017, 67, 245–253. [Google Scholar] [CrossRef]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A contemporary prostate cancer grading system: A validated alternative to the Gleason score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading Committee. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: Definition of grading patterns and proposal for a new grading system. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- American Cancer Society. Prostate Cancer Pathology. 2023. Available online: https://www.cancer.org/cancer/diagnosis-staging/tests/biopsy-and-cytology-tests/understanding-your-pathology-report/prostate-pathology/prostate-cancer-pathology.html (accessed on 10 March 2025).
- Roach, M., III; Hanks, G.; Thames, H., Jr.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef]
- Gandaglia, G.; Ploussard, G.; Valerio, M.; Marra, G.; Moschini, M.; Martini, A.; Roumiguié, M.; Fossati, N.; Stabile, A.; Beauval, J.-B.; et al. Prognostic implications of multiparametric magnetic resonance imaging and concomitant systematic biopsy in predicting biochemical recurrence after radical prostatec-tomy in prostate cancer patients diagnosed with magnetic resonance imaging–targeted biopsy. Eur. Urol. Oncol. 2020, 3, 739–747. [Google Scholar] [CrossRef]
- Li, J.; Patil, D.; Sanda, M.G.; Filson, C.P. Cancer-specific outcomes for prostate cancer patients who had prebiopsy prostate MRI. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 58.e9–58.e15. [Google Scholar] [CrossRef] [PubMed]
- Bjurlin, M.A.; Carroll, P.R.; Eggener, S.; Fulgham, P.F.; Margolis, D.J.; Pinto, P.A.; Rosenkrantz, A.B.; Rubenstein, J.N.; Rukstalis, D.B.; Taneja, S.S.; et al. Update of the standard operating procedure on the use of multiparametric magnetic resonance imaging for the diagnosis, staging and management of prostate cancer. J. Urol. 2020, 203, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Zabihollahy, F.; Naim, S.; Wibulpolprasert, P.; Reiter, R.E.; Raman, S.S.; Sung, K. Understanding spatial correlation between mul-tiparametric MRI performance and prostate cancer. J. Magn. Reson. Imaging 2024, 60, 2184–2195. [Google Scholar] [PubMed]
- Bjurlin, M.A.; Mendhiratta, N.; Wysock, J.S.; Taneja, S.S. Multiparametric MRI and targeted prostate biopsy: Improvements in cancer detection, localization, and risk assessment. Cent. Eur. J. Urol. 2016, 69, 9. [Google Scholar]
- Sosnowski, R.; Zagrodzka, M.; Borkowski, T. The limitations of multiparametric magnetic resonance imaging also must be borne in mind. Cent. Eur. J. Urol. 2016, 69, 22–23. [Google Scholar]
- Patel, H.D.; Okabe, Y.; Rac, G.; Pahouja, G.; Desai, S.; Shea, S.M.; Gorbonos, A.; Quek, M.L.; Flanigan, R.C.; Goldberg, A.; et al. MRI versus non-MRI diagnostic pathways before radical prostatectomy: Impact on nerve-sparing, positive surgical margins, and biochemical recurrence. Urol. Oncol. Semin. Orig. Investig. 2023, 41, 104.e19–104.e27. [Google Scholar] [CrossRef]
- Vickers, A.; Carlsson, S.V.; Cooperberg, M. Routine use of magnetic resonance imaging for early detection of prostate cancer is not justified by the clinical trial evidence. Eur. Urol. 2020, 78, 304–306. [Google Scholar] [CrossRef]
| Systematic Biopsy (n = 189) | Combination Biopsy (n = 329) | p-Value | |
|---|---|---|---|
| Median age year (IQR) | 65.9 (60.5–71.0) | 64.6 (59.0–69.5) | 0.071 |
| Race (%) | <0.001 | ||
| White | 91 (48.2) | 241 (73.3) | |
| African American | 89 (47.1) | 81 (24.6) | |
| Positive family history of prostate cancer (%) | 36 (19.2) | 95 (29.7) | 0.009 |
| Median PSA ng/mL (IQR) | 7.1 (5.2–10.6) | 7.0 (5.3–102) | 0.886 |
| Median prostate volume mL (IQR) | 38.2(30.0–46.0) | 39.0 (30.0–51.0) | 0.605 |
| Abnormal DRE (%) | 50 (27.0) | 55 (17.0) | 0.007 |
| Grade group (%) | 0.848 | ||
| Group 1 | 16 (8.5) | 20 (6.2) | |
| Group 2 | 91 (48.2) | 165 (51.1) | |
| Group 3 | 41 (21.7) | 65 (20.1) | |
| Group 4 | 20 (10.6) | 38 (11.8) | |
| Group 5 | 21 (11.1) | 35 (10.8) | |
| MRI highest PI-RAD score (%) | |||
| PI-RAD 2 | 1 (0.3) | ||
| PI-RAD 3 | 13 (4.4) | ||
| PI-RAD 4 | 130 (44.1) | ||
| PI-RAD 5 | 151 (51.2) |
| Biochemical Recurrence No (n = 457) | Biochemical Recurrence Yes (n = 52) | p-Value | |
|---|---|---|---|
| Median age year (IQR) | 65.2 (59.6–69.6) | 65.9 (60.4–71.3) | 0.369 |
| Race (%) | 0.459 | ||
| White | 300 (66.7) | 32 (61.5) | |
| African American | 150 (33.3) | 20 (38.5) | |
| Positive family history of prostate cancer (%) | 121 (26.5) | 10 (19.6) | 0.288 |
| Median PSA ng/mL (IQR) | 6.8 (5.2–9.9) | 9.0 (5.8–13.6) | 0.002 |
| Median prostate volume ml (IQR) | 39.0 (30.0–48.6) | 37.9 (28.4–49.2) | 0.607 |
| Abnormal DRE (%) | 97 (21.2) | 8 (15.7) | 0.354 |
| Grade group (%) | <0.001 | ||
| Group 1 | 35 (7.6) | 1 (2) | |
| Group 2 | 242 (52.5) | 14 (27.5) | |
| Group 3 | 92 (20) | 14 (27.5) | |
| Group 4 | 53 (11.5) | 5 (9.8) | |
| Group 5 | 39 (8.5) | 17 (13.3) | |
| Biopsy type | <0.001 | ||
| Systematic biopsy (%) | 158 (33.9) | 31 (59.6) | |
| Combination biopsy (%) | 308 (93.6) | 21 (40.4) |
| Variable Name | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Race | |||
| African American | 0.98 | 0.50–1.92 | 0.949 |
| Biopsy type | |||
| Combination biopsy | 0.37 | 0.20–0.70 | 0.002 |
| Abnormal DRE | 0.68 | 0.31–1.49 | 0.332 |
| Biopsy grade group | 1.61 | 1.25–2.07 | <0.001 |
| PSA value | 1.09 | 1.02–1.17 | 0.008 |
| Age | 0.99 | 0.95–1.03 | 0.517 |
| Positive family history | 0.73 | 0.35–1.52 | 0.401 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tayebi, S.; Tremblay, S.; Koehler, J.; Lazarovich, A.; Blank, F.; Hsu, W.-W.; Verma, S.; Sidana, A. Prebiopsy Magnetic Resonance Imaging Followed by Combination Biopsy for Prostate Cancer Diagnosis Is Associated with a Lower Risk of Biochemical Failure After Treatment Compared to Systematic Biopsy Alone. Diagnostics 2025, 15, 698. https://doi.org/10.3390/diagnostics15060698
Tayebi S, Tremblay S, Koehler J, Lazarovich A, Blank F, Hsu W-W, Verma S, Sidana A. Prebiopsy Magnetic Resonance Imaging Followed by Combination Biopsy for Prostate Cancer Diagnosis Is Associated with a Lower Risk of Biochemical Failure After Treatment Compared to Systematic Biopsy Alone. Diagnostics. 2025; 15(6):698. https://doi.org/10.3390/diagnostics15060698
Chicago/Turabian StyleTayebi, Shima, Samuel Tremblay, Jason Koehler, Alon Lazarovich, Fernando Blank, Wei-Wen Hsu, Sadhna Verma, and Abhinav Sidana. 2025. "Prebiopsy Magnetic Resonance Imaging Followed by Combination Biopsy for Prostate Cancer Diagnosis Is Associated with a Lower Risk of Biochemical Failure After Treatment Compared to Systematic Biopsy Alone" Diagnostics 15, no. 6: 698. https://doi.org/10.3390/diagnostics15060698
APA StyleTayebi, S., Tremblay, S., Koehler, J., Lazarovich, A., Blank, F., Hsu, W.-W., Verma, S., & Sidana, A. (2025). Prebiopsy Magnetic Resonance Imaging Followed by Combination Biopsy for Prostate Cancer Diagnosis Is Associated with a Lower Risk of Biochemical Failure After Treatment Compared to Systematic Biopsy Alone. Diagnostics, 15(6), 698. https://doi.org/10.3390/diagnostics15060698





