AI-Driven Advances in Low-Dose Imaging and Enhancement—A Review
Abstract
1. Introduction
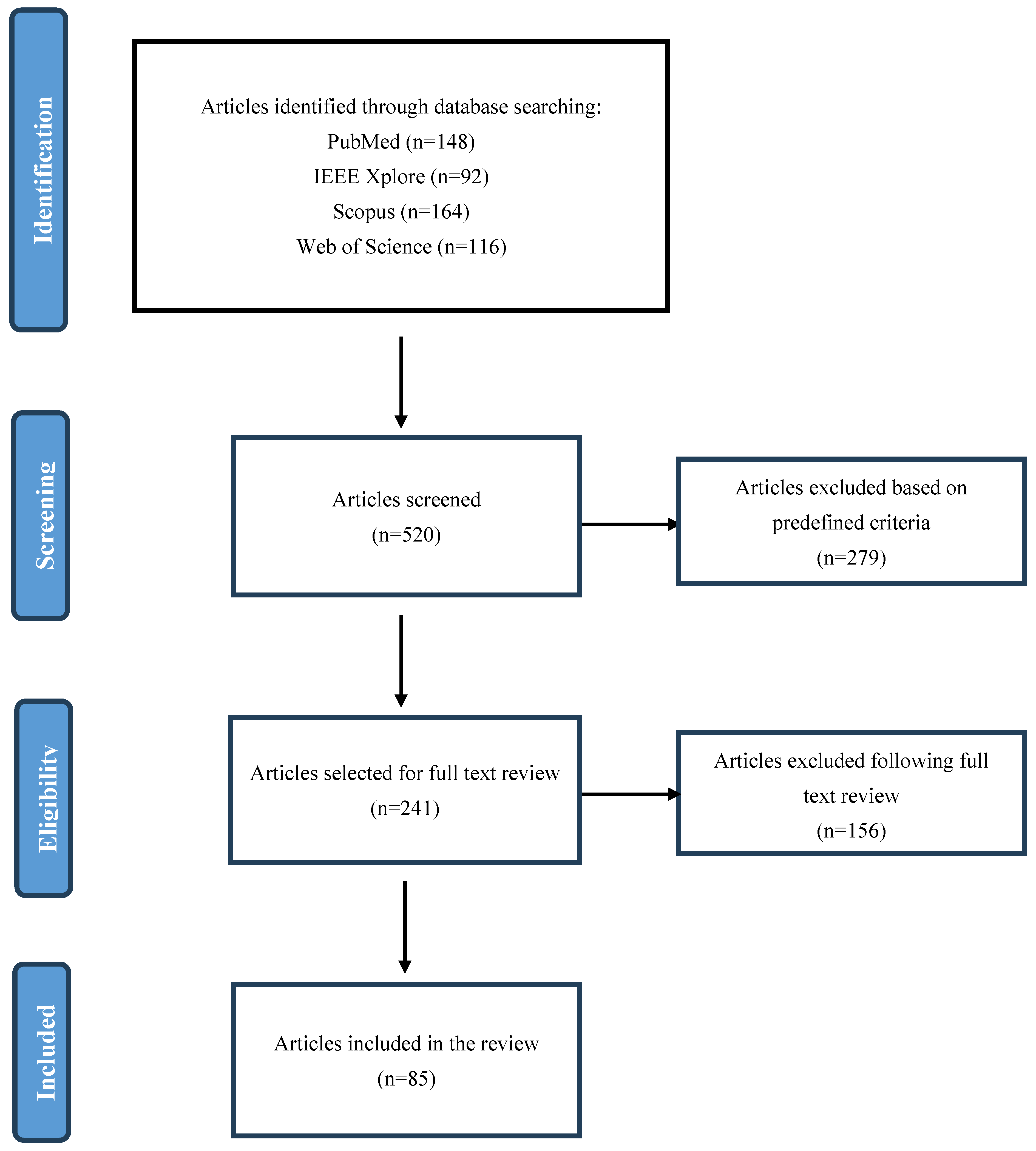
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.2.1. Inclusion Criteria
- Peer-reviewed journal articles and conference proceedings discussing AI-assisted low-dose imaging;
- Studies evaluating AI algorithms for radiation dose reduction, image enhancement, or diagnostic accuracy;
- Papers presenting clinical trials, retrospective analyses, or systematic reviews on AI in low-dose imaging;
- Research on AI applications in CT, X-ray, MRI, fluoroscopy, and PET imaging.
2.2.2. Exclusion Criteria
- Studies focusing only on AI in medical imaging without addressing radiation dose reduction;
- Non-English publications;
- Preprints, editorials, or opinion articles without experimental validation;
- Studies lacking quantitative results on AI’s impact on image quality or radiation dose.
2.3. Study Selection and Screening
2.4. Data Extraction and Synthesis
- AI techniques used (e.g., CNNs, GANs, reinforcement learning);
- Medical imaging modality and radiation dose reduction methods;
- Key findings on image quality, diagnostic accuracy, and workflow efficiency.
3. Radiation Exposure in Medical Imaging: Risks and Challenges
3.1. Risks of Ionizing Radiation
3.2. Efforts to Reduce Exposure
4. Artificial Intelligence in Medical Imaging
4.1. Overview of AI in Radiology
4.2. AI-Assisted Low-Dose Protocols
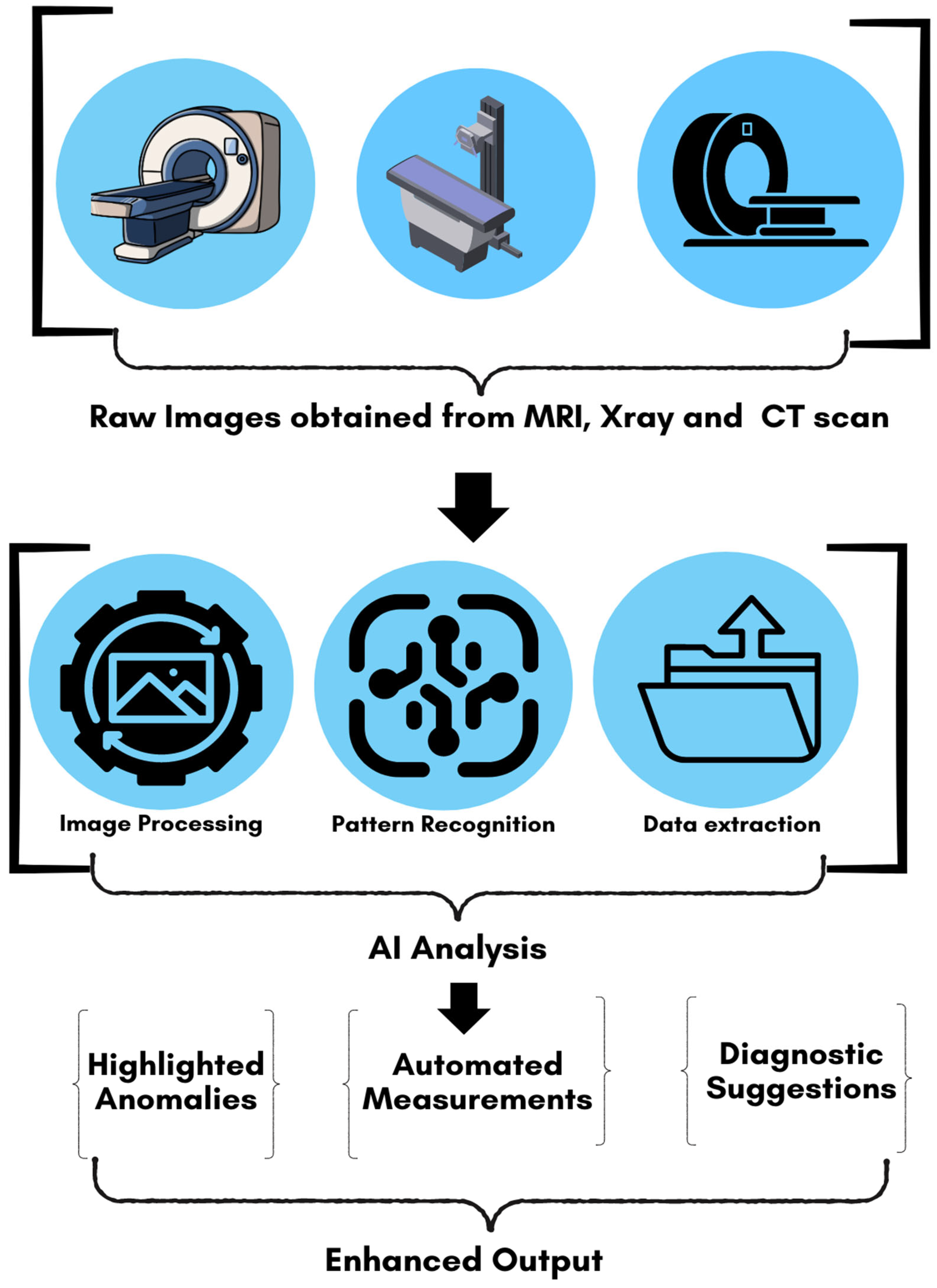
5. AI-Based Image Processing Techniques
5.1. Denoising Techniques
5.2. Artifact Reduction Techniques
5.3. Super-Resolution and Image Enhancement
5.4. Contrast Enhancement Techniques
6. Evaluation Metrics for AI-Based Low-Dose Imaging Techniques
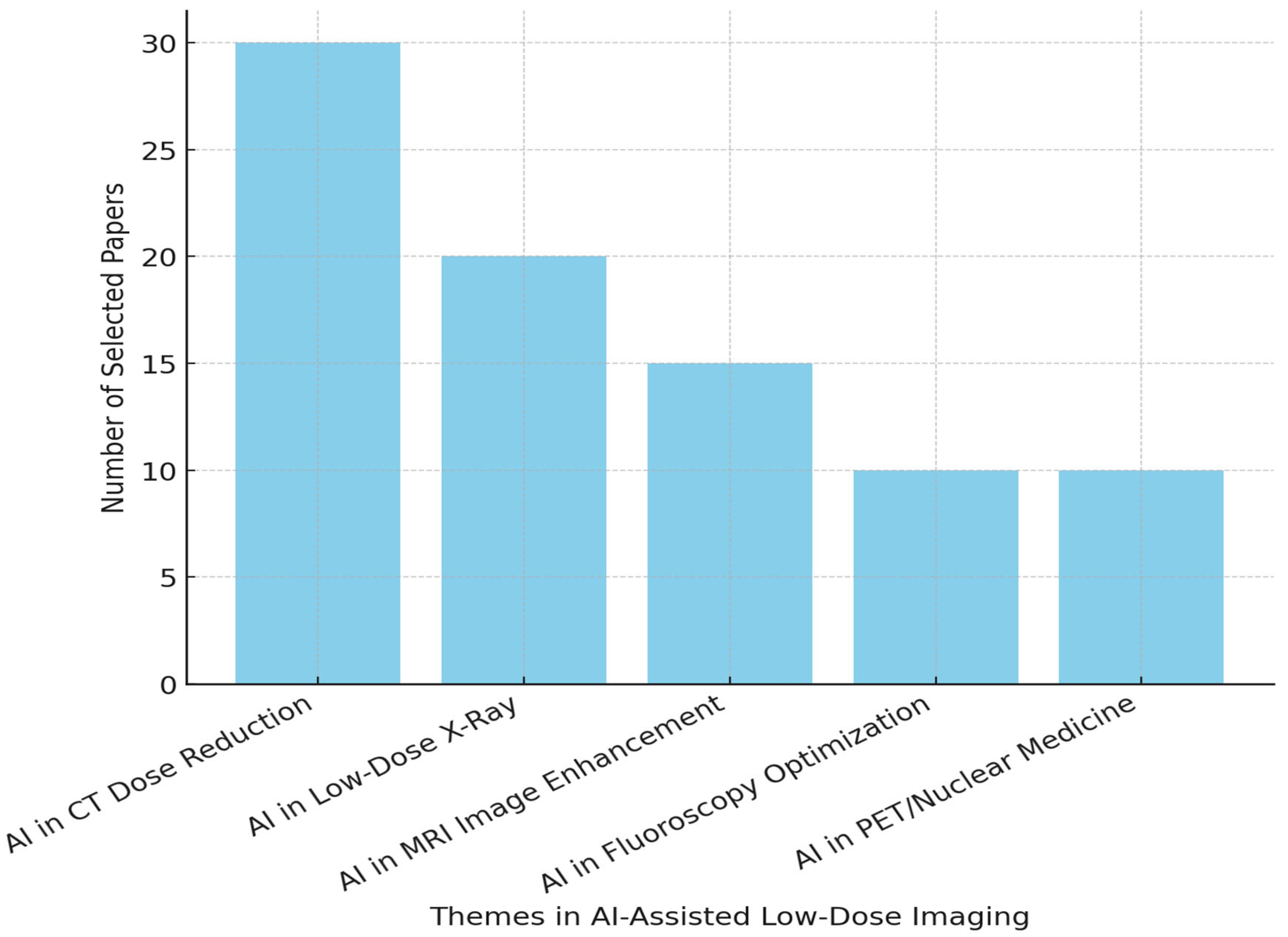
7. Key Applications of AI in Low-Dose Imaging Protocols
7.1. AI in CT
7.2. AI in X-Ray Imaging
7.3. Magnetic Resonance Imaging (MRI) and AI
8. Benefits of AI-Assisted Low-Dose Imaging
8.1. Reduced Patient Risk
8.2. Enhanced Image Quality
8.3. Cost Efficiency
9. Future Directions
9.1. Hybrid AI Systems
9.2. Personalized Imaging Protocols
9.3. Expansion to Other Modalities
10. Challenges and Limitations of AI in Low-Dose Imaging
10.1. Generalizability of AI Models
10.2. Ethical and Regulatory Considerations
10.3. Computational Demands and Practical Implementation
10.4. Open Challenges and Future Considerations
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abhisheka, B.; Biswas, S.K.; Purkayastha, B.; Das, D.; Escargueil, A. Recent trend in medical imaging modalities and their applications in disease diagnosis: A review. Multimed. Tools Appl. 2024, 83, 43035–43070. [Google Scholar] [CrossRef]
- Kissane, J.; Neutze, J.A.; Singh, H. (Eds.) Radiology Fundamentals: Introduction to Imaging & Technology; Springer Nature: Berlin Germany, 2020. [Google Scholar]
- Hussain, S.; Mubeen, I.; Ullah, N.; Shah, S.S.U.D.; Khan, B.A.; Zahoor, M.; Ullah, R.; Khan, F.A.; Sultan, M.A. Modern Diagnostic Imaging Technique Applications and Risk Factors in the Medical Field: A Review. Biomed Res. Int. 2022, 2022, 5164970. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.S.; Nasim, M.A.A.; Hossain, I.; Ullah, D.M.A.; Gupta, D.K.D.; Bhuiyan, M.M.H. Introduction of medical imaging modalities. In Data-Driven Approaches on Medical Imaging; Springer Nature: Cham, Switzerland, 2023; pp. 1–25. [Google Scholar]
- Tapper, W.; Carneiro, G.; Mikropoulos, C.; Thomas, S.A.; Evans, P.M.; Boussios, S. The Application of Radiomics and AI to Molecular Imaging for Prostate Cancer. J. Pers. Med. 2024, 14, 287. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.J.; Zhang, J.; Mak, K.C.; Liu, Z.H.; Wang, H.Q. Low Radiation X-rays: Benefiting People Globally by Reducing Cancer Risks. Int. J. Med. Sci. 2021, 18, 73–80. [Google Scholar] [CrossRef]
- Nilsson, R.; Liu, N.A. Nuclear DNA damages generated by reactive oxygen molecules (ROS) under oxidative stress and their relevance to human cancers, including ionizing radiation-induced neoplasia part I: Physical, chemical and molecular biology aspects. Radiat. Med. Prot. 2020, 1, 140–152. [Google Scholar] [CrossRef]
- Averbeck, D.; Candéias, S.; Chandna, S.; Foray, N.; Friedl, A.A.; Haghdoost, S.; Jeggo, P.A.; Lumniczky, K.; Paris, F.; Quintens, R.; et al. Establishing mechanisms affecting the individual response to ionizing radiation. Int. J. Radiat. Biol. 2020, 96, 297–323. [Google Scholar] [CrossRef]
- Bastiani, L.; Paolicchi, F.; Faggioni, L.; Martinelli, M.; Gerasia, R.; Martini, C.; Cornacchione, P.; Ceccarelli, M.; Chiappino, D.; Della Latta, D.; et al. Patient Perceptions and Knowledge of Ionizing Radiation from Medical Imaging. JAMA Netw. Open 2021, 4, e2128561. [Google Scholar] [CrossRef]
- Kruger, E.; Toraih, E.A.; Hussein, M.H.; Shehata, S.A.; Waheed, A.; Fawzy, M.S.; Kandil, E. Thyroid Carcinoma: A Review for 25 Years of Environmental Risk Factors Studies. Cancers 2022, 14, 6172. [Google Scholar] [CrossRef]
- Al Khudairi, O.A.; Alasiri, R.S.A.; Al Saiary, S.O.S.; Al-Shalail, G.A.; Hadi, S.M.A.; Alyami, S.H.H.; Al Shreeh, N.H. Radiation in Diagnostic Imaging: An In-Depth Examination. J. Surv. Fish. Sci. 2023, 10, 118–124. [Google Scholar]
- Joyce, S.; O’Connor, O.J.; Maher, M.M.; McEntee, M.F. Strategies for dose reduction with specific clinical indications during computed tomography. Radiography 2020, 26, S62–S68. [Google Scholar] [CrossRef]
- Seeram, E. Dose Optimization in Digital Radiography: Physical Principles and Quality Control. In Digital Radiography; Springer: Singapore, 2019; pp. 213–227. [Google Scholar]
- Seeram, E.; Kanade, V. Image Processing and Analysis. In Artificial Intelligence in Medical Imaging Technology: An Introduction; Springer Nature: Cham, Switzerland, 2024; pp. 83–103. [Google Scholar]
- Seeram, E.; Kanade, V. Artificial Intelligence in Medical Imaging Technology at a Glance. In Artificial Intelligence in Medical Imaging Technology: An Introduction; Springer Nature: Cham, Switzerland, 2024; pp. 1–16. [Google Scholar]
- Suliman, I.I.; Khouqeer, G.A.; Ahmed, N.A.; Abuzaid, M.M.; Sulieman, A. Low-Dose Chest CT Protocols for Imaging COVID-19 Pneumonia: Technique Parameters and Radiation Dose. Life 2023, 13, 992. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.P.; Badea, C.T. Advances in micro-CT imaging of small animals. Phys. Med. 2021, 88, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Schofield, R.; King, L.; Tayal, U.; Castellano, I.; Stirrup, J.; Pontana, F.; Earls, J.; Nicol, E. Image reconstruction: Part 1—Understanding filtered back projection, noise and image acquisition. J. Cardiovasc. Comput. Tomogr. 2020, 14, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, U.C.; Ohagwu, C.C.; Aronu, M.E.; Okafor, C.E.; Idumah, C.I.; Okokpujie, I.P.; Chukwu, N.N.; Chukwunyelu, C.E. Ionizing radiation protection and the linear No-threshold controversy: Extent of support or counter to the prevailing paradigm. J. Environ. Radioact. 2022, 253–254, 106984. [Google Scholar] [CrossRef]
- Nguyen, X.V.; Oztek, M.A.; Nelakurti, D.D.; Brunnquell, C.L.; Mossa-Basha, M.; Haynor, D.R.; Prevedello, L.M. Applying Artificial Intelligence to Mitigate Effects of Patient Motion or Other Complicating Factors on Image Quality. Top. Magn. Reason. Imaging 2020, 29, 175–180. [Google Scholar] [CrossRef]
- Kulathilake, K.A.S.H.; Abdullah, N.A.; Sabri, A.Q.M.; Lai, K.W. A review on Deep Learning approaches for low-dose Computed Tomography restoration. Complex Intell. Syst. 2023, 9, 2713–2745. [Google Scholar] [CrossRef]
- Huynh, E.; Hosny, A.; Guthier, C.; Bitterman, D.S.; Petit, S.F.; Haas-Kogan, D.A.; Kann, B.; Aerts, H.J.W.L.; Mak, R.H. Artificial intelligence in radiation oncology. Nat. Rev. Clin. Oncol. 2020, 17, 771–781. [Google Scholar] [CrossRef]
- Veit-Haibach, P.; Herrmann, K. Machine Learning in Nuclear Medicine and Hybrid Imaging; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Raymond, C. An Evaluation of a Deep Learning Approach for Radiation Dose Reduction in 18F-FDG PET/MRI Pediatric Epilepsy Imaging. Master’s Thesis, The University of Western Ontario, London, ON, Canada, 2023. [Google Scholar]
- Jenkins, N.W.; Parrish, J.M.; Sheha, E.D.; Singh, K. Intraoperative risks of radiation exposure for the surgeon and patient. Ann. Transl. Med. 2021, 9, 84. [Google Scholar] [CrossRef]
- Zhang, Z.; Phang, C.C.; Tan, R.Y.; Pang, S.C.; Chandramohan, S.; Zhuang, K.D.; Sulaiman, M.S.; Tay, K.H.; Chong, T.T.; Tan, C.S. Does reducing radiation levels for procedures affect image quality and radiation to proceduralists? A double-blinded randomised study of two protocols. Clin. Radiol. 2021, 76, 157.e1–157.e10. [Google Scholar] [CrossRef]
- Immonen, E.; Wong, J.; Nieminen, M.; Kekkonen, L.; Roine, S.; Törnroos, S.; Lanca, L.; Guan, F.; Metsälä, E. The use of deep learning towards dose optimization in low-dose computed tomography: A scoping review. Radiography 2022, 28, 208–214. [Google Scholar] [CrossRef]
- Kambadakone, A. Artificial Intelligence and CT Image Reconstruction: Potential of a New Era in Radiation Dose Reduction. J. Am. Coll. Radiol. 2020, 17, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Rehani, M.M.; Nacouzi, D. Higher patient doses through X-ray imaging procedures. Phys. Med. 2020, 79, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Panayides, A.S.; Amini, A.; Filipovic, N.D.; Sharma, A.; Tsaftaris, S.A.; Young, A.; Foran, D.; Do, N.; Golemati, S.; Kurc, T.; et al. AI in Medical Imaging Informatics: Current Challenges and Future Directions. IEEE. J. Biomed. Health Inform. 2020, 24, 1837–1857. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, O.; Pinak, M. How often does it happen? A review of unintended, unnecessary and unavoidable high-dose radiation exposures. J. Radiol. Prot. 2021, 41, R189. [Google Scholar] [CrossRef]
- Akram, S.; Chowdhury, Y.S. Radiation Exposure of Medical Imaging; StatPearls: Petersburg, FL, USA, 2020. [Google Scholar]
- Bogard, J.S.; Downing, D.J.; Coleman, R.L.; Eckerman, K.F.; Turner, J.E. Atoms, Radiation, and Radiation Protection; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Toprani, S.M.; Scheibler, C.; Mordukhovich, I.; McNeely, E.; Nagel, Z.D. Cosmic Ionizing Radiation: A DNA Damaging Agent That May Underly Excess Cancer in Flight Crews. Int. J. Mol. Sci. 2024, 25, 7670. [Google Scholar] [CrossRef]
- Brambilla, M.; Vassileva, J.; Kuchcinska, A.; Rehani, M.M. Multinational data on cumulative radiation exposure of patients from recurrent radiological procedures: Call for action. Eur. Radiol. 2020, 30, 2493–2501. [Google Scholar] [CrossRef]
- Tsalafoutas, I.A.; Hassan Kharita, M.; Al-Naemi, H.; Kalra, M.K. Radiation dose monitoring in computed tomography: Status, options and limitations. Phys. Med. 2020, 79, 1–15. [Google Scholar] [CrossRef]
- Cramer, A.A.K. Design and Applications of Cold-Cathode X-ray Imaging Systems. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2021. [Google Scholar]
- Paulis, L.E.; Schnerr, R.S.; Halton, J.; Qin, Z.Z.; Chua, A. Assessment of scattered and leakage radiation from ultra-portable digital chest X-ray systems: An independent study. arXiv 2024, arXiv:2406.10044. [Google Scholar]
- Walsh, C.; O’Reilly, G.; Murphy, D. Patient cumulative radiation exposure-the potential for unintended consequences. Eur. Radiol. 2020, 30, 4434–4437. [Google Scholar] [CrossRef]
- Abalo, K.D.; Rage, E.; Leuraud, K.; Richardson, D.B.; Le Pointe, H.D.; Laurier, D.; Bernier, M.O. Early life ionizing radiation exposure and cancer risks: Systematic review and meta-analysis. Pediatr. Radiol. 2021, 51, 45–56. [Google Scholar] [CrossRef]
- Davis, K.L.; Fox, E.; Merchant, M.S.; Reid, J.M.; Kudgus, R.A.; Liu, X.; Minard, C.G.; Voss, S.; Berg, S.L.; Weigel, B.J.; et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): A multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2020, 21, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Marcu, L.G.; Chau, M.; Bezak, E. How much is too much? Systematic review of cumulative doses from radiological imaging and the risk of cancer in children and young adults. Crit. Rev. Oncol. Hematol. 2021, 160, 103292. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Hei, T.K. Aging and age-related health effects of ionizing radiation. Radiat. Med. Prot. 2020, 1, 15–23. [Google Scholar] [CrossRef]
- Aslanian, H.R.; Lee, J.H.; Canto, M.I. AGA Clinical Practice Update on Pancreas Cancer Screening in High-Risk Individuals: Expert Review. Gastroenterology 2020, 159, 358–362. [Google Scholar] [CrossRef]
- Bryant, P.A. Radiation Protection Optimisation in New Nuclear Build: Challenges in the Application of the As Low As Reasonably Achievable’(ALARA) Principle. Ph.D. Thesis, University of Surrey, Guildford, UK, 2021. [Google Scholar]
- Aloufi, M.A.T.; Alharthi, A.K.Z.; Alzhrani, H.A.T.; Alsubhi, S.K.M.; Abassafa, A.S. Unveiling The Realities: A Comprehensive Examination of Radiation Risks In Medical Imaging. J. Surv. Fish. Sci. 2023, 10, 46–52. [Google Scholar]
- Najjar, R. Redefining Radiology: A Review of Artificial Intelligence Integration in Medical Imaging. Diagnostics 2023, 13, 2760. [Google Scholar] [CrossRef]
- Tsapaki, V. Radiation dose optimization in diagnostic and interventional radiology: Current issues and future perspectives. Phys. Med. 2020, 79, 16–21. [Google Scholar] [CrossRef]
- Vinnikov, V.; Belyakov, O. Clinical applications of biological dosimetry in patients exposed to low dose radiation due to radiological, imaging or nuclear medicine procedures. In Seminars in Nuclear Medicine; WB Saunders: Philadelphia, PA, USA, 2022; Volume 52, pp. 114–139. [Google Scholar]
- Lell, M.M.; Kachelrieß, M. Recent and Upcoming Technological Developments in Computed Tomography: High Speed, Low Dose, Deep Learning, Multienergy. Investig. Radiol. 2020, 55, 8–19. [Google Scholar] [CrossRef]
- Shao, Y.H.; Tsai, K.; Kim, S.; Wu, Y.J.; Demissie, K. Exposure to Tomographic Scans and Cancer Risks. JNCI Cancer Spectr. 2019, 4, pkz072. [Google Scholar] [CrossRef]
- Sookpeng, S.; Martin, C.J.; Krisanachinda, A. Effects of tube potential selection together with computed tomography automatic tube current modulation on CT imaging performance. J. Radiol. Prot. 2021, 41, 809. [Google Scholar] [CrossRef]
- Feldle, P.; Grunz, J.P.; Huflage, H.; Kunz, A.S.; Ergün, S.; Afat, S.; Gruschwitz, P.; Görtz, L.; Pennig, L.; Bley, T.A.; et al. Influence of helical pitch and gantry rotation time on image quality and file size in ultrahigh-resolution photon-counting detector CT. Sci. Rep. 2024, 14, 9358. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Sun, P.; Peterson, C.B.; Anderson, M.R.; Liu, X.; Morani, A.C.; Jensen, C.T. Low pitch significantly reduces helical artifacts in abdominal CT. Eur. J. Radiol. 2023, 166, 110977. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, P.; Mileto, A.; Yu, L.; Leng, S.; Guimaraes, L.S.; Missert, A.D.; Jensen, C.T.; Gong, H.; McCollough, C.H.; Fletcher, J.G. CT Noise-Reduction Methods for Lower-Dose Scanning: Strengths and Weaknesses of Iterative Reconstruction Algorithms and New Techniques. Radiographics 2021, 41, 1493–1508. [Google Scholar] [CrossRef] [PubMed]
- Ben Yedder, H.; Cardoen, B.; Hamarneh, G. Deep learning for biomedical image reconstruction: A survey. Artif. Intell. Rev. 2021, 54, 215–251. [Google Scholar] [CrossRef]
- Nagayama, Y.; Sakabe, D.; Goto, M.; Emoto, T.; Oda, S.; Nakaura, T.; Kidoh, M.; Uetani, H.; Funama, Y.; Hirai, T. Deep Learning-based Reconstruction for Lower-Dose Pediatric CT: Technical Principles, Image Characteristics, and Clinical Implementations. Radiographics 2021, 41, 1936–1953. [Google Scholar] [CrossRef]
- Arabi, H.; AkhavanAllaf, A.; Sanaat, A.; Shiri, I.; Zaidi, H. The promise of artificial intelligence and deep learning in PET and SPECT imaging. Phys. Med. 2021, 83, 122–137. [Google Scholar] [CrossRef]
- Moen, T.R.; Chen, B.; Holmes, D.R., 3rd; Duan, X.; Yu, Z.; Yu, L.; Leng, S.; Fletcher, J.G.; McCollough, C.H. Low-dose CT image and projection dataset. Med. Phys. 2021, 48, 902–911. [Google Scholar] [CrossRef]
- Mun, S.K.; Wong, K.H.; Lo, S.B.; Li, Y.; Bayarsaikhan, S. Artificial Intelligence for the Future Radiology Diagnostic Service. Front. Mol. Biosci. 2021, 7, 614258. [Google Scholar] [CrossRef]
- Ahmad, Z.; Rahim, S.; Zubair, M.; Abdul-Ghafar, J. Artificial intelligence (AI) in medicine, current applications and future role with special emphasis on its potential and promise in pathology: Present and future impact, obstacles including costs and acceptance among pathologists, practical and philosophical considerations. A comprehensive review. Diagn. Pathol. 2021, 16, 24. [Google Scholar]
- Lin, A.; Kolossváry, M.; Išgum, I.; Maurovich-Horvat, P.; Slomka, P.J.; Dey, D. Artificial intelligence: Improving the efficiency of cardiovascular imaging. Expert Rev. Med. Devices 2020, 17, 565–577. [Google Scholar] [CrossRef]
- El Naqa, I.; Haider, M.A.; Giger, M.L.; Ten Haken, R.K. Artificial Intelligence: Reshaping the practice of radiological sciences in the 21st century. Br. J. Radiol. 2020, 93, 20190855. [Google Scholar] [CrossRef] [PubMed]
- Abdou, M.A. Literature review: Efficient deep neural networks techniques for medical image analysis. Neural Comput. Appl. 2022, 34, 5791–5812. [Google Scholar] [CrossRef]
- Martín Noguerol, T.; Paulano-Godino, F.; Martín-Valdivia, M.T.; Menias, C.O.; Luna, A. Strengths, Weaknesses, Opportunities, and Threats Analysis of Artificial Intelligence and Machine Learning Applications in Radiology. J. Am. Coll. Radiol. 2019, 16, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Enlow, E.; Abbaszadeh, S. State-of-the-art challenges and emerging technologies in radiation detection for nuclear medicine imaging: A review. Front. Phys. 2023, 11, 1106546. [Google Scholar] [CrossRef]
- Wu, D.; Kim, K.; Li, Q. Low-dose CT reconstruction with Noise2Noise network and testing-time fine-tuning. Med. Phys. 2021, 48, 7657–7672. [Google Scholar] [CrossRef]
- Chen, H.; Li, Q.; Zhou, L.; Li, F. Deep learning-based algorithms for low-dose CT imaging: A review. Eur. J. Radiol. 2024, 172, 111355. [Google Scholar] [CrossRef]
- Yang, X.; De Andrade, V.; Scullin, W.; Dyer, E.L.; Kasthuri, N.; De Carlo, F.; Gürsoy, D. Low-dose X-ray tomography through a deep convolutional neural network. Sci. Rep. 2018, 8, 2575. [Google Scholar] [CrossRef]
- Chepelev, L.L.; Nicolaou, S.; Sheikh, A. AI for Medical Image Processing: Improving Quality, Accessibility, and Safety. In AI in Clinical Medicine: A Practical Guide for Healthcare Professionals; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 350–364. [Google Scholar]
- Archana, R.; Jeevaraj, P.S.E. Deep learning models for digital image processing: A review. Artif. Intell. Rev. 2024, 57, 11. [Google Scholar] [CrossRef]
- Alnaggar, O.A.M.F.; Jagadale, B.N.; Saif, M.A.N.; Ghaleb, O.A.M.; Ahmed, A.A.Q.; Aqlan, H.A.A.; Al-Arik, H.D.E. Efficient artificial intelligence approaches for medical image processing in healthcare: Comprehensive review, taxonomy, and analysis. Artif. Intell. Rev. 2024, 57, 221. [Google Scholar] [CrossRef]
- Pierre, K.; Haneberg, A.G.; Kwak, S.; Peters, K.R.; Hochhegger, B.; Sananmuang, T.; Tunlayadechanont, P.; Tighe, P.J.; Mancuso, A.; Forghani, R. Applications of artificial intelligence in the radiology roundtrip: Process streamlining, workflow optimization, and beyond. In Seminars in Roentgenology; WB Saunders: Philadelphia, PA, USA, 2023; Volume 58, pp. 158–169. [Google Scholar]
- Cellina, M.; Cacioppa, L.M.; Cè, M.; Chiarpenello, V.; Costa, M.; Vincenzo, Z.; Pais, D.; Bausano, M.V.; Rossini, N.; Bruno, A.; et al. Artificial Intelligence in Lung Cancer Screening: The Future Is Now. Cancers 2023, 15, 4344. [Google Scholar] [CrossRef]
- Sayed, I.S.; Mohd Yusof, M.I. Techniques and Strategies to Minimize Radiation Exposure in Pediatric Computed Tomography (CT) Abdominal Examinations: A Review. Cureus 2024, 16, e67494. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Coelho, L. How Artificial Intelligence Is Shaping Medical Imaging Technology: A Survey of Innovations and Applications. Bioengineering 2023, 10, 1435. [Google Scholar] [CrossRef] [PubMed]
- Lell, M.; Kachelrieß, M. Computed Tomography 2.0: New Detector Technology, AI, and Other Developments. Investig. Radiol. 2023, 58, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Barragán-Montero, A.; Javaid, U.; Valdés, G.; Nguyen, D.; Desbordes, P.; Macq, B.; Willems, S.; Vandewinckele, L.; Holmström, M.; Löfman, F.; et al. Artificial intelligence and machine learning for medical imaging: A technology review. Phys. Med. 2021, 83, 242–256. [Google Scholar] [CrossRef]
- Greffier, J.; Hamard, A.; Pereira, F.; Barrau, C.; Pasquier, H.; Beregi, J.P.; Frandon, J. Image quality and dose reduction opportunity of deep learning image reconstruction algorithm for CT: A phantom study. Eur. Radiol. 2020, 30, 3951–3959. [Google Scholar] [CrossRef]
- Koetzier, L.R.; Mastrodicasa, D.; Szczykutowicz, T.P.; van der Werf, N.R.; Wang, A.S.; Sandfort, V.; van der Molen, A.J.; Fleischmann, D.; Willemink, M.J. Deep Learning Image Reconstruction for CT: Technical Principles and Clinical Prospects. Radiology 2023, 306, e221257. [Google Scholar] [CrossRef]
- Kalita, A.J.; Boruah, A.; Das, T.; Mazumder, N.; Jaiswal, S.K.; Zhuo, G.Y.; Gogoi, A.; Kakoty, N.M.; Kao, F.J. Artificial Intelligence in Diagnostic Medical Image Processing for Advanced Healthcare Applications. In Biomedical Imaging: Advances in Artificial Intelligence and Machine Learning; Springer Nature: Singapore, 2024; pp. 1–61. [Google Scholar]
- Chen, Z.; Pawar, K.; Ekanayake, M.; Pain, C.; Zhong, S.; Egan, G.F. Deep learning for image enhancement and correction in magnetic resonance imaging—State-of-the-art and challenges. J. Digit. Imaging 2023, 36, 204–230. [Google Scholar] [CrossRef]
- Nazir, A.; Hussain, A.; Singh, M.; Assad, A. Deep learning in medicine: Advancing healthcare with intelligent solutions and the future of holography imaging in early diagnosis. Multimed. Tools Appl. 2024, 1–64. [Google Scholar] [CrossRef]
- Sadia, R.T.; Chen, J.; Zhang, J. CT image denoising methods for image quality improvement and radiation dose reduction. J. Appl. Clin. Med. Phys. 2024, 25, e14270. [Google Scholar] [CrossRef]
- Chi, J.; Wu, C.; Yu, X.; Ji, P.; Chu, H. Single low-dose CT image denoising using a generative adversarial network with modified U-Net generator and multi-level discriminator. IEEE Access 2020, 8, 133470–133487. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Li, J.; Yu, T.; Li, M.; Zhou, Z.; Peng, Y. Improving the image quality of pediatric chest CT angiography with low radiation dose and contrast volume using deep learning image reconstruction. Quant. Imaging Med. Surg. 2021, 11, 3051. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, D.; Hellwig, N.C.; Boehner, S.; Fuchs, T.; Fischer, R.; Schmidt, D. Artificial intelligence and deep learning for advancing PET image reconstruction: State-of-the-art and future directions. Nukl. -Nucl. 2023, 62, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhong, L.; Wu, H.; Li, S.; Li, Y. Remote sensing image Super-resolution reconstruction by fusing multi-scale receptive fields and hybrid transformer. Sci. Rep. 2025, 15, 2140. [Google Scholar] [CrossRef] [PubMed]
- Lohrke, J.; Frenzel, T.; Endrikat, J.; Alves, F.C.; Grist, T.M.; Law, M.; Lee, J.M.; Leiner, T.; Li, K.C.; Nikolaou, K.; et al. 25 years of contrast-enhanced MRI: Developments, current challenges and future perspectives. Adv. Ther. 2016, 33, 1–28. [Google Scholar] [CrossRef]
- Kambadakone, A.R.; Prakash, P.; Hahn, P.F.; Sahani, D.V. Low-dose CT examinations in Crohn’s disease: Impact on image quality, diagnostic performance, and radiation dose. Am. J. Roentgenol. 2010, 195, 78–88. [Google Scholar] [CrossRef]
- Seoni, S.; Shahini, A.; Meiburger, K.M.; Marzola, F.; Rotunno, G.; Acharya, U.R.; Molinari, F.; Salvi, M. All you need is data preparation: A systematic review of image harmonization techniques in Multi-center/device studies for medical support systems. Comput. Methods Programs Biomed. 2024, 250, 108200. [Google Scholar] [CrossRef]
- Tseng, H.H.; Luo, Y.; Cui, S.; Chien, J.T.; Ten Haken, R.K.; Naqa, I.E. Deep reinforcement learning for automated radiation adaptation in lung cancer. Med. Phys. 2017, 44, 6690–6705. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, G.; Liang, H.; Liu, J.; Ma, L.; Wang, T.; Guo, Y.; Chen, Y.; Yan, Z.; Chen, X.; et al. A Lung CT Foundation Model Facilitating Disease Diagnosis and Medical Imaging. medRxiv 2025. [Google Scholar] [CrossRef]
- Marcos, L.; Babyn, P.; Alirezaie, J. Generative AI in Medical Imaging and Its Application in Low Dose Computed Tomography (CT) Image Denoising. In Applications of Generative AI 2024; Springer International Publishing: Cham, Switzerland, 2024; pp. 387–401. [Google Scholar]
- Fujioka, T.; Satoh, Y.; Imokawa, T.; Mori, M.; Yamaga, E.; Takahashi, K.; Kubota, K.; Onishi, H.; Tateishi, U. Proposal to improve the image quality of short-acquisition time-dedicated breast positron emission tomography using the pix2pix generative adversarial network. Diagnostics 2022, 12, 3114. [Google Scholar] [CrossRef]
- Suriyan, K.; Ramaingam, N.; Rajagopal, S.; Sakkarai, J.; Asokan, B.; Alagarsamy, M. Performance analysis of peak signal-to-noise ratio and multipath source routing using different denoising method. Bull. Electr. Eng. Inform. 2022, 11, 286–292. [Google Scholar] [CrossRef]
- Yamashita, K.; Markov, K. Medical image enhancement using super-resolution methods. In Proceedings of the Computational Science–ICCS 2020: 20th International Conference, Amsterdam, The Netherlands, 3–5 June 2020; Springer International Publishing: Cham, Switzerland, 2020; pp. 496–508. [Google Scholar]
- Isgut, M.; Gloster, L.; Choi, K.; Venugopalan, J.; Wang, M.D. Systematic review of advanced AI methods for improving healthcare data quality in post COVID-19 Era. IEEE Rev. Biomed. Eng. 2022, 16, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Niu, T.; Tang, S.; Yang, X.; Kadom, N.; Tang, X. Content-oriented sparse representation (COSR) for CT denoising with preservation of texture and edge. Med. Phys. 2018, 45, 4942–4954. [Google Scholar] [CrossRef] [PubMed]
- Karthik, A.; Aggarwal, K.; Kapoor, A.; Singh, D.; Hu, L.; Gandhamal, A.; Kumar, D. Comprehensive assessment of imaging quality of artificial intelligence-assisted compressed sensing-based MR images in routine clinical settings. BMC Med. Imaging 2024, 24, 284. [Google Scholar] [CrossRef]
- Kazimierczak, W.; Kazimierczak, N.; Wilamowska, J.; Wojtowicz, O.; Nowak, E.; Serafin, Z. Enhanced visualization in endoleak detection through iterative and AI-noise optimized spectral reconstructions. Sci. Rep. 2024, 14, 3845. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, D.V.; Maindola, M.; Jose, R.; Kaliappan, S.; Patel, M.; Maranan, R. AI-Powered Multi View Face Video Super-Resolution Techniques for Real-Time Video Processing. In Proceedings of the 2024 International Conference on Integrated Intelligence and Communication Systems (ICIICS), Kalaburagi, India, 22–23 November 2024; IEEE: Piscateville, NJ, USA, 2024; pp. 1–7. [Google Scholar]
- Wang, B.; Dabbaghjamanesh, M.; Kavousi-Fard, A.; Yue, Y. AI-enhanced multi-stage learning-to-learning approach for secure smart cities load management in IoT networks. Ad Hoc Netw. 2024, 164, 103628. [Google Scholar] [CrossRef]
- Vranješ, M.; Rimac-Drlje, S.; Vranješ, D. Foveation-based content adaptive root mean squared error for video quality assessment. Multimed. Tools Appl. 2018, 77, 21053–21082. [Google Scholar] [CrossRef]
- Arabboev, M.; Begmatov, S.; Rikhsivoev, M.; Nosirov, K.; Saydiakbarov, S. A comprehensive review of image super-resolution metrics: Classical and AI-based approaches. Acta IMEKO 2024, 13, 1–8. [Google Scholar] [CrossRef]
- Sachdeva, I.; Ramesh, S.; Chadha, U.; Punugoti, H.; Selvaraj, S.K. Computational AI models in VAT photopolymerization: A review, current trends, open issues, and future opportunities. Neural Comput. Appl. 2022, 34, 17207–17229. [Google Scholar] [CrossRef]
- Graham, S.; Minhas, F.; Bilal, M.; Ali, M.; Tsang, Y.W.; Eastwood, M.; Wahab, N.; Jahanifar, M.; Hero, E.; Dodd, K.; et al. Screening of normal endoscopic large bowel biopsies with interpretable graph learning: A retrospective study. Gut 2023, 72, 1709–1721. [Google Scholar] [CrossRef]
- Ong, W.; Lee, A.; Tan, W.C.; Fong, K.T.; Lai, D.D.; Tan, Y.L.; Low, X.Z.; Ge, S.; Makmur, A.; Ong, S.J.; et al. Oncologic applications of artificial intelligence and deep learning methods in CT spine imaging—A systematic review. Cancers 2024, 16, 2988. [Google Scholar] [CrossRef]
- Dedeene, L.; Van Elslande, J.; Dewitte, J.; Martens, G.; De Laere, E.; De Jaeger, P.; De Smet, D. An artificial intelligence-driven support tool for predicting urine culture test results. Clin. Chim. Acta 2024, 562, 119854. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.; Carrasquillo, J.A.; Turkbey, E.B.; Mena, E.; Lindenberg, L.; Eclarinal, P.C.; Nilubol, N.; Choyke, P.L.; Floudas, C.S.; Lin, F.I.; et al. An automated pheochromocytoma and paraganglioma lesion segmentation AI-model at whole-body 68Ga-DOTATATE PET/CT. EJNMMI Res. 2024, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Turcas, A.; Leucuta, D.; Balan, C.; Clementel, E.; Gheara, C.; Kacso, A.; Kelly, S.M.; Tanasa, D.; Cernea, D.; Achimas-Cadariu, P. Deep-learning magnetic resonance imaging-based automatic segmentation for organs-at-risk in the brain: Accuracy and impact on dose distribution. Phys. Imaging Radiat. Oncol. 2023, 27, 100454. [Google Scholar] [CrossRef] [PubMed]
- Polymeri, E.; Johnsson, Å.A.; Enqvist, O.; Ulén, J.; Pettersson, N.; Nordström, F.; Kindblom, J.; Trägårdh, E.; Edenbrandt, L.; Kjölhede, H. Artificial Intelligence-Based Organ Delineation for Radiation Treatment Planning of Prostate Cancer on Computed Tomography. Adv. Radiat. Oncol. 2024, 9, 101383. [Google Scholar] [CrossRef]
- Doman, K.; Konishi, T.; Mekada, Y. Lesion Image Synthesis Using DCGANs for Metastatic Liver Cancer Detection. Adv. Exp. Med. Biol. 2020, 1213, 95–106. [Google Scholar]
- Kuang, Y.; Lan, T.; Peng, X.; Selasi, G.E.; Liu, Q.; Zhang, J. Unsupervised Multi-Discriminator Generative Adversarial Network for Lung Nodule Malignancy Classification. IEEE Access 2020, 8, 77725–77734. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.; Wang, M.; Shao, C.; Shi, L.; Yang, S.; Zhang, Z.; Feng, M.; Shan, F.; Liu, L. Combination of generative adversarial network and convolutional neural network for automatic subcentimeter pulmonary adenocarcinoma classification. Quant. Imaging Med. Surg. 2020, 10, 1249–1264. [Google Scholar] [CrossRef]
- Barakat, N.; Awad, M.; Abu-Nabah, B.A. A machine learning approach on chest X-rays for pediatric pneumonia detection. Digit. Health 2023, 9, 20552076231180008. [Google Scholar] [CrossRef]
- Morcos, G.; Yi, P.H.; Jeudy, J. Applying Artificial Intelligence to Pediatric Chest Imaging: Reliability of Leveraging Adult-Based Artificial Intelligence Models. J. Am. Coll. Radiol. 2023, 20, 742–747. [Google Scholar] [CrossRef]
- Ieracitano, C.; Mammone, N.; Versaci, M.; Varone, G.; Ali, A.R.; Armentano, A.; Calabrese, G.; Ferrarelli, A.; Turano, L.; Tebala, C.; et al. A fuzzy-enhanced deep learning approach for early detection of COVID-19 pneumonia from portable chest X-ray images. Neurocomputing 2022, 481, 202–215. [Google Scholar] [CrossRef]
- Baker, J.; Smith, R.; Taylor, L. The impact of AI on patient experience in imaging. J. Med. Imaging 2021, 28, 112–120. [Google Scholar]
- Johnson, M.; Lewis, A.; Kim, H. Reducing repeat scans through advanced imaging techniques: A review. Radiol. Innov. 2022, 5, 45–52. [Google Scholar]
- Salaudeen, H.D.; Aleem, A.O.; Solomon, E.U. Mitigating Radiation Effects and Enhancing Patient Comfort Through Image Processing and Data Analysis: Utilizing Deep Learning Models and State-Of-The-Art Technology in Medical Imaging. Int. Res. J. Mod. Eng. Technol. Sci. 2024, 6, 2582–5208. [Google Scholar]
- Maicas, G.; Carneiro, G.; Bradley, A.P.; Nascimento, J.C.; Reid, I. Deep reinforcement learning for active breast lesion detection from DCE-MRI. In International Conference on Medical Image Computing and Computer-Assisted Intervention; Springer International Publishing: Cham, Switzerland, 2017; pp. 665–673. [Google Scholar]
- Huda, W. CT radiation exposure: An overview. Curr. Radiol. Rep. 2015, 3, 80. [Google Scholar] [CrossRef]
- Lv, W.; Zhu, W.; Wang, M.; Hou, Y.; Xian, J.; Cao, D.; Wang, F.; Huang, G.; Xue, C.; Yang, Q.; et al. Application of Artificial Intelligence in Optimizing Medical Imaging Workflows. In Artificial Intelligence in Medical Imaging in China; Springer Nature: Singapore, 2024; pp. 111–131. [Google Scholar]
- Granata, C.; Sofia, C.; Francavilla, M.; Kardos, M.; Kasznia-Brown, J.; Nievelstein, R.A.; Olteanu, B.S.; Owens, C.; Salerno, S.; Sorantin, E.; et al. Let’s talk about radiation dose and radiation protection in children. Pediatr. Radiol. 2025, 55, 386–396. [Google Scholar] [CrossRef]
- Ahmad, I.S.; Li, N.; Wang, T.; Liu, X.; Dai, J.; Chan, Y.; Liu, H.; Zhu, J.; Kong, W.; Lu, Z.; et al. COVID-19 Detection via Ultra-Low-Dose X-ray Images Enabled by Deep Learning. Bioengineering 2023, 10, 1314. [Google Scholar] [CrossRef]
- Fantini, I.; Yasuda, C.; Bento, M.; Rittner, L.; Cendes, F.; Lotufo, R. Automatic MR image quality evaluation using a Deep CNN: A reference-free method to rate motion artifacts in neuroimaging. Comput. Med. Imaging Graph. 2021, 90, 101897. [Google Scholar] [CrossRef]
- Runge, V.M.; Richter, J.K.; Heverhagen, J.T. Speed in Clinical Magnetic Resonance. Investig. Radiol. 2017, 52, 1–17. [Google Scholar] [CrossRef]
- Tsui, B.; Calabrese, E.; Zaharchuk, G.; Rauschecker, A.M. Reducing Gadolinium Contrast With Artificial Intelligence. J. Magn. Reason. Imaging 2024, 60, 848–859. [Google Scholar] [CrossRef]
- Gupta, R.V.; Kalra, M.K.; Ebrahimian, S.; Kaviani, P.; Primak, A.; Bizzo, B.; Dreyer, K.J. Complex Relationship Between Artificial Intelligence and CT Radiation Dose. Acad. Radiol. 2022, 29, 1709–1719. [Google Scholar] [CrossRef]
- Palmer, J.D.; Tsang, D.S.; Tinkle, C.L.; Olch, A.J.; Kremer, L.C.M.; Ronckers, C.M.; Gibbs, I.C.; Constine, L.S. Late effects of radiation therapy in pediatric patients and survivorship. Pediatr. Blood Cancer 2021, 68, e28349. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.K. Artificial intelligence for radiation dose optimization in pediatric radiology: A systematic review. Children 2022, 14, 1044. [Google Scholar] [CrossRef]
- Ihlis, R.L.; Kadesjö, N.; Tsilingaridis, G.; Benchimol, D.; Shi, X.Q. Image quality assessment of low-dose protocols in cone beam computed tomography of the anterior maxilla. Oral Surg. Oral Med. Oral Pathol. Oral. Radiol. 2022, 133, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.W.; Siddiq, A.; Khan, I.R. A comparative study of medical image enhancement algorithms and quality assessment metrics on COVID-19 CT images. Signal Image Video Process. 2023, 17, 915–924. [Google Scholar] [CrossRef]
- Yuan, N.; Rao, S.; Chen, Q.; Sensoy, L.; Qi, J.; Rong, Y. Head and neck synthetic CT generated from ultra-low-dose cone-beam CT following Image Gently Protocol using deep neural network. Med. Phys. 2022, 49, 3263–3277. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.J.; Johnson, P.M.; Knoll, F.; Lui, Y.W. Artificial Intelligence for MR Image Reconstruction: An Overview for Clinicians. J. Magn. Reason. Imaging 2021, 53, 1015–1028. [Google Scholar] [CrossRef]
- Potočnik, J.; Foley, S.; Thomas, E. Current and potential applications of artificial intelligence in medical imaging practice: A narrative review. J. Med. Imaging Radiat. Sci. 2023, 54, 376–385. [Google Scholar] [CrossRef]
- Recht, M.P.; Dewey, M.; Dreyer, K.; Langlotz, C.; Niessen, W.; Prainsack, B.; Smith, J.J. Integrating artificial intelligence into the clinical practice of radiology: Challenges and recommendations. Eur. Radiol. 2020, 30, 3576–3584. [Google Scholar] [CrossRef]
- Dodda, S.; Narne, S.; Chintala, S.; Kanungo, S.; Adedoja, T.; Sharma, S. Exploring AI-driven Innovations in Image Communication Systems for Enhanced Medical Imaging Applications. J. Electr. Syst. 2024, 20, 949–959. [Google Scholar]
- Shiyam Sundar, L.K.; Muzik, O.; Buvat, I.; Bidaut, L.; Beyer, T. Potentials and caveats of AI in hybrid imaging. Methods 2021, 188, 4–19. [Google Scholar] [CrossRef]
- Pain, C.D.; Egan, G.F.; Chen, Z. Deep learning-based image reconstruction and post-processing methods in positron emission tomography for low-dose imaging and resolution enhancement. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3098–3118. [Google Scholar] [CrossRef] [PubMed]
- Swathi, B.; Polyakov, S.V.; Kandavalli, S.R.; Singh, D.K.; Murthy, M.Y.B.; Gopi, A. Enhancing hybrid manufacturing with AI-driven real-time adaptive process control: Integrating machine learning models and robotic systems. Int. J. Adv. Manuf. Technol. 2024, 1–9. [Google Scholar] [CrossRef]
- Brendlin, A.S.; Plajer, D.; Chaika, M.; Wrazidlo, R.; Estler, A.; Tsiflikas, I.; Artzner, C.P.; Afat, S.; Bongers, M.N. AI Denoising Significantly Improves Image Quality in Whole-Body Low-Dose Computed Tomography Staging. Diagnostics 2022, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Schindera, S.T.; Diedrichsen, L.; Müller, H.C.; Rusch, O.; Marin, D.; Schmidt, B.; Raupach, R.; Vock, P.; Szucs-Farkas, Z. Iterative reconstruction algorithm for abdominal multidetector CT at different tube voltages: Assessment of diagnostic accuracy, image quality, and radiation dose in a phantom study. Radiology 2011, 260, 454–462. [Google Scholar] [CrossRef]
- Papp, L.; Spielvogel, C.P.; Rausch, I.; Hacker, M.; Beyer, T. Personalizing Medicine Through Hybrid Imaging and Medical Big Data Analysis. Front. Phys. 2018, 6, 51. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Zhang, Y.; Ming, X.; Yu, J.; Carlson, D.J.; Kim, J.; Deng, J. Is it the time for personalized imaging protocols in cancer radiation therapy? Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 659–660. [Google Scholar] [CrossRef]
- Venkataraman, V.; Browning, T.; Pedrosa, I.; Abbara, S.; Fetzer, D.; Toomay, S.; Peshock, R.M. Implementing Shared, Standardized Imaging Protocols to Improve Cross-Enterprise Workflow and Quality. J. Digit. Imaging 2019, 32, 880–887. [Google Scholar] [CrossRef]
- Sharma, P.S.; Saindane, A.M. Standardizing Magnetic Resonance Imaging Protocols Across a Large Radiology Enterprise: Barriers and Solutions. Curr. Probl. Diagn. Radiol. 2020, 49, 312–316. [Google Scholar] [CrossRef]
- Gichoya, J.W.; Banerjee, I.; Bhimireddy, A.R.; Burns, J.L.; Celi, L.A.; Chen, L.C.; Correa, R.; Dullerud, N.; Ghassemi, M.; Huang, S.C.; et al. AI recognition of patient race in medical imaging: A modelling study. Lancet Digit. Health 2022, 4, e406–e414. [Google Scholar] [CrossRef]
- Almohammed, H.I.; Elshami, W.; Hamd, Z.Y.; Abuzaid, M. Optimizing CT Abdomen-Pelvis Scan Radiation Dose: Examining the Role of Body Metrics (Waist Circumference, Hip Circumference, Abdominal Fat, and Body Mass Index) in Dose Efficiency. Tomography 2024, 10, 643–653. [Google Scholar] [CrossRef]
- Rehani, M.M.; Miller, D.L.; Baliyan, V. High-Dose Fluoroscopically Guided Procedures in Patients: Radiation Management Recommendations for Interventionalists. Cardiovasc. Intervent. Radiol. 2021, 44, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Shiyam Sundar, L.K.; Gutschmayer, S.; Maenle, M.; Beyer, T. Extracting value from total-body PET/CT image data—The emerging role of artificial intelligence. Cancer Imaging 2024, 24, 51. [Google Scholar] [CrossRef] [PubMed]
- Reader, A.J.; Pan, B. AI for PET image reconstruction. Br. J. Radiol. 2023, 96, 20230292. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, R.; Shah, A.D.; Akin, O.; Do, R.K.G.; Konar, A.S.; Hatzoglou, V.; Mahmood, U.; Lee, N.; Wong, R.J.; Banerjee, S.; et al. Artificial Intelligence in CT and MR Imaging for Oncological Applications. Cancers 2023, 15, 2573. [Google Scholar] [CrossRef]
- Yang, J.; Clifton, L.; Dung, N.T.; Phong, N.T.; Yen, L.M.; Thy, D.B.; Soltan, A.A.; Thwaites, L.; Clifton, D.A. Mitigating machine learning bias between high income and low–middle income countries for enhanced model fairness and generalizability. Sci. Rep. 2024, 14, 13318. [Google Scholar] [CrossRef]
- Khan, M.S.; Umer, H.; Faruqe, F. Artificial intelligence for low income countries. Humanit. Soc. Sci. Commun. 2024, 11, 1422. [Google Scholar] [CrossRef]
- Sanida, T.; Dasygenis, M. A novel lightweight CNN for chest X-ray-based lung disease identification on heterogeneous embedded system. Appl. Intell. 2024, 54, 4756–4780. [Google Scholar] [CrossRef]

| Metric | Description | Typical Use Case | Performance Range in AI Models |
|---|---|---|---|
| Structural Similarity Index (SSIM) | Measures structural similarity between AI-enhanced and full-dose images. SSIM values range from 0 to 1, with higher values indicating a closer resemblance to reference images [93]. | Used in evaluating AI-based denoising and artifact reduction in CT and MRI [94]. | AI-based low-dose imaging models often achieve SSIM scores of ≥0.90, indicating near-full-dose image quality [95]. |
| Peak Signal-to-Noise Ratio (PSNR) | Evaluates the ratio between signal power and noise level in decibels (dB). Higher PSNR values indicate lower noise and better image clarity [96]. | Applied in noise suppression and super-resolution image enhancement [97]. | AI-driven reconstruction techniques typically improve PSNR by 4–6 dB over conventional low-dose methods [98]. |
| Contrast-to-Noise Ratio (CNR) | Measures how well contrast is preserved while suppressing noise, which is critical for detecting lesions and fine anatomical details [99]. | Used in AI-assisted contrast enhancement techniques [100]. | AI-based methods enhance CNR by 20–30%, improving lesion detectability [101]. |
| Mean Squared Error (MSE) | Quantifies pixel-level differences between AI-enhanced images and reference full-dose images. Lower MSE values indicate better reconstruction accuracy [93]. | Used in evaluating AI-driven super-resolution and artifact removal methods [102]. | AI-enhanced image processing techniques have shown 40% lower MSE compared to conventional approaches [103]. |
| Root Mean Squared Error (RMSE) | A variation of MSE that gives higher weight to larger pixel deviations, providing a more comprehensive assessment of image accuracy [104]. | Applied in evaluating AI-based image restoration methods [105]. | RMSE values are significantly reduced in AI-optimized imaging, leading to more reliable reconstructions [106]. |
| Area Under the Receiver Operating Characteristic Curve (AUC-ROC) | Assesses the ability of AI models to distinguish between normal and abnormal cases, often used in AI-based diagnostic classification [107]. | Used in AI-assisted lesion detection in low-dose CT and MRI [108]. | AI-driven detection models report AUC-ROC values of ≥0.95, demonstrating high diagnostic reliability [109]. |
| Dice Similarity Coefficient (DSC) | Measures segmentation accuracy by comparing AI-identified regions to expert-annotated reference regions. Values closer to 1 indicate better segmentation [110]. | Applied in AI-based segmentation tasks in CT and MRI [111]. | AI-based segmentation models achieve DSC values ≥ 0.85, ensuring precise anatomical delineation [112]. |
| Imaging Modality | AI Technique | Function | Benefits | Example/Study |
|---|---|---|---|---|
| CT | CNNs | Denoising, artifact reduction, image reconstruction | Improve LDCT image quality by reducing noise, enhancing resolution, and maintaining diagnostic accuracy even at lower radiation doses | A study found that AI models effectively denoised LDCT images and improved visualization of lung nodules without increasing radiation exposure [74]. |
| GANs | Image synthesis and enhancement | Generate high-quality images by learning from full-dose counterparts, offering significant dose reductions without sacrificing diagnostic utility. GANs have been explored to augment the training data for distinction capabilities of disease detection models. | The use of GANs increased the detection rate of metastatic liver lesions in abdominal CT scans from 65% to 95% [113]. The use of GANs increased the anomaly score on malignant images from 91.6% to 95.32% [114]. A study found that the use of GANs for data augmentation improved the detection accuracy of sub-centimetric pulmonary adenocarcinoma by about 8%, increasing from 53.2% to 60.5% [115]. | |
| X-ray Imaging | ML Models | Denoising, contrast enhancement, feature extraction | Allows for lower radiation doses, especially in pediatric, emergency and dental settings, by reducing noise and improving anatomical visualization | A study demonstrated that the Quadratic SVM model achieved a detection accuracy of 97.58% for pneumonia in the pediatric age group [116]. An AI algorithm from TorchXRayVision achieved an accuracy of 76.54% when applied to a publicly available pediatric chest X-ray dataset [117]. |
| DL-based models | Segmentation, feature detection for abnormalities | Enhance the detection of subtle fractures, lung lesions, or infections with low-dose X-rays, improving speed and accuracy in diagnostic workflows | A study using a fuzzy enhanced deep learning-based framework to differentiate between chest X-rays of COVID-19 pneumonia and interstitial pneumonias not caused by COVID-19 achieved a classification accuracy of up to 81% [118]. | |
| MRI | AI-driven reconstruction algorithms | Motion artifact correction, noise reduction, acceleration of image acquisition | Reduce scan times and patient discomfort, improve image resolution, reduce the need for contrast agents in certain cases | The integration of advanced image processing algorithms and deep learning models reduced scan time by an average of 20–30%, resulting in quicker and more efficient experience [119]. AI-enhanced imaging techniques improved image quality at lower radiation doses, reducing the need for repeat scans and leading to a 25% reduction in repeat scan rates, directly enhancing patient experiences [120]. A study collected quantitative data from surveys and found that over 80% of patients reported a positive overall experience with the improved imaging procedures, which led to a 25% reduction in repeat scan rates, directly enhancing patient experiences [121]. |
| Reinforcement Learning | Optimization of scan parameters during acquisition | Adapts scan settings in real-time based on patient-specific factors, leading to more efficient scans and reduced need for operator intervention | A novel algorithm for breast lesion detection from DCE-MRI achieved optimal detection accuracy with reduced run time complexity [122]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clement David-Olawade, A.; Olawade, D.B.; Vanderbloemen, L.; Rotifa, O.B.; Fidelis, S.C.; Egbon, E.; Akpan, A.O.; Adeleke, S.; Ghose, A.; Boussios, S. AI-Driven Advances in Low-Dose Imaging and Enhancement—A Review. Diagnostics 2025, 15, 689. https://doi.org/10.3390/diagnostics15060689
Clement David-Olawade A, Olawade DB, Vanderbloemen L, Rotifa OB, Fidelis SC, Egbon E, Akpan AO, Adeleke S, Ghose A, Boussios S. AI-Driven Advances in Low-Dose Imaging and Enhancement—A Review. Diagnostics. 2025; 15(6):689. https://doi.org/10.3390/diagnostics15060689
Chicago/Turabian StyleClement David-Olawade, Aanuoluwapo, David B. Olawade, Laura Vanderbloemen, Oluwayomi B. Rotifa, Sandra Chinaza Fidelis, Eghosasere Egbon, Akwaowo Owoidighe Akpan, Sola Adeleke, Aruni Ghose, and Stergios Boussios. 2025. "AI-Driven Advances in Low-Dose Imaging and Enhancement—A Review" Diagnostics 15, no. 6: 689. https://doi.org/10.3390/diagnostics15060689
APA StyleClement David-Olawade, A., Olawade, D. B., Vanderbloemen, L., Rotifa, O. B., Fidelis, S. C., Egbon, E., Akpan, A. O., Adeleke, S., Ghose, A., & Boussios, S. (2025). AI-Driven Advances in Low-Dose Imaging and Enhancement—A Review. Diagnostics, 15(6), 689. https://doi.org/10.3390/diagnostics15060689









