Detection of Respiratory Disease Based on Surface-Enhanced Raman Scattering and Multivariate Analysis of Human Serum
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Spectra Measurement
2.2. Blood Serum Samples
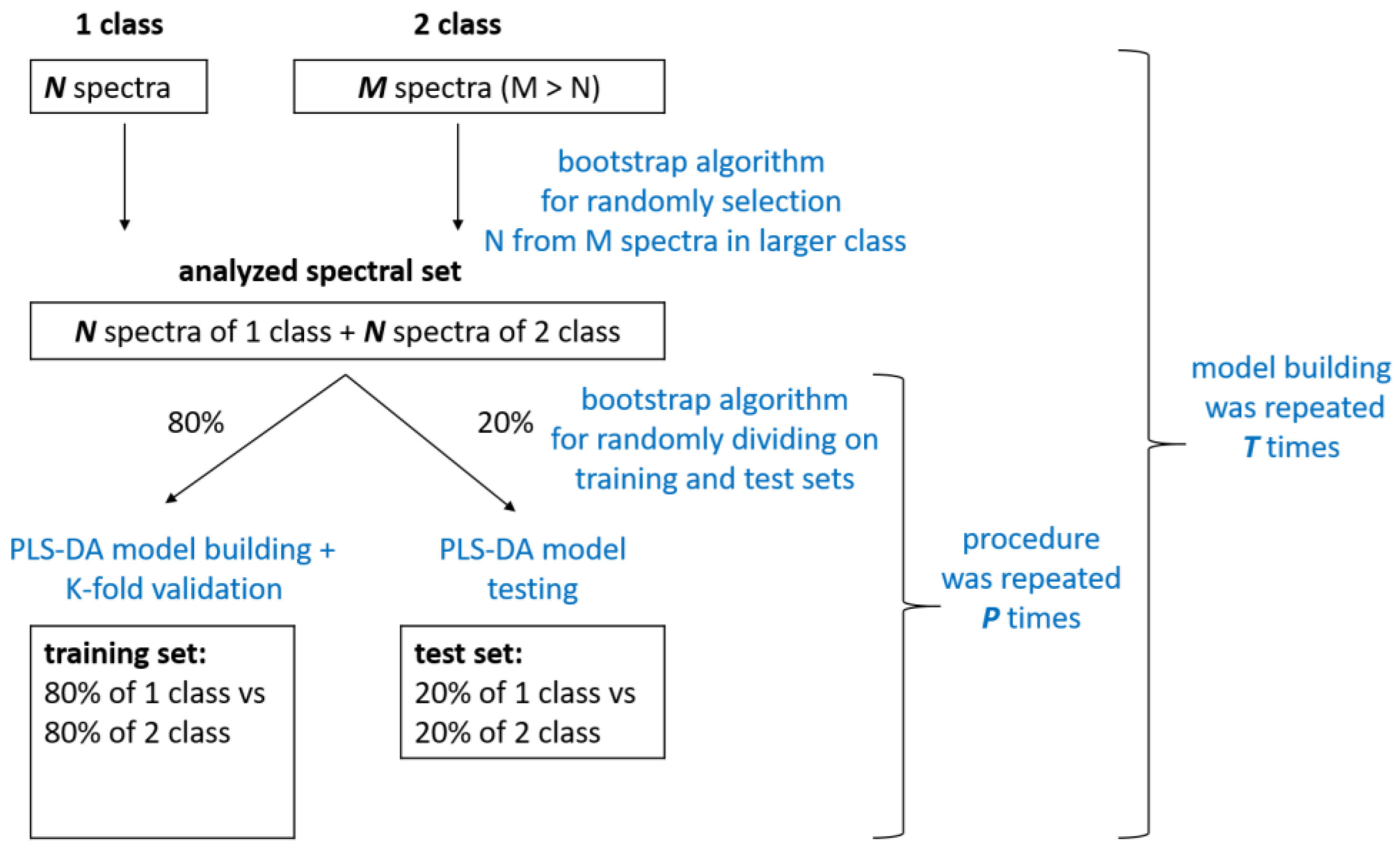
2.3. Spectra Preprocessing and Multivariate Statistical Analysis
- (1)
- “respiratory diseases (COPD + BA+ COPD&BA) vs. pathological referent group (CHF)”;
- (2)
- “COPD vs. BA”.
3. Results and Discussion
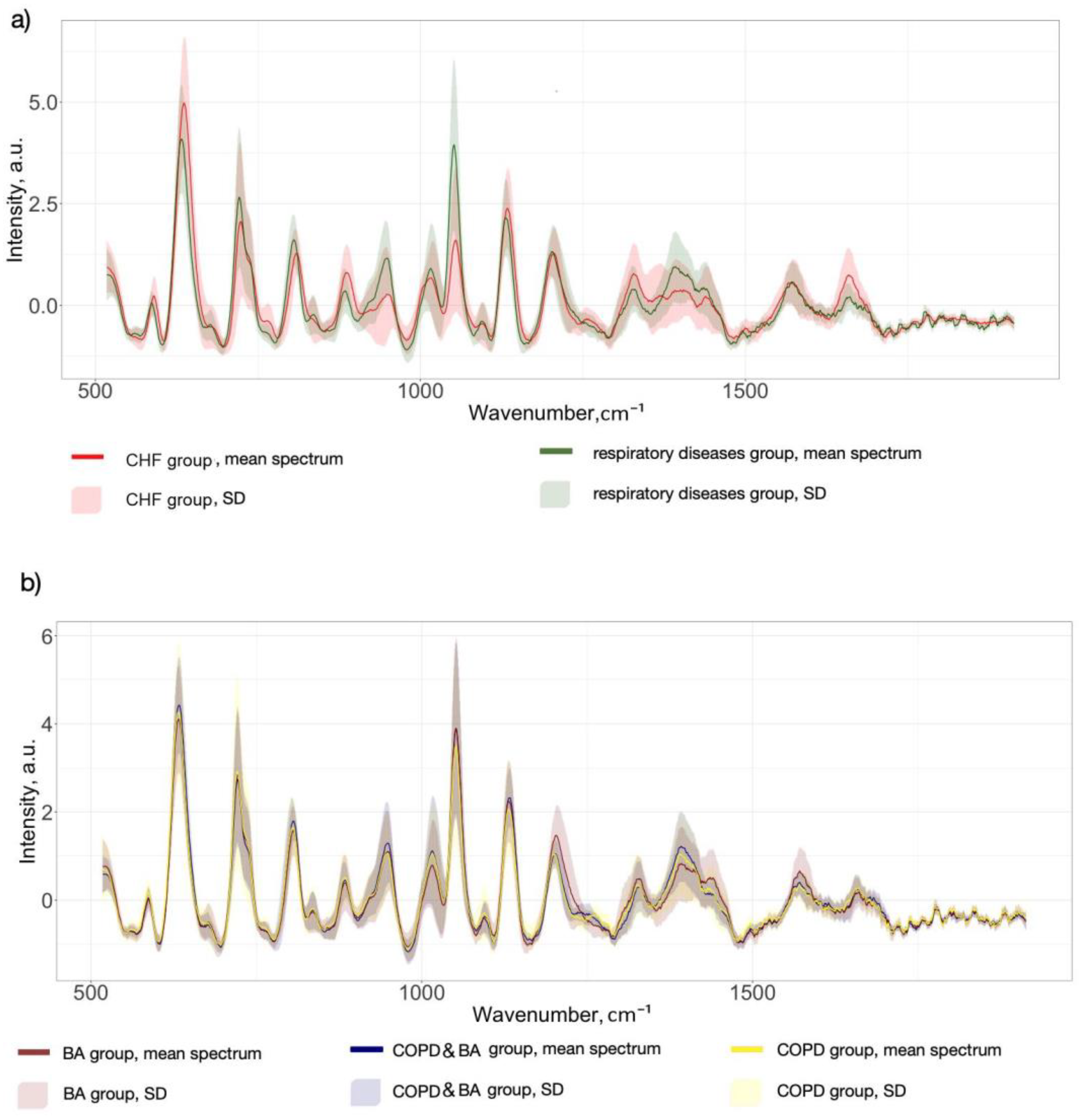
3.1. SERS Serum Spectra
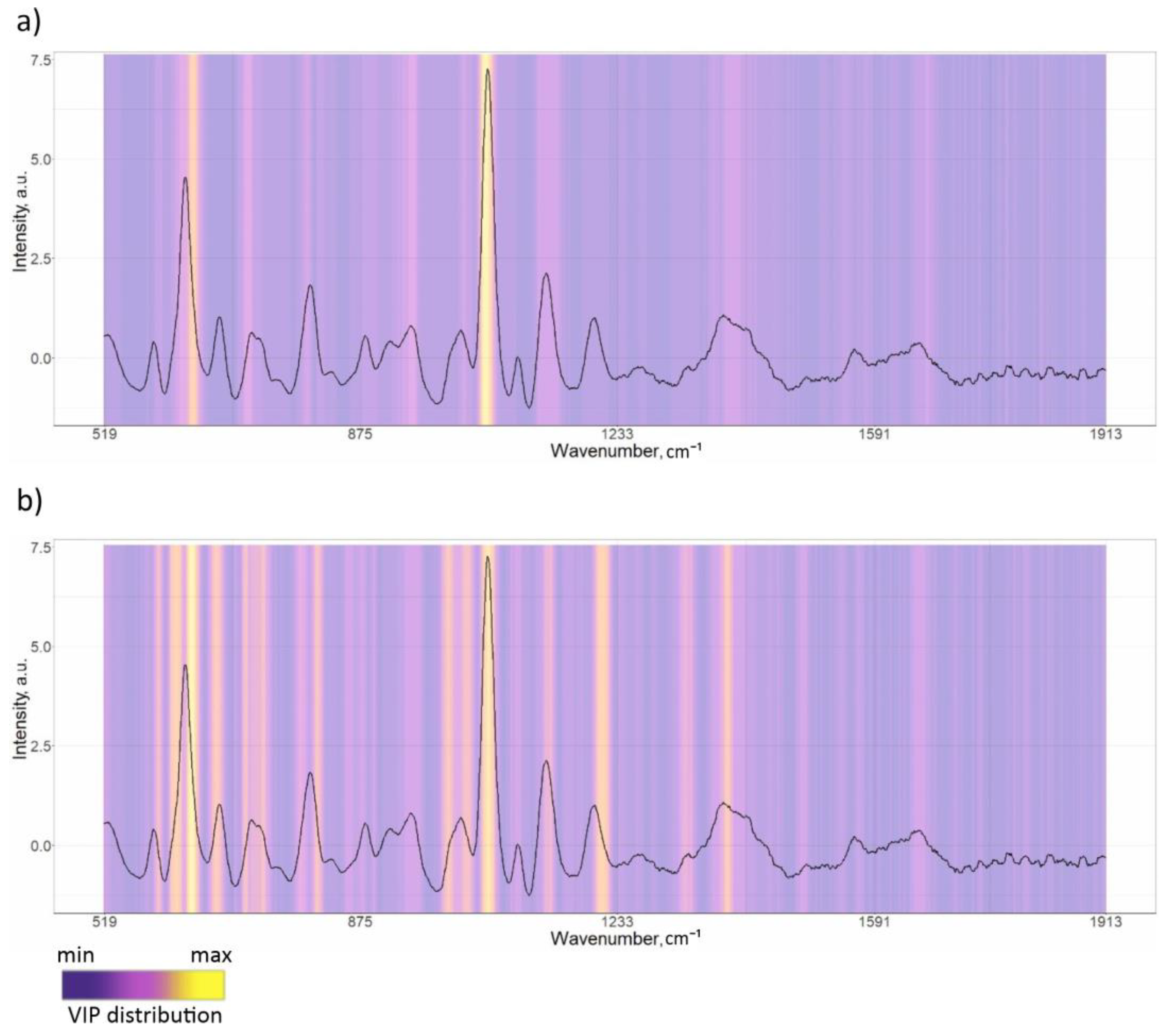
3.2. PLS-DA Classification Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chronic Obstructive Pulmonary Disease (COPD). Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 6 January 2024).
- Yoon, H.I. Biomarkers of COPD. In COPD: Heterogeneity and Personalized Treatment; Lee, S.-D., Ed.; Springer: Berlin, Heidelberg, 2017; pp. 129–143. ISBN 9783662471777. [Google Scholar]
- Agnew, M. Spirometry in Clinical Use: Practical Issues. Breathe 2010, 6, 196–203. [Google Scholar] [CrossRef]
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Carlomagno, C.; Gualerzi, A.; Picciolini, S.; Rodà, F.; Banfi, P.I.; Lax, A.; Bedoni, M. Characterization of the COPD Salivary Fingerprint through Surface Enhanced Raman Spectroscopy: A Pilot Study. Diagnostics 2021, 11, 508. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Tian, Y.; Liang, X.; Piao, C. Early Diagnosis of High-Risk Chronic Obstructive Pulmonary Disease Based on Quantitative High-Resolution Computed Tomography Measurements. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 3099–3114. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Santos, S.; Yang, Z.; Yang, S.; Kirkhus, N.E. Sputum and Salivary Protein Biomarkers and Point-of-Care Biosensors for the Management of COPD. Analyst 2020, 145, 1583–1604. [Google Scholar] [CrossRef]
- Khristoforova, Y.; Bratchenko, L.; Bratchenko, I. Raman-Based Techniques in Medical Applications for Diagnostic Tasks: A Review. Int. J. Mol. Sci. 2023, 24, 15605. [Google Scholar] [CrossRef] [PubMed]
- Ralbovsky, N.M.; Lednev, I.K. Raman Spectroscopy and Chemometrics: A Potential Universal Method for Diagnosing Cancer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 463–487. [Google Scholar] [CrossRef]
- Chisanga, M.; Muhamadali, H.; Ellis, D.I.; Goodacre, R. Enhancing Disease Diagnosis: Biomedical Applications of Surface-Enhanced Raman Scattering. Appl. Sci. 2019, 9, 1163. [Google Scholar] [CrossRef]
- Bratchenko, L.A.; Khristoforova, Y.A.; Pimenova, I.A.; Snegerev, M.S.; Kupaev, V.I.; Lebedev, P.A.; Kistenev, Y.V.; Bratchenko, I.A. Comparative Study Into the Effect of Detector Noises and Sensitivity on the Serum SERS Analysis: Example of Non-Communicable Diseases Discrimination. J. Biophotonics 2025, e202400475. [Google Scholar] [CrossRef]
- Khristoforova, Y.A.; Bratchenko, L.A.; Skuratova, M.A.; Lebedeva, E.A.; Lebedev, P.A.; Bratchenko, I.A. Raman Spectroscopy in Chronic Heart Failure Diagnosis Based on Human Skin Analysis. J. Biophotonics 2023, 16, e202300016. [Google Scholar] [CrossRef]
- Blake, N.; Gaifulina, R.; Griffin, L.D.; Bell, I.M.; Thomas, G.M.H. Machine Learning of Raman Spectroscopy Data for Classifying Cancers: A Review of the Recent Literature. Diagnostics 2022, 12, 1491. [Google Scholar] [CrossRef]
- Hanna, K.; Krzoska, E.; Shaaban, A.M.; Muirhead, D.; Abu-Eid, R.; Speirs, V. Raman Spectroscopy: Current Applications in Breast Cancer Diagnosis, Challenges and Future Prospects. Br. J. Cancer 2022, 126, 1125–1139. [Google Scholar] [CrossRef]
- Carota, A.G.; Campanella, B.; Del Carratore, R.; Bongioanni, P.; Giannelli, R.; Legnaioli, S. Raman Spectroscopy and Multivariate Analysis as Potential Tool to Follow Alzheimer’s Disease Progression. Anal. Bioanal. Chem. 2022, 414, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Ralbovsky, N.M.; Fitzgerald, G.S.; McNay, E.C.; Lednev, I.K. Towards Development of a Novel Screening Method for Identifying Alzheimer’s Disease Risk: Raman Spectroscopy of Blood Serum and Machine Learning. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 254, 119603. [Google Scholar] [CrossRef] [PubMed]
- Ryzhikova, E.; Ralbovsky, N.M.; Sikirzhytski, V.; Kazakov, O.; Halamkova, L.; Quinn, J.; Zimmerman, E.A.; Lednev, I.K. Raman Spectroscopy and Machine Learning for Biomedical Applications: Alzheimer’s Disease Diagnosis Based on the Analysis of Cerebrospinal Fluid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119188. [Google Scholar] [CrossRef]
- Shipp, D.W.; Sinjab, F.; Notingher, I. Raman Spectroscopy: Techniques and Applications in the Life Sciences. Adv. Opt. Photon. 2017, 9, 315–428. [Google Scholar] [CrossRef]
- Al-Sammarraie, S.Z.; Bratchenko, L.A.; Tupikova, E.N.; Skuratova, M.A.; Wang, S.; Lebedev, P.A.; Bratchenko, I.A. Human Blood Plasma SERS Analysis Using Silver Nanoparticles for Cardiovascular Diseases Detection. J. Biomed. Photon Eng. 2024, 10, 010301. [Google Scholar] [CrossRef]
- Yang, B.; Liu, J.; Zhang, F.; Wang, Y.; Liu, X.; Niu, S.; Yuan, Y.; Bi, S. Sensitive Detection of Dextromethorphan Hydrobromide Based on Portable Raman Spectrometer and CuO@AgNPs Nano Composite SERS Substrate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 308, 123798. [Google Scholar] [CrossRef]
- Shin, H.; Choi, B.H.; Shim, O.; Kim, J.; Park, Y.; Cho, S.K.; Kim, H.K.; Choi, Y. Single Test-Based Diagnosis of Multiple Cancer Types Using Exosome-SERS-AI for Early Stage Cancers. Nat. Commun. 2023, 14, 1644. [Google Scholar] [CrossRef]
- Sultangaziyev, A.; Ilyas, A.; Dyussupova, A.; Bukasov, R. Trends in Application of SERS Substrates beyond Ag and Au, and Their Role in Bioanalysis. Biosensors 2022, 12, 967. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, S.; Farman, F.; Bilal, M.; Krafft, C.; Shahzad, S. Demonstrating the Application of Raman Spectroscopy Together with Chemometric Technique for Screening of Asthma Disease. Biomed. Opt. Express 2019, 10, 600–609. [Google Scholar] [CrossRef]
- Guleken, Z.; Tuyji Tok, Y.; Jakubczyk, P.; Paja, W.; Pancerz, K.; Shpotyuk, Y.; Cebulski, J.; Depciuch, J. Development of Novel Spectroscopic and Machine Learning Methods for the Measurement of Periodic Changes in COVID-19 Antibody Level. Measurement 2022, 196, 111258. [Google Scholar] [CrossRef]
- Goulart, A.C.C.; Zângaro, R.A.; Carvalho, H.C.; Silveira, L. Diagnosing COVID-19 in Human Sera with Detected Immunoglobulins IgM and IgG by Means of Raman Spectroscopy. J. Raman Spectrosc. 2021, 52, 2671–2682. [Google Scholar] [CrossRef] [PubMed]
- Carlomagno, C.; Bertazioli, D.; Gualerzi, A.; Picciolini, S.; Banfi, P.I.; Lax, A.; Messina, E.; Navarro, J.; Bianchi, L.; Caronni, A.; et al. COVID-19 Salivary Raman Fingerprint: Innovative Approach for the Detection of Current and Past SARS-CoV-2 Infections. Sci. Rep. 2021, 11, 4943. [Google Scholar] [CrossRef] [PubMed]
- Goulart, A.C.C.; Silveira, L.; Carvalho, H.C.; Dorta, C.B.; Pacheco, M.T.T.; Zângaro, R.A. Diagnosing COVID-19 in Human Serum Using Raman Spectroscopy. Lasers Med. Sci. 2022, 37, 2217–2226. [Google Scholar] [CrossRef] [PubMed]
- Rufino, R.; Silva, J.R.L.E. Bases Celulares e Bioquímicas Da Doença Pulmonar Obstrutiva Crônica. J. Bras. Pneumol. 2006, 32, 241–248. [Google Scholar] [CrossRef]
- Vicol, C.; Buculei, I.; Melinte, O.E.; Dobrin, M.E.; Stavarache, E.I.; Gavrilescu, C.-M.; Postolache, P.; Matei, D.; Trofor, A. The Lipid Profile and Biochemical Parameters of COPD Patients in Relation to Smoking Status. Biomedicines 2022, 10, 2936. [Google Scholar] [CrossRef]
- Rutten, F.H.; Cramer, M.M.; Lammers, J.J.; Grobbee, D.E.; Hoes, A.W. Heart Failure and Chronic Obstructive Pulmonary Disease: An Ignored Combination? Eur. J. Heart Fail. 2006, 8, 706–711. [Google Scholar] [CrossRef]
- Fabbri, L.M.; Luppi, F.; Beghé, B.; Rabe, K.F. Complex Chronic Comorbidities of COPD. Eur. Respir. J. 2008, 31, 204–212. [Google Scholar] [CrossRef]
- Al-Sammarraie, S.Z.; Bratchenko, L.A.; Typikova, E.N.; Lebedev, P.A.; Zakharov, V.P.; Bratchenko, I.A. Silver Nanoparticles-Based Substrate for Blood Serum Analysis under 785 Nm Laser Excitation. J. Biomed. Photon Eng. 2022, 8, 010301. [Google Scholar] [CrossRef]
- Marriott, P.; Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap. J. R. Stat. Soc. Ser. A 1995, 158, 347. [Google Scholar] [CrossRef]
- Bratchenko, L.A.; Bratchenko, I.A. Avoiding Overestimation and the ‘Black Box’ Problem in Biofluids Multivariate Analysis by Raman Spectroscopy: Interpretation and Transparency With the SP-LIME Algorithm. J. Raman Spectrosc. 2024. [Google Scholar] [CrossRef]
- Kucheryavskiy, S. “mdatools”: Multivariate Data Analysis for Chemometrics, R Package Version 0.9.4; R Foundation for Statistical Computing: Vienna, Austria, 2019.
- Zhang, K.; Liu, X.; Man, B.; Yang, C.; Zhang, C.; Liu, M.; Zhang, Y.; Liu, L.; Chen, C. Label-Free and Stable Serum Analysis Based on Ag-NPs/PSi Surface-Enhanced Raman Scattering for Noninvasive Lung Cancer Detection. Biomed. Opt. Express 2018, 9, 4345–4358. [Google Scholar] [CrossRef]
- Kralova, K.; Kral, M.; Vrtelka, O.; Setnicka, V. Comparative Study of Raman Spectroscopy Techniques in Blood Plasma-Based Clinical Diagnostics: A Demonstration on Alzheimer’s Disease. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 304, 123392. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, A.; Dalla Marta, S.; Spizzo, R.; Cervo, S.; Steffan, A.; Colombatti, A.; Sergo, V. Surface-Enhanced Raman Spectroscopy of Blood Plasma and Serum Using Ag and Au Nanoparticles: A Systematic Study. Anal. Bioanal. Chem. 2014, 406, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Avci, E.; Yilmaz, H.; Sahiner, N.; Tuna, B.G.; Cicekdal, M.B.; Eser, M.; Basak, K.; Altıntoprak, F.; Zengin, I.; Dogan, S.; et al. Label-Free Surface Enhanced Raman Spectroscopy for Cancer Detection. Cancers 2022, 14, 5021. [Google Scholar] [CrossRef]
- Ndrepepa, G. Uric Acid and Cardiovascular Disease. Clin. Chim. Acta 2018, 484, 150–163. [Google Scholar] [CrossRef]
- Saito, Y.; Tanaka, A.; Node, K.; Kobayashi, Y. Uric Acid and Cardiovascular Disease: A Clinical Review. J. Cardiol. 2021, 78, 51–57. [Google Scholar] [CrossRef]
- Jung, S.W.; Kim, S.-M.; Kim, Y.G.; Lee, S.-H.; Moon, J.-Y. Uric Acid and Inflammation in Kidney Disease. Am. J. Physiol. Ren. Physiol. 2020, 318, F1327–F1340. [Google Scholar] [CrossRef]
- Sarangi, R. Serum Uric Acid in Chronic Obstructive Pulmonary Disease: A Hospital Based Case Control Study. J. Clin. Diagn. Res. 2017, 11, BC09–BC13. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Xiao, S.; Dai, C.; Wen, X.; Wu, F.; Peng, J.; Tian, H.; Zhou, Y.; Ran, P. Association Between Serum Uric Acid and Lung Function in People with and without Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, B.; Angus, R.M.; Agarwal, S.; Lane, S.; Calverley, P.M.A. Hyperglycaemia as a Predictor of Outcome during Non-Invasive Ventilation in Decompensated COPD. Thorax 2009, 64, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Kubysheva, N.; Boldina, M.; Eliseeva, T.; Soodaeva, S.; Klimanov, I.; Khaletskaya, A.; Bayrasheva, V.; Solovyev, V.; Villa-Vargas, L.A.; Ramírez-Salinas, M.A.; et al. Relationship of Serum Levels of IL-17, IL-18, TNF-α, and Lung Function Parameters in Patients with COPD, Asthma-COPD Overlap, and Bronchial Asthma. Mediat. Inflamm. 2020, 2020, 4652898. [Google Scholar] [CrossRef]
- Chen, Z.; Bai, F.-F.; Han, L.; Zhu, J.; Zheng, T.; Zhu, Z.; Zhou, L.-F. Targeting Neutrophils in Severe Asthma via Siglec-9. Int. Arch. Allergy Immunol. 2018, 175, 5–15. [Google Scholar] [CrossRef] [PubMed]
| Group of Subjects | Number of Patients | Mean Age (Min–Max) | Total Number of Spectra |
|---|---|---|---|
| Respiratory diseases (COPD + BA + COPD&BA) | 41 (21 male, 20 female) | 61 (39–74) | 143 |
| Chronic heart failure (CHF) | 103 (76 male, 27 female) | 65 (43–74) | 309 |
| BA, n = 20 | COPD, n = 11 | COPD&BA, n = 10 | p-Value | |||
|---|---|---|---|---|---|---|
| Mean ± SD | ||||||
| Smoker’s index (packs/years) | 0.032 ± 1.66 | 14.46 ± 16.63 | 27.38 ± 12.33 | 0.001 | 0.001 | 0.012 |
| Body mass index | 28.37 ± 4.96 | 26.77 ± 3.72 | 29.20 ± 5.70 | 0.146 | 0.409 | 0.057 |
| Experience of the BA, year | 13.73 ± 8.77 | – | 9.15 ± 8.81 | – | 0.070 | – |
| Experience of the COPD, year | – | 5.50 ± 5.05 | 7.31 ± 4.75 | – | – | 0.289 |
| IGS, μg/day | 301.75 ± 258.98 | 102.50 ± 216.63 | 326.15 ± 261.71 | 0.002 | 0.729 | 0.008 |
| The number of exacerbations per year | 1.55 ± 0.75 | 1.69 ± 1.08 | 2.31 ± 2.06 | 0.728 | 0.273 | 0.525 |
| ACT, scores | 16.82 ± 5.71 | – | 13.15 ± 4.58 | – | 0.047 | – |
| CAT, scores | – | 20.47 ± 8.06 | 22.75 ± 5.40 | – | – | 0.494 |
| FEV1 (%) | 77.40 ± 20.45 | 53.55 ± 28.06 | 53.48 ± 15.24 | 0.006 | 0.002 | 0.956 |
| FVC (%) | 79.17 ± 20.69 | 74.66 ± 34.76 | 65.53 ± 15.26 | 0.632 | 0.051 | 0.505 |
| FEV1/FVC | 0.79 ± 0.09 | 0.62 ± 0.15 | 0.64 ± 0.13 | 0.001 | 0.005 | 0.720 |
| Classification Models | Specificity Mean (Min–Max) | Sensitivity Mean (Min–Max) | Accuracy Mean (Min–Max) | ROC AUC Mean (Min–Max) | |
|---|---|---|---|---|---|
| Respiratory diseases (COPD + BA+ COPD&BA) vs. CHF (pathological referent group) | Training set | 0.95 (0.92–1.0) | 0.94 (0.91–0.99) | 0.95 (0.94–0.98) | 0.97 (0.96–1.0) |
| Test set | 0.97 (0.86–1.0) | 0.85 (0.70–1.0) | 0.92 (0.82–1) | 0.96 (0.85–1.0) | |
| COPD vs. BA | Training set | 0.92 (0.86–1.0) | 0.86 (0.75–0.92) | 0.89 (0.85–0.96) | 0.93 (0.78–0.99) |
| Test set | 0.57 (0.17–1.0) | 0.64 (0.0–1.0) | 0.61 (0.1–1.0) | 0.72 (0.53–1.0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khristoforova, Y.; Bratchenko, L.; Kupaev, V.; Senyushkin, D.; Skuratova, M.; Wang, S.; Lebedev, P.; Bratchenko, I. Detection of Respiratory Disease Based on Surface-Enhanced Raman Scattering and Multivariate Analysis of Human Serum. Diagnostics 2025, 15, 660. https://doi.org/10.3390/diagnostics15060660
Khristoforova Y, Bratchenko L, Kupaev V, Senyushkin D, Skuratova M, Wang S, Lebedev P, Bratchenko I. Detection of Respiratory Disease Based on Surface-Enhanced Raman Scattering and Multivariate Analysis of Human Serum. Diagnostics. 2025; 15(6):660. https://doi.org/10.3390/diagnostics15060660
Chicago/Turabian StyleKhristoforova, Yulia, Lyudmila Bratchenko, Vitalii Kupaev, Dmitry Senyushkin, Maria Skuratova, Shuang Wang, Petr Lebedev, and Ivan Bratchenko. 2025. "Detection of Respiratory Disease Based on Surface-Enhanced Raman Scattering and Multivariate Analysis of Human Serum" Diagnostics 15, no. 6: 660. https://doi.org/10.3390/diagnostics15060660
APA StyleKhristoforova, Y., Bratchenko, L., Kupaev, V., Senyushkin, D., Skuratova, M., Wang, S., Lebedev, P., & Bratchenko, I. (2025). Detection of Respiratory Disease Based on Surface-Enhanced Raman Scattering and Multivariate Analysis of Human Serum. Diagnostics, 15(6), 660. https://doi.org/10.3390/diagnostics15060660






