Positron Emission Tomography–Magnetic Resonance Imaging, a New Hybrid Imaging Modality for Dentomaxillofacial Malignancies—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Eligibility Criteria
2.2.1. Types of Outcome Measure
2.2.2. Study Design
2.2.3. Inclusion Criteria
2.2.4. Exclusion Criteria
2.3. Information Sources
2.4. Search Strategy
2.5. Study Selection
2.6. Data Collection and Data Items
2.7. Risk of Bias Assessment in Included Studies
2.8. Effect Measures and Data Synthesis
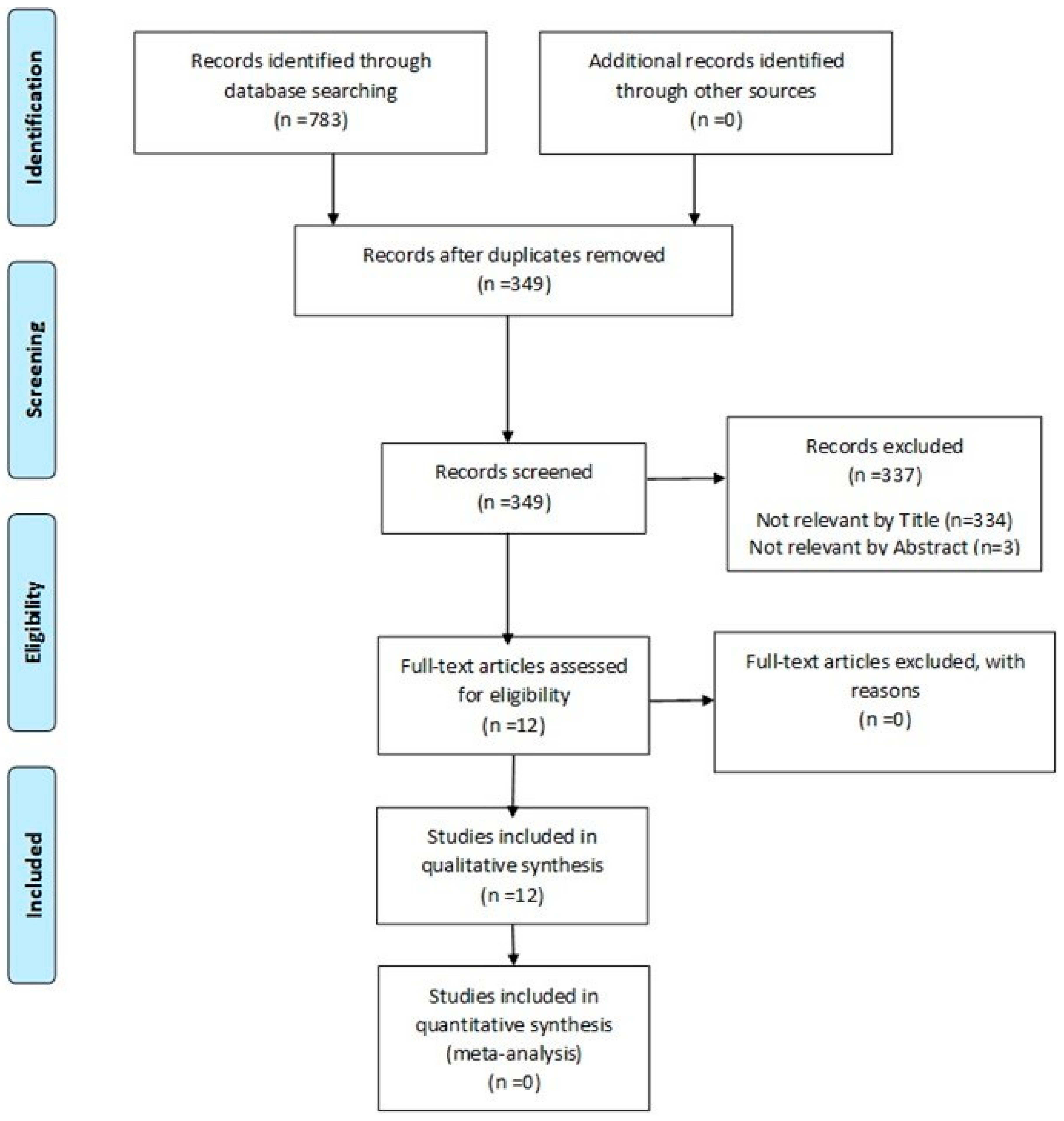
3. Results
3.1. Description of Studies
3.2. Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rumboldt, Z.; Gordon, L.; Bonsall, R.; Ackermann, S. Imaging in head and neck cancer. Curr. Treat. Opt. Oncol. 2006, 7, 23–34. [Google Scholar] [CrossRef]
- Sekine, T.; De Galiza Barbosa, F.; Kuhn, F.P.; Burger, I.A.; Stolzmann, P.; Huber, G.F. PET + MR versus PET/CT in the initial staging of head and neck cancer, using a trimodality PET/CT+MR system. Clin. Imaging 2017, 42, 232–239. [Google Scholar] [CrossRef]
- Farina, E.; Ferioli, M.; Castellucci, P.; Farina, A.; Rambaldi, G.Z.; Cilla, S.; Cammelli, S.; Fanti, S.; Morganti, A.G. 18F-Fdg-PET-guided planning and replanning (adaptive) radiotherapy in head and neck cancer: Current state of art. Anticancer Res. 2017, 37, 6523–6532. [Google Scholar] [CrossRef] [PubMed]
- Lwin, C.T.; Hanlon, R.; Lowe, D.; Brown, J.S.; Woolgar, J.A.; Triantafyllou, A.; Rogers, S.N.; Bekiroglu, F.; Lewis-Jones, H.; Wieshmann, H.; et al. Accuracy of MRI in prediction of tumour thickness and nodal stage in oral squamous cell carcinoma. Oral. Oncol. 2012, 48, 149–154. [Google Scholar] [PubMed]
- King, A.D.; Tse, G.M.K.; Yuen, E.H.Y.; To, E.W.H.; Vlantis, A.C.; Zee, B.; Chan, A.B.; van Hasselt, A.C.; Ahuja, A.T. Comparison of CT and MR imaging for the detection of extranodal neoplastic spread in metastatic neck nodes. Eur. J. Radiol. 2004, 52, 264–270. [Google Scholar] [CrossRef]
- Offiah, C.; Hall, E. Post-treatment imaging appearances in head and neck cancer patients. Clin. Radiol. 2011, 66, 13–24. [Google Scholar] [CrossRef]
- Ng, S.H.; Chan, S.C.; Yen, T.C.; Liao, C.T.; Lin, C.Y.; Tung-Chieh Chang, J.; Ko, S.F.; Wang, H.M.; Chang, K.P.; Fan, K.H. PET/CT and 3-T whole-body MRI in the detection of malignancy in treated oropharyngeal and hypopharyngeal carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.H.; Yoon, D.Y.; Park, C.H.; Chang, S.; Lim, K.J.; Seo, Y.L.; Yun, E.J.; Choi, C.S.; Bae, S.H. CT, MR, 18F-FDG PET/CT, and their combined use for the assessment of mandibular invasion by squamous cell carcinomas of the oral cavity. Acta Radiol. 2010, 51, 1111–1119. [Google Scholar] [CrossRef]
- Imaizumi, A.; Yoshino, N.; Yamada, I.; Nagumo, K.; Amagasa, T.; Omura, K.; Okada, N.; Kurabayashi, T. A potential pitfall of MR imaging for assessing mandibular invasion of squamous cell carcinoma in the oral cavity. AJNR Am. J. 2006, 27, 114–122. Available online: http://www.ajnr.org/content/27/1/114 (accessed on 1 November 2024).
- Kuhn, F.P.; Hüllner, M.; Mader, C.E.; Kastrinidis, N.; Huber, G.F.; Von Schulthess, G.K.; Kollias, S.; Veit-Haibach, P. Contrast-Enhanced PET/MR Imaging Versus ContrastEnhanced PET/CT in Head and Neck Cancer: How Much MR Information Is Needed? J. Nucl. Med. 2014, 55, 551–558. [Google Scholar] [CrossRef]
- Ladefoged, C.N.; Hansen, A.E.; Keller, S.H.; Fischer, B.M.; Rasmussen, J.H.; Law, I.; Kjær, A.; Højgaard, L.; Lauze, F.; Beyer, T.; et al. Dental artifacts in the head and neck region: Implications for Dixon-based attenuation correction in PET/MR. EJNMMI Phys. 2015, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Anderla, A.; Culibrk, D.; Delso, G.; Mirkovic, M. MR Image Based Approach for Metal Artifact Reduction in X-Ray CT. Sci. World J. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Hagiwara, M.; Nusbaum, A.; Schmidt, B.L. MR Assessment of Oral Cavity Carcinomas. Magn. Reson. Imaging Clin. N. Am. 2012, 20, 473–494. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.W.; Djulbegovic, B.; Soares, H.P.; Siegel, B.A.; Lowe, V.J.; Lyman, G.H.; Coleman, R.E.; Wahl, R.; Paschold, J.C.; Avril, N.; et al. Recommendations on the Use of 18 F-FDG PET in Oncology. J. Nucl. Med. 2008, 49, 480–488. [Google Scholar] [CrossRef]
- Kyzas, P.A.; Evangelou, E.; Denaxa-Kyza, D.; Ioannidis, J.P.A. 18FFluorodeoxyglucose Positron Emission Tomography to Evaluate Cervical Node Metastases in Patients With Head and Neck Squamous Cell Carcinoma: A Meta-analysis. J. Natl. Cancer Inst. 2008, 100, 712–720. [Google Scholar] [CrossRef]
- Wax, M.K.; Myers, L.L.; Gona, J.M.; Husain, S.S.; Nabi, H.A. The Role of Positron Emission Tomography in the Evaluation of the N-Positive Neck. Otolaryngol. Head Neck Surg. 2003, 129, 163–177. [Google Scholar] [CrossRef]
- Yen, T.C.; Chang, J.T.C.; Ng, S.H.; Chang, Y.C.; Chan, S.C.; Wang, H.M.; See, L.C.; Chen, T.M.; Kang, C.J.; Wu, Y.F.; et al. Staging of untreated squamous cell carcinoma of buccal mucosa with 18FFDG PET: Comparison with head and neck CT/MRI and histopathology. J. Nucl. Med. 2005, 46, 775–781. [Google Scholar] [PubMed]
- Appenzeller, P.; Mader, C.; Huellner, M.W.; Schmidt, D.; Schmid, D.; Boss, A.; von Schulthess, G.; Veit-Haibach, P. PET/CT versus body coil PET/MRI: How low can you go? Insights Imaging 2013, 4, 481–490. [Google Scholar] [CrossRef]
- Samarin, A.; Burger, C.; Wollenweber, S.D.; Crook, D.W.; Burger, I.A.; Schmid, D.T.; on Schulthess, G.K.; Kuhn, F.P. PET/MR imaging of bone lesions—Implications for PET quantification from imperfect attenuation correction. Eur. J. Nucl. Med. Mol. Imaging 2013, 39, 1154–1160. [Google Scholar] [CrossRef]
- Queiroz, M.A.; Huellner, M.W. PET/MR in Cancers of the Head and Neck. Semin. Nucl. Med. 2015, 45, 248–255. [Google Scholar] [CrossRef]
- Saito, N.; Nadgir, R.N.; Nakahira, M.; Takahashi, M.; Uchino, A.; Kimura, F.; Truong, M.T.; Sakai, O. Posttreatment CT and MR Imaging in Head and Neck Cancer: What the Radiologist Needs to Know. Radiographics 2012, 32, 1261–1262. [Google Scholar] [CrossRef]
- Nakamoto, Y.; Tamai, K.; Saga, T.; Higashi, T.; Hara, T.; Suga, T.; Koyama, T.; Togashi, K. Clinical Value of Image Fusion from MR and PET in Patients with Head and Neck Cancer. Mol. Imaging Biol. 2009, 11, 46–53. [Google Scholar] [CrossRef]
- Kanda, T.; Kitajima, K.; Suenaga, Y.; Konishi, J.; Sasaki, R.; Morimoto, K.; Saito, M.; Otsuki, N.; Nibu, K.; Sugimura, K. Value of retrospective image fusion of 18F-FDG PET and MRI for preoperative staging of head and neck cancer: Comparison with PET/CT and contrast-enhanced neck, MRI. Eur. J. Radiol. 2013, 82, 2005–2010. [Google Scholar] [CrossRef]
- Huang, S.H.; Chien, C.Y.; Lin, W.C.; Fang, F.M.; Wang, P.W.; Lui, C.C.; Huang, Y.C.; Hung, B.T.; Tu, M.C.; Chang, C.C. A Comparative Study of Fused FDG PET/MRI, PET/CT, MRI, and CT Imaging for Assessing Surrounding Tissue Invasion of Advanced Buccal Squamous Cell Carcinoma. Clin. Nucl. Med. 2011, 36, 518–525. [Google Scholar] [CrossRef]
- Becker, M.; Zaidi, H. Imaging in head and neck squamous cell carcinoma: The potential role of PET/MRI. Br. J. Radiol. 2014, 87, 20130677. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Souvatzoglou, M.; Pickhard, A.; Loeffelbein, D.J.; Knopf, A.; Holzapfel, K.; Martinez-Möller, A.; Nekolla, S.G.; Scherer, E.Q.; Schwaiger, M.; et al. Simulation of a MR–PET protocol for staging of head-and-neck cancer including Dixon MR for attenuation correction. Eur. J. Radiol. 2012, 81, 2658–2665. [Google Scholar] [CrossRef]
- Loeffelbein, D.J.; Souvatzoglou, M.; Wankerl, V.; Martinez-Möller, A.; Dinges, J.; Schwaiger, M.; Beer, A.J. PET-MRI Fusion in Head-and-Neck Oncology: Current Status and Implications for Hybrid PET/MRI. J. Oral Maxillofac. Surg. 2012, 70, 473–483. [Google Scholar] [CrossRef]
- Boss, A.; Stegger, L.; Bisdas, S.; Kolb, A.; Schwenzer, N.; Pfister, M.; Claussen, C.D.; Pichler, B.J.; Pfannenberg, C. Feasibility of simultaneous PET/MR imaging in the head and upper neck area. Eur. Radiol. 2011, 21, 1439–1446. [Google Scholar] [CrossRef]
- Chan, S.C.; Yeh, C.H.; Yen, T.C.; Ng, S.H.; Chang, J.T.C.; Lin, C.Y.; Yen-Ming, T.; Fan, K.H.; Huang, B.S.; Hsu, C.L.; et al. Clinical utility of simultaneous whole-body 18F-FDG PET/MRI as a single-step imaging modality in the staging of primary nasopharyngeal carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1297–1298. [Google Scholar] [CrossRef]
- Kaltoft, N.S.; Marner, L.; Larsen, V.A.; Hasselbalch, S.G.; Law, I.; Henriksen, O.M. Hybrid FDG PET/MRI vs. FDG PET and CT in patients with suspected dementia—A comparison of diagnostic yield and propagated influence on clinical diagnosis and patient management. PLoS ONE 2019, 14, e0216409. [Google Scholar] [CrossRef]
- Freihat, O.; Tóth, Z.; Pintér, T.; Kedves, A.; Sipos, D.; Cselik, Z.; Lippai, N.; Repa, I.; Kovács, Á. Pretreatment PET/MRI based FDG and DWI imaging parameters for predicting HPV status and tumor response to chemoradiotherapy in primary oropharyngeal squamous cell carcinoma (OPSCC). Oral Oncol. 2021, 116, 105239. [Google Scholar] [CrossRef] [PubMed]
- Meller, J.; Köster, G.; Liersch, T.; Siefker, U.; Lehmann, K.; Meyer, I.; Schreiber, K.; Altenvoerde, G.; Becker, W. Chronic bacterial osteomyelitis: Prospective comparison of 18F-FDG imaging with a dual-head coincidence camera and 111In-labelled autologous leucocyte scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Van Der Bruggen, W.; Bleeker-Rovers, C.P.; Boerman, O.C.; Gotthardt, M.; Oyen, W.J.G. PET and SPECT in Osteomyelitis and Prosthetic Bone and Joint Infections: A Systematic Review. Semin. Nucl. Med. 2010, 40, 3–15. [Google Scholar] [CrossRef]
- Sharma, P.; Mukherjee, A.; Karunanithi, S.; Bal, C.; Kumar, R. Potential Role of 18 F-FDG PET/CT in Patients With Fungal Infections. Am. J. Roentgenol. 2014, 203, 180–189. [Google Scholar] [CrossRef]
- Coviello, V.; Stevens, M.R. Contemporary Concepts in the Treatment of Chronic Osteomyelitis. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 523–524. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in a non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar]
- Reinert, C.P.; Pfannenberg, C.; Dittmann, H.; Gückel, B.; La Fougère, C.; Nikolaou, K.; Hoefert, S. [18F] Fluoride Positron-Emission Tomography (PET) and [18F]FDG PET for Assessment of Osteomyelitis of the Jaw in Comparison to Computed Tomography (CT) and Magnetic Resonance Imaging (MRI): A Prospective PET/CT and PET/MRI Pilot Study. J. Clin. Med. 2022, 11, 3998. [Google Scholar] [CrossRef]
- Park, J.; Pak, K.; Yun, T.J.; Lee, E.K.; Ryoo, I.; Lee, J.Y.; Hwang, I.; Yoo, R.E.; Kang, K.M.; Choi, S.H. Diagnostic Accuracy and Confidence of [18F] FDG PET/MRI in comparison with PET or MRI alone in Head and Neck Cancer. Sci. Rep. 2020, 10, 9490. [Google Scholar] [CrossRef]
- Schaarschmidt, B.M.; Gomez, B.; Buchbender, C.; Grueneisen, J.; Nensa, F.; Sawicki, L.M.; Ruhlmann, V.; Wetter, A.; Antoch, G.; Heusch, P. Is integrated 18F-FDG PET/MRI superior to 18F-FDG PET/CT in the differentiation of incidental tracer uptake in the head and neck area? Diagn. Interv. Radiol. 2017, 23, 127–132. [Google Scholar] [CrossRef]
- Queiroz, M.A.; Hüllner, M.; Kuhn, F.; Huber, G.; Meerwein, C.; Kollias, S.; von Schulthess, G.; Veit-Haibach, P. Use of diffusion-weighted imaging (DWI) in PET/MRI for head and neck cancer evaluation. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2212–2231. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Kikuchi, M.; Imai, Y.; Yamashita, D.; Hino, M.; Ito, K.; Shimizu, K.; Harada, H.; Shinohara, S. Clinical Value of Fused PET/MRI for Surgical Planning in Patients With Oral/Oropharyngeal Carcinoma. Laryngoscope 2019, 130, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Kanno, M.; Tsujikawa, T.; Narita, N.; Ito, Y.; Makino, A.; Imamura, Y.; Kimura, H.; Okazawa, H.; Fujieda, S. Comparison of diagnostic accuracy between [18F]FDG PET/MRI and contrast-enhanced MRI in T staging for oral tongue cancer. Ann. Nucl. Med. 2020, 34, 952–959. [Google Scholar] [CrossRef]
- Loeffelbein, D.J.; Souvatzoglou, M.; Wankerl, V.; Dinges, J.; Ritschl, L.M.; Mücke, T.; Pickhard, A.; Eiber, M.; Schwaiger, M.; Beer, A.J. Diagnostic value of retrospective PET-MRI fusion in head-and-neck cancer. BMC Cancer 2014, 14, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Schaarschmidt, B.M.; Heusch, P.; Buchbender, C.; Ruhlmann, M.; Bergmann, C.; Ruhlmann, V.; Schlamann, M.; Antoch, G.; Forsting, M.; Wetter, A. Locoregional tumour evaluation of squamous cell carcinoma in the head and neck area: A comparison between MRI, PET/CT and integrated PET/MRI. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 92–102. [Google Scholar] [CrossRef]
- Samołyk-Kogaczewska, N.; Sierko, E.; Zuzda, K.; Gugnacki, P.; Szumowski, P.; Mojsak, M.; Burzyńska-Śliwowska, J.; Wojtukiewicz, M.Z.; Szczecina, K.; Jurgilewicz, D.H. PET/MRI-guided GTV delineation during radiotherapy planning in patients with squamous cell carcinoma of the tongue. Strahlenther. Und Onkol. 2019, 195, 780–781. [Google Scholar] [CrossRef]
- Tsujikawa, T.; Kanno, M.; Ito, Y.; Oikawa, H.; Rahman, M.G.M.; Narita, N.; Fujieda, S.; Okazawa, H. Zero Echo Time–Based PET/MRI Attenuation Correction in Patients With Oral Cavity Cancer: Initial Experience. Clin. Nucl. Med. 2020, 45, 501–505. [Google Scholar] [CrossRef]
- Samołyk-Kogaczewska, N.; Sierko, E.; Dziemianczyk-Pakiela, D.; Nowaszewska, K.B.; Lukasik, M.; Reszec, J. Usefulness of Hybrid PET/MRI in Clinical Evaluation of Head and Neck Cancer Patients. Cancers 2020, 12, 511. [Google Scholar] [CrossRef]
- Grosu, A.L.; Piert, M.; Weber, W.A.; Jeremic, B.; Picchio, M.; Schratzenstaller, U.; Zimmermann, F.B.; Schwaiger, M.; Molls, M. Positron Emission Tomography for Radiation Treatment Planning. Strahlenther. Onkol. 2005, 181, 483–489. [Google Scholar] [CrossRef]
- Ligtenberg, H.; Jager, E.A.; Caldas-Magalhaes, J.; Schakel, T.; Pameijer, F.A.; Kasperts, N.; Willems, S.M.; Terhaard, C.H.; Raaijmakers, C.P.; Philippens, M.E. Modality-specific target definition for laryngeal and hypopharyngeal cancer on FDG-PET, CT and MRI. Radiother. Oncol. 2017, 123, 63–70. [Google Scholar] [CrossRef]
- Lonneux, M.; Hamoir, M.; Reychler, H.; Maingon, P.; Duvillard, C.; Calais, G.; Bridji, B.; Digue, L.; Toubeau, M.; Grégoire, V. Positron Emission Tomography With [18F] Fluorodeoxyglucose Improves Staging and Patient Management in Patients With Head and Neck Squamous Cell Carcinoma: A Multicenter Prospective Study. J. Clin. Oncol. 2010, 28, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.L.; Harris, J.; Yao, M.; Rosenthal, D.I.; Opanowski, A.; Levering, A.; Ang, K.K.; Trotti, A.M.; Garden, A.S.; Jones, C.U.; et al. Metabolic Tumor Volume as a Prognostic Imaging-Based Biomarker for Head-and-Neck Cancer: Pilot Results From Radiation Therapy Oncology Group Protocol 0522. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 721–729. [Google Scholar] [CrossRef]
- Tahari, A.K.; Alluri, K.C.; Quon, H.; Koch, W.; Wahl, R.L.; Subramaniam, R.M. FDG PET/CT Imaging of Oropharyngeal Squamous Cell Carcinoma: Characteristics of Human Papillomavirus–Positive and –Negative Tumors. Clin. Nucl. Med. 2014, 39, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, H.; Srinivasan, A. Diffusion imaging of the head and neck. Curr. Radiol. Rep. 2014, 2, 49. [Google Scholar] [CrossRef]
- Jansen, J.F.A.; Koutcher, J.A.; Shukla-Dave, A. Non-invasive imaging of angiogenesis in head and neck squamous cell carcinoma. Angiogenesis 2010, 13, 149–160. [Google Scholar] [CrossRef]
- Vandecaveye, V.; Dirix, P.; De Keyzer, F.; Op De Beeck, K.; Vander Poorten, V.; Hauben, E.; Lambrecht, M.; Nuyts, S.; Hermans, R. Diffusion-Weighted Magnetic Resonance Imaging Early After Chemoradiotherapy to Monitor Treatment Response in Head-andNeck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Ravanelli, M.; Grammatica, A.; Tononcelli, E.; Morello, R.; Leali, M.; Battocchio, S.; Agazzi, G.M.; Buglione di Monale E Bastia, M.; Maroldi, R.; Nicolai, P.; et al. Correlation between Human Papillomavirus Status and Quantitative MR Imaging Parameters including Diffusion-Weighted Imaging and Texture Features in Oropharyngeal Carcinoma. AJNR Am. J. Neuroradiol. 2018, 39, 1878–1883. [Google Scholar] [CrossRef]
- Nakahira, M.; Saito, N.; Yamaguchi, H.; Kuba, K.; Sugasawa, M. Use of quantitative diffusion-weighted magnetic resonance imaging to predict human papilloma virus status in patients with oropharyngeal squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2014, 271, 1219–1225. [Google Scholar] [CrossRef]
- Payabvash, S.; Chan, A.; Jabehdar Maralani, P.; Malhotra, A. Quantitative diffusion magnetic resonance imaging for prediction of human papillomavirus status in head and neck squamous-cell carcinoma: A systematic review and meta-analysis. Neuroradiol. J. 2019, 32, 232–340. [Google Scholar] [CrossRef]
- Tsujikawa, T.; Narita, N.; Kanno, M.; Takabayashi, T.; Fujieda, S.; Okazawa, H. Role of PET/MRI in oral cavity and oropharyngeal cancers based on the 8th edition of the AJCC cancer staging system: A pictorial essay. Ann. Nucl. Med. 2018, 32, 239–249. [Google Scholar] [CrossRef]
- Queiroz, M.A.; Hüllner, M.; Kuhn, F.; Huber, G.; Meerwein, C.; Kollias, S.; von Schulthess, G.; Veit-Haibach, P. PET/MRI and PET/CT in follow-up of head and neck cancer patients. Eur. J. Nucl Med. Mol. Imaging 2014, 41, 1066–1075. [Google Scholar] [CrossRef]
- Ng, S.H.; Yen, T.C.; Liao, C.T.; Chang, J.T.C.; Chan, S.C.; Ko, S.F.; Wang, H.M.; Wong, H.F. 18F-FDG PET and CT/MRI in oral cavity squamous cell carcinoma: A prospective study of 124 patients with histologic correlation. J. Nucl. Med. 2005, 46, 1136–1143. [Google Scholar]
- Schaaf, W.E.; Patel, Z.; Retrouvey, M.; Cunningham, T.D.; Johnson, L.S. Frequency and clinical relevance of PET/CT incidentalomas. Abdom. Imaging 2014, 39, 657–662. [Google Scholar] [CrossRef]
- Wong, W.L.; Gibson, D.; Sanghera, B.; Goodchild, K.; Saunders, M. Evaluation of normal FDG uptake in palatine tonsil and its potential value for detecting occult head and neck cancers: A PET CT study. Nucl. Med. Commun. 2007, 28, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Sebro, R.; Aparici, C.M.; Pampaloni, M.H. Frequency and clinical implications of incidental new primary cancers detected on true whole-body 18F-FDG PET/CT studies. Nucl. Med. Commun. 2013, 34, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakami, H.A.; Makis, W.; Anand, S.; Mlynarek, A.; Black, M.J.; Stern, J.; Payne, R.J.; Hier, M.P. Head and neck incidentalomas on positron emission tomographic scanning:ignore or investigate? J. Otolaryngol. Head Neck Surg. 2011, 40, 384–390. [Google Scholar] [PubMed]
- Baltensperger, M.; Eyrich, G. Osteomyelitis of the Jaws: Definition and Classification. In Osteomyelitis of the Jaws; Baltensperger, M.M., Eyrich, G.K.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 5–56. Available online: http://link.springer.com/10.1007/978-3-540-28766-7_2 (accessed on 1 November 2024).
- Yang, X.; Zhou, K.; Shang, W.; Song, K. Oral administration of alendronate and vitamin D3 for the treatment of chronic non-bacterial osteomyelitis of the jaw. Int. J. Oral Maxillofac. Surg. 2020, 49, 1595–1598. [Google Scholar] [CrossRef]
- Timme, M.; Bohner, L.; Huss, S.; Kleinheinz, J.; Hanisch, M. Response of Different Treatment Protocols to Treat Chronic Non-Bacterial Osteomyelitis (CNO) of the Mandible in Adult Patients: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 1737. [Google Scholar] [CrossRef] [PubMed]
- Platzek, I.; Beuthien-Baumann, B.; Schneider, M.; Gudziol, V.; Langner, J.; Schramm, G.; Laniado, M.; Kotzerke, J.; van den Hoff, J. PET/MRI in head and neck cancer: Initial experience. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 6–11. [Google Scholar] [CrossRef]
- Geraldine, B.E.; Pyatigorskaya, N.; De Laroche, R.; Herve, G.; Zaslavsky, C.; Bertaux, M.; Giron, A.; Soret, M.; Bertolus, C.; Sahli-Amor, M.; et al. Diagnostic performance of 18F-FDG PET/MR in head and neck malignancies. J. Nucl. Med. 2017, 58, 280. [Google Scholar]
- Lee, S.J.; Seo, H.J.; Cheon, G.J.; Kim, J.H.; Kim, E.E.; Kang, K.W.; Paeng, J.C.; Chung, J.K.; Lee, D.S. Usefulness of Integrated PET/MRI in Head and Neck Cancer: A Preliminary Study. Nucl. Med. Mol. Imaging 2014, 48, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Kubiessa, K.; Purz, S.; Gawlitza, M.; Kühn, A.; Fuchs, J.; Steinhoff, K.G.; Boehm, A.; Sabri, O.; Kluge, R.; Kahn, T.; et al. Initial clinical results of simultaneous 18F-FDG PET/MRI in comparison to 18F-FDG PET/CT in patients with head and neck cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 639–648. [Google Scholar] [CrossRef]
- Schlittenbauer, T.; Zeilinger, M.; Nkenke, E.; Kreißel, S.; Wurm, M.C.; Lell, M.; Kuwert, T.; Beck, M. Positron emission tomography-computed tomography versus positron emission tomography-magnetic resonance imaging for diagnosis of oral squamous cell carcinoma: A pilot study. J. Craniomaxillofac. Surg. 2015, 43, 2129–2135. [Google Scholar] [CrossRef]
- De Bondt, R.B.J.; Nelemans, P.J.; Bakers, F.; Casselman, J.W.; Peutz-Kootstra, C.; Kremer, B.; Hofman, P.A.; Beets-Tan, R.G. Morphological MRI criteria improve the detection of lymph node metastases in head and neck squamous cell carcinoma: Multivariate logistic regression analysis of MRI features of cervical lymph nodes. Eur. Radiol. 2009, 19, 626–633. [Google Scholar] [CrossRef]
- Partovi, S.; Kohan, A.; Vercher-Conejero, J.L.; Rubbert, C.; Margevicius, S.; Schluchter, M.D.; Gaeta, C.; Faulhaber, P.; Robbin, M.R. Qualitative and Quantitative Performance of 18 FFDG-PET/MRI versus 18 F-FDG-PET/CT in Patients with Head and Neck Cancer. Am. J. Neuroradiol. 2014, 35, 1970–1975. [Google Scholar] [CrossRef] [PubMed]
- Platzek, I.; Beuthien-Baumann, B.; Schneider, M.; Gudziol, V.; Kitzler, H.H.; Maus, J.; Schramm, G.; Popp, M.; Laniado, M.; Kotzerke, J.; et al. FDG PET/MR for lymph node staging in head and neck cancer. Eur. J. Radiol. 2014, 83, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Nakamatsu, S.; Matsusue, E.; Miyoshi, H.; Kakite, S.; Kaminou, T.; Ogawa, T. Correlation of apparent diffusion coefficients measured by diffusionweighted MR imaging and standardized uptake values from FDG PET/CT in metastatic neck lymph nodes of head and neck squamous cell carcinomas. Clin. Imaging 2012, 36, 90–97. [Google Scholar] [CrossRef][Green Version]
- Baba, A.; Hashimoto, K.; Kayama, R.; Yamauchi, H.; Ikeda, K.; Ojiri, H. Radiological approach for the newly incorporated T staging factor, depth of invasion (DOI), of the oral tongue cancer in the 8th edition of American Joint Committee on Cancer (AJCC) staging manual: Assessment of the necessity for elective neck dissection. Jpn. J. Radiol. 2020, 38, 821–822. [Google Scholar] [CrossRef]
- Murakami, R.; Shiraishi, S.; Yoshida, R.; Sakata, J.; Yamana, K.; Hirosue, A.; Uchiyama, Y.; Nakayama, H.; Yamashita, Y. Reliability of MRI-Derived Depth of Invasion of Oral Tongue Cancer. Acad. Radiol. 2019, 26, e180–e186. [Google Scholar] [CrossRef]
- Ahmed, M.; Schmidt, M.; Sohaib, A.; Kong, C.; Burke, K.; Richardson, C.; Usher, M.; Brennan, S.; Riddell, A.; Davies, M.; et al. The value of magnetic resonance imaging in target volume delineation of base of tongue tumours—A study using flexible surface coils. Radiother. Oncol. 2010, 94, 161–167. [Google Scholar] [CrossRef]
- Delouya, G.; Igidbashian, L.; Houle, A.; Bélair, M.; Boucher, L.; Cohade, C.; Beaulieu, S.; Filion, E.J.; Coulombe, G.; Hinse, M.; et al. 18F-FDG-PET imaging in radiotherapy tumor volume delineation in treatment of head and neck cancer. Radiother. Oncol. 2011, 101, 362–368. [Google Scholar] [CrossRef]
- Leclerc, M.; Lartigau, E.; Lacornerie, T.; Daisne, J.F.; Kramar, A.; Grégoire, V. Primary tumor delineation based on (18)FDG PET for locally advanced head and neck cancer treated by chemo-radiotherapy. Radiother. Oncol. 2015, 116, 87–93. [Google Scholar] [CrossRef]
- Guido, A.; Fuccio, L.; Rombi, B.; Castellucci, P.; Cecconi, A.; Bunkheila, F.; Fuccio, C.; Spezi, E.; Angelini, A.L.; Barbieri, E. Combined 18F-FDG-PET/CT imaging in radiotherapy target delineation for head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Nagamachi, S.; Nishii, R.; Wakamatsu, H.; Mizutani, Y.; Kiyohara, S.; Fujita, S.; Futami, S.; Sakae, T.; Furukoji, E.; Tamura, S.; et al. The usefulness of (18)F-FDG PET/MRI fusion image in diagnosing pancreatic tumor: Comparison with (18)F-FDG PET/CT. Ann. Nucl. Med. 2013, 27, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Suenaga, Y.; Ueno, Y.; Kanda, T.; Maeda, T.; Takahashi, S.; Ebina, Y.; Miyahara, Y.; Yamada, H.; Sugimura, K. Value of fusion of PET and MRI for staging of endometrial cancer: Comparison with 18F-FDG contrast-enhanced PET/CT and dynamic contrast-enhanced pelvic MRI. Eur. J. Radiol. 2013, 82, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Pfluger, T.; Melzer, H.I.; Mueller, W.P.; Coppenrath, E.; Bartenstein, P.; Albert, M.H.; Schmid, I. Diagnostic value of combined ¹⁸F-FDG PET/MRI for staging and restaging in paediatric oncology. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1745–1755. [Google Scholar] [CrossRef]
- Antoch, G. Whole-Body Dual-Modality PET/CT and Whole-Body MRI for Tumor Staging in Oncology. JAMA 2003, 290, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Antoch, G.; Saoudi, N.; Kuehl, H.; Dahmen, G.; Mueller, S.P.; Beyer, T.; Bockisch, A.; Debatin, J.F.; Freudenberg, L.S. Accuracy of whole-body dual-modality fluorine-18-2-fluoro-2-deoxy-Dglucose positron emission tomography and computed tomography (FDGPET/CT) for tumor staging in solid tumors: Comparison with CT and PET. J. Clin. Oncol. 2004, 22, 4357–4358. [Google Scholar] [CrossRef]
- Rosenbaum, S.J.; Lind, T.; Antoch, G.; Bockisch, A. False-Positive FDG PET Uptake−the Role of PET/CT. Eur. Radiol. 2006, 16, 1054–1055. [Google Scholar] [CrossRef]
| Authors | Publication Year | Title | Journal | Age Range | Software | Gender | Sample Size | PET CT | PET MRI | Contrast Enhance-ment | Type of Cancer | Tumor Region | Tesla | Origin | SUV PET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hayashi et al. [42]. | 2019 | Clinical Value of Fused PET/MRI for Surgical Planning in Patients with Oral/ Oropharyngeal Carcinoma | Laryngo- scope | 54–85 yrs | GE Advantage Workstation 4.5 | 8 M 3 F | 11 participants | Discovery 600 Motion System | 1.5 T Avanto Siemens | lack of contrast- enhancement | Squamous cell carcinoma of mandible, lower gingiva, buccal mucosa | mandible, medial pterygoid muscle | 1.5 T | Japan | Not mentioned |
| Kanno et al. [43]. | 2020 | Comparison of diagnostic accuracy between [18F] FDG PET/MRI and contrast-enhanced MRI in T staging for oral tongue cancer | Annals of Nuclear Medicine | 38–88 yrs | GE Advantage Workstation 4.5 | 7 M 11 F | 18 participants | Biograph mCT Flow (Siemens) | 3T PET/MR GE Healthcare | Gadolinium- diethylene- triamine | oral tongue cancer | tongue | 3 T | Japan | 0–10 |
| Loeffelbein et al. [44]. | 2014 | Diagnostic value of retrospective PET-MRI fusion in head-and-neck cancer | BMC Cancer | 27–72 yrs | Not Mentioned | 21 M 12 F | 33 participants | Siemens Biograph Sensation 64 PET/CT | Magnetom Verio 3T Siemens | Contrast agent (I.V) (Imeron 300, 80–120 mL) | head and neck region (primary malignant neoplasm, recurrent tumor disease, lymph node metastasis) | oropharynx, tongue, the mouth floor, hypopharynx, buccal mucosa, vallecula, tonsil, salivary gland | 3 T | Germany | SUV of five (5) |
| Schaarschmidt et al. [45]. | 2015 | Locoregional tumour evaluation of squamous cell carcinoma in the head and neck area: a comparison between MRI, PET/CT and Integrated PET/MRI | Eur J Nucl Med Mol Imaging | 56.5 ± 8.6 yrs | OsiriX Workstation (Pixmeo SARL, Bernex, Switzerland) | 23 M 2 F | 25 participants | Biograph mCT (Siemens) | Biograph mMR Siemens | Contrast agent (I.V) (Ultravist, 60 mL) | head and neck squamous cell carcinoma (HNSCC) | head and neck squamous cell carcinoma (HNSCC) | N/M | Germany | Primary 10.5 ± 3.0 (PET/CT), 13.6 ± 6.1 (PET/MR), Recurrent 8.0 ± 3.8 (PET/CT), 8.6 ± 4.4 (PET/MR) |
| Samołyk- Kogaczewska et al. [46]. | 2019 | PET/MRI-guided GTV delineation during radiotherapy planning in patients with squamous cell carcinoma of the tongue | Strahlenther Onkol | 36–66 yrs | Siemens syngo.via VB10B | 5 M 5 F | 10 participants | 320-slices CT scanner Aquilion ON | 3T Siemens Biograph mMRI Siemens | Contrast agent (I.V) (Ultravist 300, 1 mL/kg) | squamous cell carcinoma of tongue (SCC) | squamous cell carcinoma of tongue | 3T | Poland | SUVmax 4.66–20.6 SUVmean 2.61–12.4 |
| Tsujikawa et al. [47]. | 2020 | Zero Echo Time-Based PET/MRI Attenuation Correction in Patients With Oral Cavity Cancer | Clinical Nuclear Medicine | 45–90 yrs | Not Mentioned | 8 M 5 F | 13 participants | Biograph mCT Flow Siemens | 3T Signa PET/MR Healthcare | NA | oral cavity cancer | tongue, mandibular gingival, maxillary gingival, buccal mucosa, oral floor | 3T | Japan | PRIMARY OCC: 14.4 ± 8.0 SUV(CT) 14.5 ± 8.6 SUV(Dixon) 15.6 ± 8.8 SUV(ZTE) CLNs 6.3 ± 3.0 SUV(CT) 8.0 ± 4.0 SUV(Dixon) 7.6 ± 3.9 SUV(ZTE) |
| Schaarschmidt et al. [40]. | 2017 | Is integrated 18F-FDG PET/MRI superior to 18F-FDG PET/CT in the differentiation of incidental tracer uptake in the head and neck area? | Diagn Interv Radiol | 54.4 ± 15 yrs | OsiriX (Pixmeo SARL). | 41 M 40 F | 81 participants | Biograph mCTTM Siemens | Magnetom Verio 3 T Siemens | Ultravist Bayer Healthcare (I.V, 70 mL) | head and neck region (primary malignant neoplasm, recurrent tumour disease, lymph node metastasis) | salivary glands, oral and nasal cavity, thyroid, unknown primary tumor | Not men- tioned | Germany | mean SUVmax, 5.0 ± 1.9 (PET/CT) 5.9 ± 3.0 (PET/MRI) |
| Samołyk- Kogaczewska et al. [48]. | 2020 | Usefulness of Hybrid PET/MRI in Clinical Evaluation of Head and Neck Cancer Patients | Cancers | 36–74 yrs | Not Mentioned | 9 M 12 F | 21 participants | Aquilion CX | Siemens Healthcare GmbH | Ultravist (300, 1 mL/kg) | squamous cell carcinoma | tongue, buccal mucosa, lower gingiva, base tongue maxillary alveolar ridge, submandibular salivary gland | 3 T | Poland | NI |
| Freihat et al. [31]. | 2021 | Pre-treatment PET/MRI based FDG and DWI imaging Parameters for predicting HPV status and tumor response to chemoradiotherapy in primary oropharyngeal squamous cell carcinoma (OPSCC) | Oral Oncology | 61.4 ± 0.7 yrs | Siemens Syngo Via (20VB) | 23 M 19 F | 33 participants | NA | Biograph mMR Siemens | Gadovist Bayer Healthcare | oro- pharyngeal squamous cell carcinoma | HPV | 3 T | Hungary | Primary tumors: 12.61 ± 0.5 |
| Queiroz et al. [41]. | 2014 | PET/MRI and PET/CT in follow-up of head and neck cancer patients | Eur J Nucl Med Mol Imaging | 24–90 yrs | Not Mentioned | 68 M 19 F | 87 participants | GE Healthcare | GE Healthcare | Visipaque 320 (I.V, 70 mL), GE Healthcare (0.2 mL/kg) | Head and neck Region (primary malignant neoplasm, recurrent tumour disease, lymph node metastasis) | squamous cell carcinoma, adeno- carcinoma, oropharynx, oral cavity, larynx, epipharynx, hypopharynx | 3T | Switzer- land | Positive If their SUVmax was at least two-fold higher than surrounding background activity |
| Reinert et al. [38]. | 2022 | [18F] Fluoride Positron-Emission Tomography (PET) and [18F]FDG PET for Assessment of Osteomyelitis of the Jaw in Comparison to Computed Tomography (CT) and Magnetic Resonance Imaging (MRI): A Prospective PET/CT and PET/MRI Pilot Study | J Clin Med | 55.3 ± 10.0 yrs | Not Mentioned | 6 F | 6 participants | Biograph mCT Siemens | Biograph mMR Siemens Healthcare | Gadovist, Bayer Vital | Osteomye- litis | OMJ | Not men- tioned | Germany | healthy jawbone SUVmean 15.4 ± 4.2, [18F] FDG uptake was moderately higher SUVmean 1.9 ± 0.7) |
| Park et al. [39] | 2020 | Diagnostic Accuracy and Confidence of [18F] FDG PET/MRI in comparison with PET or MRI alone in Head and Neck Cancer | Scientific Reports | 18–83 yrs | Not Mentioned | 71 M 37 F | 108 participants | NA | Biograph mMR Siemens Healthcare | Dotarem (0.1 mmol/kg) | Head and neck region (primary malignant neoplasm, recurrent tumour disease, lymph node metastasis) | pharynx, oral cavity, sinonasal cavity, parotid gland, larynx, infrate- mporal fossa, squamous cell carcinoma, lymphoma, adeno- carcinoma | 3 T | Korea | Definitely benign SUV < 2.5, Score of 2 probably benign: SUV ≥ 2.5 Score of 4, probably malignant (2.5 ≤ SUV < 5) Score of 5 definitely malignant SUV ≥ 5 |
| Bias Due to | ||||||||
|---|---|---|---|---|---|---|---|---|
| Confounding | Selection of Participants for the Study | Classification of Interventions | Deviations from Intended Interventions | Missing Data | Measurement of Outcomes | Selection of the Reported Result | Overall | |
| Hayashi et al. 2019 [42] | Low | Moderate | Low | Moderate | Moderate | Moderate | Moderate | Moderate |
| Kanno et al. 2020 [43] | Low | Moderate | Moderate | Moderate | Moderate | Moderate | Low | Moderate |
| Loeffelbein et al. 2014 [44] | Low | Moderate | Low | Moderate | Low | Low | Low | Moderate |
| Schaarschmidt et al. 2015 [45] | Low | Serious | Serious | Moderate | Low | Moderate | Moderate | Serious |
| Samołyk -Kogaczewska et al. 2019 [46] | Low | Moderate | Low | Serious | Moderate | Moderate | Moderate | Serious |
| Tsujikawa et al. 2020 [47] | Low | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| Samołyk -Kogaczewska et al. 2020 [48] | Low | Moderate | Moderate | Moderate | Low | Low | Moderate | Moderate |
| Freihat et al. 2021 [31] | Low | Moderate | Moderate | Low | Moderate | Low | Low | Moderate |
| Schaarschmidt et al. 2017 [40] | Low | Low | Moderate | Low | Low | Moderate | Moderate | Moderate |
| Queiroz et al. 2014 [41] | Low | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Park et al. 2020 [39] | Low | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Reinert et al. 2022 [38] | Low | Moderate | Low | Low | Low | Low | Moderate | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsea, A.; Christoloukas, N.; Koutsipetsidou, S.; Papavasileiou, P.; Oikonomou, G.; Angelopoulos, C. Positron Emission Tomography–Magnetic Resonance Imaging, a New Hybrid Imaging Modality for Dentomaxillofacial Malignancies—A Systematic Review. Diagnostics 2025, 15, 654. https://doi.org/10.3390/diagnostics15060654
Mitsea A, Christoloukas N, Koutsipetsidou S, Papavasileiou P, Oikonomou G, Angelopoulos C. Positron Emission Tomography–Magnetic Resonance Imaging, a New Hybrid Imaging Modality for Dentomaxillofacial Malignancies—A Systematic Review. Diagnostics. 2025; 15(6):654. https://doi.org/10.3390/diagnostics15060654
Chicago/Turabian StyleMitsea, Anastasia, Nikolaos Christoloukas, Spyridoula Koutsipetsidou, Periklis Papavasileiou, Georgia Oikonomou, and Christos Angelopoulos. 2025. "Positron Emission Tomography–Magnetic Resonance Imaging, a New Hybrid Imaging Modality for Dentomaxillofacial Malignancies—A Systematic Review" Diagnostics 15, no. 6: 654. https://doi.org/10.3390/diagnostics15060654
APA StyleMitsea, A., Christoloukas, N., Koutsipetsidou, S., Papavasileiou, P., Oikonomou, G., & Angelopoulos, C. (2025). Positron Emission Tomography–Magnetic Resonance Imaging, a New Hybrid Imaging Modality for Dentomaxillofacial Malignancies—A Systematic Review. Diagnostics, 15(6), 654. https://doi.org/10.3390/diagnostics15060654





