Cornea Oculomics: A Clinical Blueprint for Extending Corneal Diagnostics and Artificial Intelligence in Systemic Health Insights
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Cornea Oculomics
4.1.1. Endocrine and Metabolic Diseases
4.1.2. Infectious Diseases
4.1.3. Neurological and Neuromuscular Disorders
4.1.4. Autoimmune and Rheumatologic Disorders
4.1.5. Genetic Diseases
4.1.6. Hematologic and Oncologic Disorders
4.2. Review of Corneal Diagnostic Modalities
4.3. Applications and Future Directions in Cornea Oculomics
4.3.1. Diagnostic Value of Corneal Biomarkers
4.3.2. Potential for Early Disease Detection
4.3.3. Artificial Intelligence and Applications in Marginalized Areas
4.3.4. Challenges and Limitations
4.3.5. Future Research Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANN | Artificial neural network |
| AS-OCT | Anterior segment optical coherence tomography |
| IVCM | In vivo confocal microscopy |
| CH | Corneal hysteresis |
| CNFL | Corneal nerve fiber length |
| CNFD | Corneal nerve fiber density |
| CNBD | Corneal nerve branch density |
| CNBT | Corneal nerve branch thickness |
| CNN | Convolutional neural network |
| DC | Dendritic cell |
| MEN | Multiple endocrine neoplasia |
| PUK | Peripheral ulcerative keratitis |
| SLE | Systemic lupus erythematosus |
References
- Shah, R.; Amador, C.; Tormanen, K.; Ghiam, S.; Saghizadeh, M.; Arumugaswami, V.; Kumar, A.; Kramerov, A.A.; Ljubimov, A.V. Systemic Diseases and the Cornea. Exp. Eye Res. 2021, 204, 108455. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, S.; Whelchel, A.; Nicholas, S.; Sharif, R.; Riaz, K.; Karamichos, D. Diabetic Keratopathy: Insights and Challenges. Surv. Ophthalmol. 2020, 65, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; He, Y.; Ren, Y.-R.; Chen, B.-H. Corneal Alteration and Pathogenesis in Diabetes Mellitus. Int. J. Ophthalmol. 2019, 12, 1939–1950. [Google Scholar] [CrossRef]
- Selter, J.H.; Gire, A.I.; Sikder, S. The Relationship between Graves’ Ophthalmopathy and Dry Eye Syndrome. Clin. Ophthalmol. 2014, 9, 57–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soleymanzadeh, M.; Rafizadeh, S.M.; Ghochani, G.; Mafi, A.R.; Nazari, M.; Rajabi, M.T. Biomechanical Changes of the Cornea after Orbital Decompression in Thyroid-Associated Orbitopathy Measured by Corvis ST. Sci. Rep. 2024, 14, 16930. [Google Scholar] [CrossRef] [PubMed]
- Petrie, I.; Knox Cartwright, N.; Roberts, H.; Kyrodimou, E.; Moudiotis, C.; Owens, M.; Cleaver, R.; Smith, J.; Vaidya, B. Multiple Endocrine Neoplasia Type 2A Syndrome Presenting with Corneal Nerve Thickening. QJM Int. J. Med. 2024, 117, 226–228. [Google Scholar] [CrossRef]
- Yin, L.; Wang, Y.; Zhu, J.; Tan, C.; Sun, C.; Yao, Y. Prominent Corneal Nerves in Pure Mucosal Neuroma Syndrome, a Clinical Phenotype Distinct from Multiple Endocrine Neoplasia Type 2B. BMC Ophthalmol. 2023, 23, 260. [Google Scholar] [CrossRef]
- Kinoshita, S.; Tanaka, F.; Ohashi, Y.; Ikeda, M.; Takai, S. Incidence of Prominent Corneal Nerves in Multiple Endocrine Neoplasia Type 2A. Am. J. Ophthalmol. 1991, 111, 307–311. [Google Scholar] [CrossRef]
- Javadi, M.-A.; Kanavi, M.R.; Faramarzi, A.; Feizi, S.; Azizi, F.; Javadi, F. Confocal Scan Imaging and Impression Cytology of the Cornea in a Case of Multiple Endocrine Neoplasia Type-2b. J. Ophthalmic Vis. Res. 2012, 7, 176–179. [Google Scholar]
- Moramarco, A.; Sacchetti, M.; Franzone, F.; Segatto, M.; Cecchetti, D.; Miraglia, E.; Roberti, V.; Iacovino, C.; Giustini, S. Ocular Surface Involvement in Patients with Neurofibromatosis Type 1 Syndrome. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1757–1762. [Google Scholar] [CrossRef]
- Golan, A.; Savir, H.; Bar-Meir, S.; Oliver, I.; De Vries, A. Band Keratopathy Due to Hyperparathyroidism. Ophthalmologica 1975, 171, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Abeysiri, P.; Sinha, A. An Unusual Pattern of Corneal Calcification in Tertiary Hyperparathyroidism. Arch. Ophthalmol. 2006, 124, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Babenko, B.; Traynis, I.; Chen, C.; Singh, P.; Uddin, A.; Cuadros, J.; Daskivich, L.P.; Maa, A.Y.; Kim, R.; Kang, E.Y.-C.; et al. A Deep Learning Model for Novel Systemic Biomarkers in Photographs of the External Eye: A Retrospective Study. Lancet Digit. Health 2023, 5, e257–e264. [Google Scholar] [CrossRef]
- Porter, R.; Crombie, A.L. Corneal and Conjunctival Calcification in Chronic Renal Failure. Br. J. Ophthalmol. 1973, 57, 339–343. [Google Scholar] [CrossRef]
- Shah, P.G.; Shields, C.L.; Shields, J.A.; Di Marco, C. Band Keratopathy Secondary to an Iris Melanoma. Cornea 1991, 10, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Khanam, N.; Kumar, R. Recent Applications of Artificial Intelligence in Early Cancer Detection. Curr. Med. Chem. 2022, 29, 4410–4435. [Google Scholar] [CrossRef]
- Puthiyedath, R.; Kakkanatt, A.C.V.; Mathai, M.T.; Ramachandran, L.; Sunny, A.; Arun, S. A Comparative Study on Ocular Manifestations in Patients with Polycystic Ovary Syndrome and Healthy Volunteers. Kerala J. Ophthalmol. 2022, 34, 250. [Google Scholar] [CrossRef]
- Karaca Adıyeke, S.; Karaca, I.; Yıldırım, S.; Adıyeke, M.; Uyar, İ.; Türe, G. Anterior Segment Findings in Women with Polycystic Ovary Syndrome. Turk. J. Ophthalmol. 2017, 47, 24–27. [Google Scholar] [CrossRef]
- Cengiz Ozturk, T.G.; Akcam, H.T.; Ellibes Kaya, A. Pentacam Corneal Topography and Densitometry Features of PCOS Patients. BMC Ophthalmol. 2023, 23, 5. [Google Scholar] [CrossRef]
- Danthuluri, V.; Grant, M.B. Update and Recommendations for Ocular Manifestations of COVID-19 in Adults and Children: A Narrative Review. Ophthalmol. Ther. 2020, 9, 853–875. [Google Scholar] [CrossRef]
- Siedlecki, J.; Brantl, V.; Schworm, B.; Mayer, W.J.; Gerhardt, M.; Michalakis, S.; Kreutzer, T.; Priglinger, S. COVID-19: Ophthalmological Aspects of the SARS-CoV 2 Global Pandemic. Klin. Monbl. Augenheilkd. 2020, 237, 675–680. [Google Scholar] [CrossRef]
- Sawant, O.B.; Singh, S.; Wright, R.E.; Jones, K.M.; Titus, M.S.; Dennis, E.; Hicks, E.; Majmudar, P.A.; Kumar, A.; Mian, S.I. Prevalence of SARS-CoV-2 in Human Post-Mortem Ocular Tissues. Ocul. Surf. 2021, 19, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.; Queiruga-Piñeiro, J.; Lozano-Sanroma, J.; Alcalde, I.; Gallar, J.; Fernández-Vega Cueto, L.; Alfonso, J.F.; Quirós, L.M.; Merayo-Lloves, J. Small Fiber Neuropathy in the Cornea of Covid-19 Patients Associated with the Generation of Ocular Surface Disease. Ocul. Surf. 2022, 23, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Bitirgen, G.; Korkmaz, C.; Zamani, A.; Ozkagnici, A.; Zengin, N.; Ponirakis, G.; Malik, R.A. Corneal Confocal Microscopy Identifies Corneal Nerve Fibre Loss and Increased Dendritic Cells in Patients with Long COVID. Br. J. Ophthalmol. 2022, 106, 1635–1641. [Google Scholar] [CrossRef]
- Tuli, S.S. Fungal Keratitis. Clin. Ophthalmol. 2011, 5, 275–279. [Google Scholar] [CrossRef]
- Cho, Y.W.; Kang, H.J.; Kim, G.N.; Kim, H.A.; Chung, I.Y.; Kim, S.J. Epstein-Barr Virus-Related Corneal Endotheliitis Accompanied with Secondary Glaucoma. J. Korean Ophthalmol. Soc. 2020, 61, 205–208. [Google Scholar] [CrossRef]
- Ando, K.; Ishihara, M.; Kusumoto, Y.; Shibuya, E.; Nakamura, S.; Mizuki, N. A Case of Corneal Endotheliitis with Mumps Virus RNA in Aqueous Humor Detected by RT-PCR. Ocul. Immunol. Inflamm. 2013, 21, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Sodhi, P.K. Mumps-Induced Corneal Endotheliitis. Cornea 2004, 23, 400. [Google Scholar] [CrossRef]
- Mersch, J.; Mouthuy, M.; Otjacques, L.; Forez, S.; Öztürk, N. Unilateral Mumps-Associated Corneal Endotheliitis: A Case Report of a Rare Ocular Complication of Mumps. J. Français D’ophtalmologie 2021, 44, e609–e611. [Google Scholar] [CrossRef]
- Singh, A.K.; Verma, S. Use of Ocular Biomarkers as a Potential Tool for Early Diagnosis of Alzheimer’s Disease. Indian J. Ophthalmol. 2020, 68, 555–561. [Google Scholar] [CrossRef]
- Örnek, N.; Dağ, E.; Örnek, K. Corneal Sensitivity and Tear Function in Neurodegenerative Diseases. Curr. Eye Res. 2015, 40, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Ponirakis, G.; Al Hamad, H.; Sankaranarayanan, A.; Khan, A.; Chandran, M.; Ramadan, M.; Tosino, R.; Gawhale, P.V.; Alobaidi, M.; AlSulaiti, E.; et al. Association of Corneal Nerve Fiber Measures with Cognitive Function in Dementia. Ann. Clin. Transl. Neurol. 2019, 6, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Al-Janahi, E.; Ponirakis, G.; Al Hamad, H.; Vattoth, S.; Elsotouhy, A.; Petropoulos, I.N.; Khan, A.; Gad, H.; Chandran, M.; Sankaranarayanan, A.; et al. Corneal Nerve and Brain Imaging in Mild Cognitive Impairment and Dementia. J. Alzheimer’s Dis. 2020, 77, 1533–1543. [Google Scholar] [CrossRef]
- Ponirakis, G.; Al Hamad, H.; Omar, D.A.M.; Petropoulos, I.N.; Khan, A.; Gad, H.; Chandran, M.; Gadelseed, M.; Elsotouhy, A.; Ramadan, M.; et al. Corneal Nerve Loss Predicts Dementia in Patients with Mild Cognitive Impairment. Ann. Clin. Transl. Neurol. 2023, 10, 599–609. [Google Scholar] [CrossRef]
- Che, N.-N.; Chen, S.; Jiang, Q.-H.; Chen, S.-Y.; Zhao, Z.-X.; Li, X.; Malik, R.A.; Ma, J.-J.; Yang, H.-Q. Corneal Confocal Microscopy Differentiates Patients with Parkinson’s Disease with and without Autonomic Involvement. npj Park. Dis. 2022, 8, 114. [Google Scholar] [CrossRef]
- Lim, S.H.; Ferdousi, M.; Kalteniece, A.; Mahfoud, Z.R.; Petropoulos, I.N.; Malik, R.A.; Kobylecki, C.; Silverdale, M. Corneal Confocal Microscopy Identifies Parkinson’s Disease with More Rapid Motor Progression. Mov. Disord. 2021, 36, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Yin, P.; Guan, C.; Shao, Q.; Cui, G.; Zan, K.; Xu, C. Corneal Confocal Microscopy May Help to Distinguish Multiple System Atrophy from Parkinson’s Disease. NPJ Park. Dis. 2024, 10, 63. [Google Scholar] [CrossRef]
- Bitirgen, G.; Akpinar, Z.; Malik, R.A.; Ozkagnici, A. Use of Corneal Confocal Microscopy to Detect Corneal Nerve Loss and Increased Dendritic Cells in Patients With Multiple Sclerosis. JAMA Ophthalmol. 2017, 135, 777–782. [Google Scholar] [CrossRef]
- Petropoulos, I.N.; Kamran, S.; Li, Y.; Khan, A.; Ponirakis, G.; Akhtar, N.; Deleu, D.; Shuaib, A.; Malik, R.A. Corneal Confocal Microscopy: An Imaging Endpoint for Axonal Degeneration in Multiple Sclerosis. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3677–3681. [Google Scholar] [CrossRef]
- Suh, A.; Ong, J.; Kamran, S.A.; Waisberg, E.; Paladugu, P.; Zaman, N.; Sarker, P.; Tavakkoli, A.; Lee, A.G. Retina Oculomics in Neurodegenerative Disease. Ann. Biomed. Eng. 2023, 51, 2708–2721. [Google Scholar] [CrossRef]
- Fu, J.; He, J.; Zhang, Y.; Liu, Z.; Wang, H.; Li, J.; Chen, L.; Fan, D. Small Fiber Neuropathy for Assessment of Disease Severity in Amyotrophic Lateral Sclerosis: Corneal Confocal Microscopy Findings. Orphanet J. Rare Dis. 2022, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, D.; Doğru, A.; Baykal, T. Assessment of Corneal Parameters in Patients with Rheumatoid Arthritis. Indian J. Ophthalmol. 2024, 72, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Ozcura, F.; Aksakalli, E. Central Corneal Thickness and Corneal Curvature in Patients with Rheumatoid Arthritis. Acta Ophthalmol. 2010, 88. [Google Scholar] [CrossRef]
- Gupta, Y.; Kishore, A.; Kumari, P.; Balakrishnan, N.; Lomi, N.; Gupta, N.; Vanathi, M.; Tandon, R. Peripheral Ulcerative Keratitis. Surv. Ophthalmol. 2021, 66, 977–998. [Google Scholar] [CrossRef]
- Versura, P.; Frigato, M.; Cellini, M.; Mulè, R.; Malavolta, N.; Campos, E.C. Diagnostic Performance of Tear Function Tests in Sjogren’s Syndrome Patients. Eye 2007, 21, 229–237. [Google Scholar] [CrossRef]
- Goto, E.; Matsumoto, Y.; Kamoi, M.; Endo, K.; Ishida, R.; Dogru, M.; Kaido, M.; Kojima, T.; Tsubota, K. Tear Evaporation Rates in Sjögren Syndrome and Non-Sjögren Dry Eye Patients. Am. J. Ophthalmol. 2007, 144, 81–85.e1. [Google Scholar] [CrossRef]
- Villani, E.; Galimberti, D.; Viola, F.; Mapelli, C.; Ratiglia, R. The Cornea in Sjogren’s Syndrome: An in Vivo Confocal Study. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Ding, Y.; Li, X. Alterations in Corneal Epithelial Dendritic Cell in Sjogren’s Syndrome Dry Eye and Clinical Correlations. Sci. Rep. 2022, 12, 11167. [Google Scholar] [CrossRef]
- Palejwala, N.V.; Walia, H.S.; Yeh, S. Ocular Manifestations of Systemic Lupus Erythematosus: A Review of the Literature. Autoimmune Dis. 2012, 2012, 290898. [Google Scholar] [CrossRef]
- Yazici, A.T.; Kara, N.; Yüksel, K.; Altinkaynak, H.; Baz, O.; Bozkurt, E.; Demirok, A. The Biomechanical Properties of the Cornea in Patients with Systemic Lupus Erythematosus. Eye 2011, 25, 1005–1009. [Google Scholar] [CrossRef]
- Saldaña-Garrido, J.D.; Cantó-Cerdán, M.; Gil-Guillén, V.F.; Alfaro-Beltrá, M.L.; Sivera, F. Association between Central Corneal Thickness and Systemic Lupus Erythematosus: A Cross-Sectional Study Protocol. Front. Med. 2024, 11, 1483930. [Google Scholar] [CrossRef] [PubMed]
- Eissa, I.M.; Nassar, G.A.; Arfeen, S.A.; Dahab, A.A. Evaluation of Central and Peripheral Corneal Thicknesses in Patients with Systemic Lupus Erythematosus. Clin. Ophthalmol. 2020, 14, 589–595. [Google Scholar] [CrossRef]
- Icoz, M.; Yildiz Tasci, Y.; Erten, Ş.; Sarac, O. Tomographic, Biomechanical, and In Vivo Confocal Microscopic Changes in Cornea in Chronic Gout Disease. Ocul. Immunol. Inflamm. 2024, 32, 2428–2435. [Google Scholar] [CrossRef]
- Gillan, W.D.H. The Cornea in Systemic Disease. Afr. Vision. Eye Health 2005, 64, 97–101. [Google Scholar] [CrossRef][Green Version]
- Nemet, A.Y.; Assia, E.I.; Apple, D.J.; Barequet, I.S. Current Concepts of Ocular Manifestations in Marfan Syndrome. Surv. Ophthalmol. 2006, 51, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, H.; Ansari, S.; Mohammad-Rabei, H.; Mets, M.B. Management Strategies of Ocular Abnormalities in Patients with Marfan Syndrome: Current Perspective. J. Ophthalmic Vis. Res. 2019, 14, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Asanad, S.; Bayomi, M.; Brown, D.; Buzzard, J.; Lai, E.; Ling, C.; Miglani, T.; Mohammed, T.; Tsai, J.; Uddin, O.; et al. Ehlers-Danlos Syndromes and Their Manifestations in the Visual System. Front. Med. 2022, 9, 996458. [Google Scholar] [CrossRef]
- Villani, E.; Garoli, E.; Bassotti, A.; Magnani, F.; Tresoldi, L.; Nucci, P.; Ratiglia, R. The Cornea in Classic Type Ehlers-Danlos Syndrome: Macro- and Microstructural Changes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8062–8068. [Google Scholar] [CrossRef][Green Version]
- Gharbiya, M.; Moramarco, A.; Castori, M.; Parisi, F.; Celletti, C.; Marenco, M.; Mariani, I.; Grammatico, P.; Camerota, F. Ocular Features in Joint Hypermobility Syndrome/Ehlers-Danlos Syndrome Hypermobility Type: A Clinical and in Vivo Confocal Microscopy Study. Am. J. Ophthalmol. 2012, 154, 593–600.e1. [Google Scholar] [CrossRef]
- Sridhar, M.S.; Rangaraju, A.; Anbarasu, K.; Reddy, S.P.; Daga, S.; Jayalakshmi, S.; Shaik, B. Evaluation of Kayser–Fleischer Ring in Wilson Disease by Anterior Segment Optical Coherence Tomography. Indian J. Ophthalmol. 2017, 65, 354–357. [Google Scholar] [CrossRef]
- Yang, Q.; Ju, G.; He, Y. Corneal Densitometry: A New Evaluation Indicator for Corneal Diseases. Surv. Ophthalmol. 2024, 70, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Cankurtaran, V.; Tekin, K.; Cakmak, A.I.; Inanc, M.; Turgut, F.H. Assessment of Corneal Topographic, Tomographic, Densitometric, and Biomechanical Properties of Fabry Patients with Ocular Manifestations. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1057–1064. [Google Scholar] [CrossRef]
- Alio, J.L.; Vega-Estrada, A.; Sanz, P.; Osman, A.A.; Kamal, A.M.; Mamoon, A.; Soliman, H. Corneal Morphologic Characteristics in Patients With Down Syndrome. JAMA Ophthalmol. 2018, 136, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Serefoglu Cabuk, K.; Esen, B.; Atalay, K.; Kirgiz, A.; Aydin, R. Alterations in Biomechanical Properties of the Cornea among Patients with Polycystic Kidney Disease. Int. Ophthalmol. 2018, 38, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Bouazza, M.; Youssefi, H.; Bouanani, N. Ocular Manifestations in Hematological Disorders. Cureus 2022, 14, e27941. [Google Scholar] [CrossRef]
- Hoehn, M.E.; Calderwood, J.; Gannon, E.; Cook, B.; Rochester, R.; Hartford, C.; Triplett, B.; Sunkara, A.; Kang, G.; Walton, R.C. Ocular Complications in a Young Pediatric Population Following Bone Marrow Transplantation. J. AAPOS 2018, 22, 102–106.e1. [Google Scholar] [CrossRef]
- Hoehn, M.E.; Vestal, R.; Calderwood, J.; Gannon, E.; Cook, B.; Rochester, R.; Hartford, C.; Triplett, B.; Sunkara, A.; Kang, G.; et al. Ocular Complications in School-Age Children and Adolescents after Allogeneic Bone Marrow Transplantation. Am. J. Ophthalmol. 2020, 213, 153–160. [Google Scholar] [CrossRef]
- Bhandare, N.; Moiseenko, V.; Song, W.Y.; Morris, C.G.; Bhatti, M.T.; Mendenhall, W.M. Severe Dry Eye Syndrome After Radiotherapy for Head-and-Neck Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1501–1508. [Google Scholar] [CrossRef]
- Ebeid, W.M.; Kenny, M.A.; Badran, T.A. Evaluation of Corneal Epithelial Thickness and Dry Eye Disease Tests in Thalassemic Adolescents. Clin. Ophthalmol. 2021, 15, 1425–1431. [Google Scholar] [CrossRef]
- Khan, S.A.; Naqvi, S.A.H.; Saeed, A.; Khan, W.A.; Moqeet, M.A.; Ali, W.; Khan, F.U. Peripheral Neuropathy in Beta-Thalassemia: Corneal Confocal Microscopy-Based Study. Cureus 2022, 14, e32122. [Google Scholar] [CrossRef]
- Hanna, D.; Atfy, M.; Bor’i, A.; Elsayed, M.; el-Sattar, E.M.A.; Gameil, D. Corneal and Lens Densitometry Evaluation with Pentacam HR in Children and Adolescents with Beta Thalassemia Major: A Case–Control Study. Egypt. Pediatr. Assoc. Gaz. 2024, 72, 96. [Google Scholar] [CrossRef]
- Utsunomiya, T.; Nagaoka, T.; Hanada, K.; Omae, T.; Yokota, H.; Abiko, A.; Haneda, M.; Yoshida, A. Imaging of the Corneal Subbasal Whorl-like Nerve Plexus: More Accurate Depiction of the Extent of Corneal Nerve Damage in Patients With Diabetes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5417–5423. [Google Scholar] [CrossRef]
- Hashemi, A.; Khabazkhoob, M.; Narooei, F.; Mortazavi, A.; Hashemi, H. Corneal Topographic Indices of Scheimpflug Camera in Type 2 Diabetic and Non-Diabetic Elderly Populations. BMC Ophthalmol. 2023, 23, 427. [Google Scholar] [CrossRef] [PubMed]
- Midena, E.; Brugin, E.; Ghirlando, A.; Sommavilla, M.; Avogaro, A. Corneal Diabetic Neuropathy: A Confocal Microscopy Study. J. Refract. Surg. 2006, 22, S1047–S1052. [Google Scholar] [CrossRef] [PubMed]
- Quadrado, M.J.; Popper, M.; Morgado, A.M.; Murta, J.N.; Van Best, J.A. Diabetes and Corneal Cell Densities in Humans by In Vivo Confocal Microscopy. Cornea 2006, 25, 761. [Google Scholar] [CrossRef] [PubMed]
- Hossain, P.; Sachdev, A.; Malik, R.A. Early Detection of Diabetic Peripheral Neuropathy with Corneal Confocal Microscopy. Lancet 2005, 366, 1340–1343. [Google Scholar] [CrossRef]
- Kallinikos, P.; Berhanu, M.; O’Donnell, C.; Boulton, A.J.M.; Efron, N.; Malik, R.A. Corneal Nerve Tortuosity in Diabetic Patients with Neuropathy. Investig. Ophthalmol. Vis. Sci. 2004, 45, 418–422. [Google Scholar] [CrossRef]
- Misra, S.L.; Slater, J.A.; McGhee, C.N.J.; Pradhan, M.; Braatvedt, G.D. Corneal Confocal Microscopy in Type 1 Diabetes Mellitus: A Six-Year Longitudinal Study. Transl. Vision. Sci. Technol. 2022, 11, 17. [Google Scholar] [CrossRef]
- Su, D.H.W.; Wong, T.Y.; Wong, W.-L.; Saw, S.-M.; Tan, D.T.H.; Shen, S.Y.; Loon, S.-C.; Foster, P.J.; Aung, T. Diabetes, Hyperglycemia, and Central Corneal Thickness: The Singapore Malay Eye Study. Ophthalmology 2008, 115, 964–968.e1. [Google Scholar] [CrossRef]
- Ramm, L.; Spoerl, E.; Pillunat, L.E.; Terai, N. Corneal Densitometry in Diabetes Mellitus. Cornea 2020, 39, 968. [Google Scholar] [CrossRef]
- Jiang, M.-S.; Yuan, Y.; Gu, Z.-X.; Zhuang, S.-L. Corneal Confocal Microscopy for Assessment of Diabetic Peripheral Neuropathy: A Meta-Analysis. Br. J. Ophthalmol. 2016, 100, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczak, J.; Zimmermann, H.; Kheirkhah, A.; Kadas, E.M.; Oberwahrenbrock, T.; Muller, R.; Ren, A.; Kuchling, J.; Dietze, H.; Prüss, H.; et al. Patients with Multiple Sclerosis Demonstrate Reduced Subbasal Corneal Nerve Fibre Density. Mult. Scler. 2017, 23, 1847–1853. [Google Scholar] [CrossRef]
- Dericioğlu, V.; Akkaya Turhan, S.; Erdem, H.E.; Sevik, M.O.; Erdil, E.; Sünter, G.; Ağan, K.; Toker, E. In Vivo Corneal Confocal Microscopy in Multiple Sclerosis: Can It Differentiate Disease Relapse in Multiple Sclerosis? Am. J. Ophthalmol. 2023, 250, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, I.S.J.; Konttinen, Y.T.; Vesaluoma, M.H.; Moilanen, J.A.O.; Helintö, M.; Tervo, T.M.T. Corneal Innervation and Morphology in Primary Sjögren’s Syndrome. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2545–2549. [Google Scholar] [CrossRef]
- Shih, K.C.; Tse, R.H.-K.; Lau, Y.T.-Y.; Chan, T.C.-Y. Advances in Corneal Imaging: Current Applications and Beyond. Asia-Pac. J. Ophthalmol. 2019, 8, 105. [Google Scholar] [CrossRef]
- Salah-Mabed, I.; Saad, A.; Gatinel, D. Topography of the Corneal Epithelium and Bowman Layer in Low to Moderately Myopic Eyes. J. Cataract. Refract. Surg. 2016, 42, 1190. [Google Scholar] [CrossRef]
- Ahn, H.; Jun, I.; Seo, K.Y.; Kim, E.K.; Kim, T. Artificial Intelligence for the Estimation of Visual Acuity Using Multi-Source Anterior Segment Optical Coherence Tomographic Images in Senile Cataract. Front. Med. 2022, 9, 871382. [Google Scholar] [CrossRef]
- Fu, H.; Baskaran, M.; Xu, Y.; Lin, S.; Wong, D.W.K.; Liu, J.; Tun, T.A.; Mahesh, M.; Perera, S.A.; Aung, T. A Deep Learning System for Automated Angle-Closure Detection in Anterior Segment Optical Coherence Tomography Images. Am. J. Ophthalmol. 2019, 203, 37–45. [Google Scholar] [CrossRef]
- Cruzat, A.; Pavan-Langston, D.; Hamrah, P. In Vivo Confocal Microscopy of Corneal Nerves: Analysis and Clinical Correlation. Semin. Ophthalmol. 2010, 25, 171–177. [Google Scholar] [CrossRef]
- Chiang, J.C.B.; Roy, M.; Kim, J.; Markoulli, M.; Krishnan, A.V. In-Vivo Corneal Confocal Microscopy: Imaging Analysis, Biological Insights and Future Directions. Commun. Biol. 2023, 6, 652. [Google Scholar] [CrossRef]
- Shareef, O.; Soleimani, M.; Tu, E.; Jacobs, D.; Ciolino, J.; Rahdar, A.; Cheraqpour, K.; Ashraf, M.; Habib, N.B.; Greenfield, J.; et al. A Novel Artificial Intelligence Model for Diagnosing Acanthamoeba Keratitis through Confocal Microscopy. Ocul. Surf. 2024, 34, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.M.; Borroni, D.; Liu, R.; Zhao, Y.; Zhang, J.; Lim, J.; Ma, B.; Romano, V.; Qi, H.; Ferdousi, M.; et al. An Artificial Intelligence-Based Deep Learning Algorithm for the Diagnosis of Diabetic Neuropathy Using Corneal Confocal Microscopy: A Development and Validation Study. Diabetologia 2020, 63, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, H.; Tan, H.C.; Lin, M.T.-Y.; Mehta, J.S.; Liu, Y.-C. Diabetic Corneal Neuropathy. J. Clin. Med. 2020, 9, 3956. [Google Scholar] [CrossRef]
- Biswas, S.; Alzahrani, K.; Radhakrishnan, H. Corneal Densitometry to Assess the Corneal Cystine Deposits in Patients with Cystinosis. Cornea 2023, 42, 313–319. [Google Scholar] [CrossRef]
- Ho, Y.-J.; Sun, C.-C.; Chen, H.-C. Cataract Surgery in Patients with Corneal Opacities. BMC Ophthalmol. 2018, 18, 106. [Google Scholar] [CrossRef]
- Petropoulos, I.N.; Alam, U.; Fadavi, H.; Marshall, A.; Asghar, O.; Dabbah, M.A.; Chen, X.; Graham, J.; Ponirakis, G.; Boulton, A.J.M.; et al. Rapid Automated Diagnosis of Diabetic Peripheral Neuropathy with in Vivo Corneal Confocal Microscopy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2071–2078. [Google Scholar] [CrossRef]
- Chen, X.; Graham, J.; Dabbah, M.A.; Petropoulos, I.N.; Ponirakis, G.; Asghar, O.; Alam, U.; Marshall, A.; Fadavi, H.; Ferdousi, M.; et al. Small Nerve Fiber Quantification in the Diagnosis of Diabetic Sensorimotor Polyneuropathy: Comparing Corneal Confocal Microscopy with Intraepidermal Nerve Fiber Density. Diabetes Care 2015, 38, 1138–1144. [Google Scholar] [CrossRef]
- D’Andrea, L.; Montorio, D.; Concilio, M.; Giordano, M.; Cennamo, G.; Costagliola, C. Anterior Segment-Optical Coherence Tomography and Diabetic Retinopathy: Could It Be an Early Biomarker? Photodiagn. Photodyn. Ther. 2022, 39, 102995. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.; Moeini, A.M.; Nabi-Afjadi, M. Specular Microscopy Findings in Diabetic Patients: A Systematic Review and Meta-Analysis. Clin. Diabetes Endocrinol. 2024, 10, 44. [Google Scholar] [CrossRef]
- Dehghani, C.; Pritchard, N.; Edwards, K.; Vagenas, D.; Russell, A.W.; Malik, R.A.; Efron, N. Morphometric Stability of the Corneal Subbasal Nerve Plexus in Healthy Individuals: A 3-Year Longitudinal Study Using Corneal Confocal Microscopy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3195–3199. [Google Scholar] [CrossRef]
- Wells, A.P.; Garway-Heath, D.F.; Poostchi, A.; Wong, T.; Chan, K.C.Y.; Sachdev, N. Corneal Hysteresis but Not Corneal Thickness Correlates with Optic Nerve Surface Compliance in Glaucoma Patients. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3262–3268. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, S.; Samsudin, A.; Ramli, N.; Tan, C.T.; Mimiwati, Z. Corneal Hysteresis Is Reduced in Obstructive Sleep Apnea Syndrome. Optom. Vis. Sci. 2017, 94, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Baskaran, M.; Werkmeister, R.M.; Chua, J.; Schmidl, D.; Aranha dos Santos, V.; Garhöfer, G.; Mehta, J.S.; Schmetterer, L. Anterior Segment Optical Coherence Tomography. Progress Retin. Eye Res. 2018, 66, 132–156. [Google Scholar] [CrossRef]
- Zhang, B.; Shweikh, Y.; Khawaja, A.P.; Gallacher, J.; Bauermeister, S.; Foster, P.J.; UKBiobank Eye and Vision Consortium. Associations with Corneal Hysteresis in a Population Cohort: Results from 96 010 UK Biobank Participants. Ophthalmology 2019, 126, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Channa, R.; Wolf, R.M.; Dong, Y.; Liang, M.; Wang, J.; Abramoff, M.D.; Liu, T.Y.A. Autonomous Artificial Intelligence for Diabetic Eye Disease Increases Access and Health Equity in Underserved Populations. npj Digit. Med. 2024, 7, 196. [Google Scholar] [CrossRef]
- Abdullah, Y.I.; Schuman, J.S.; Shabsigh, R.; Caplan, A.; Al-Aswad, L.A. Ethics of Artificial Intelligence in Medicine and Ophthalmology. Asia Pac. J. Ophthalmol. 2021, 10, 289–298. [Google Scholar] [CrossRef]
- Mittermaier, M.; Raza, M.M.; Kvedar, J.C. Bias in AI-Based Models for Medical Applications: Challenges and Mitigation Strategies. npj Digit. Med. 2023, 6, 113. [Google Scholar] [CrossRef]
- Seyyed-Kalantari, L.; Zhang, H.; McDermott, M.B.A.; Chen, I.Y.; Ghassemi, M. Underdiagnosis Bias of Artificial Intelligence Algorithms Applied to Chest Radiographs in Under-Served Patient Populations. Nat. Med. 2021, 27, 2176–2182. [Google Scholar] [CrossRef]
- Roy, N.S.; Wei, Y.; Kuklinski, E.; Asbell, P.A. The Growing Need for Validated Biomarkers and Endpoints for Dry Eye Clinical Research. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO1–BIO19. [Google Scholar] [CrossRef] [PubMed]
- Tamhane, M.; Cabrera-Ghayouri, S.; Abelian, G.; Viswanath, V. Review of Biomarkers in Ocular Matrices: Challenges and Opportunities. Pharm. Res. 2019, 36, 40. [Google Scholar] [CrossRef]
- Villani, E.; Bonsignore, F.; Cantalamessa, E.; Serafino, M.; Nucci, P. Imaging Biomarkers for Dry Eye Disease. Eye Contact Lens 2020, 46, S141. [Google Scholar] [CrossRef] [PubMed]
- Abràmoff, M.D.; Cunningham, B.; Patel, B.; Eydelman, M.B.; Leng, T.; Sakamoto, T.; Blodi, B.; Grenon, S.M.; Wolf, R.M.; Manrai, A.K.; et al. Foundational Considerations for Artificial Intelligence Using Ophthalmic Images. Ophthalmology 2022, 129, e14–e32. [Google Scholar] [CrossRef] [PubMed]
- Garcia Marin, Y.F.; Alonso-Caneiro, D.; Vincent, S.J.; Collins, M.J. Anterior Segment Optical Coherence Tomography (AS-OCT) Image Analysis Methods and Applications: A Systematic Review. Comput. Biol. Med. 2022, 146, 105471. [Google Scholar] [CrossRef]
- Price, W.N.; Cohen, I.G. Privacy in the Age of Medical Big Data. Nat. Med. 2019, 25, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Hong, M.; Lam, W.S.T.; Zhang, Z.; Teo, Z.L.; Liu, Y.; Ng, W.Y.; Foo, L.L.; Ting, D.S.W. Novel Technical and Privacy-Preserving Technology for Artificial Intelligence in Ophthalmology. Curr. Opin. Ophthalmol. 2022, 33, 174. [Google Scholar] [CrossRef]
- Poplin, R.; Varadarajan, A.V.; Blumer, K.; Liu, Y.; McConnell, M.V.; Corrado, G.S.; Peng, L.; Webster, D.R. Prediction of Cardiovascular Risk Factors from Retinal Fundus Photographs via Deep Learning. Nat. Biomed. Eng. 2018, 2, 158–164. [Google Scholar] [CrossRef]
- Rim, T.H.; Lee, G.; Kim, Y.; Tham, Y.-C.; Lee, C.J.; Baik, S.J.; Kim, Y.A.; Yu, M.; Deshmukh, M.; Lee, B.K.; et al. Prediction of Systemic Biomarkers from Retinal Photographs: Development and Validation of Deep-Learning Algorithms. Lancet Digit. Health 2020, 2, e526–e536. [Google Scholar] [CrossRef]

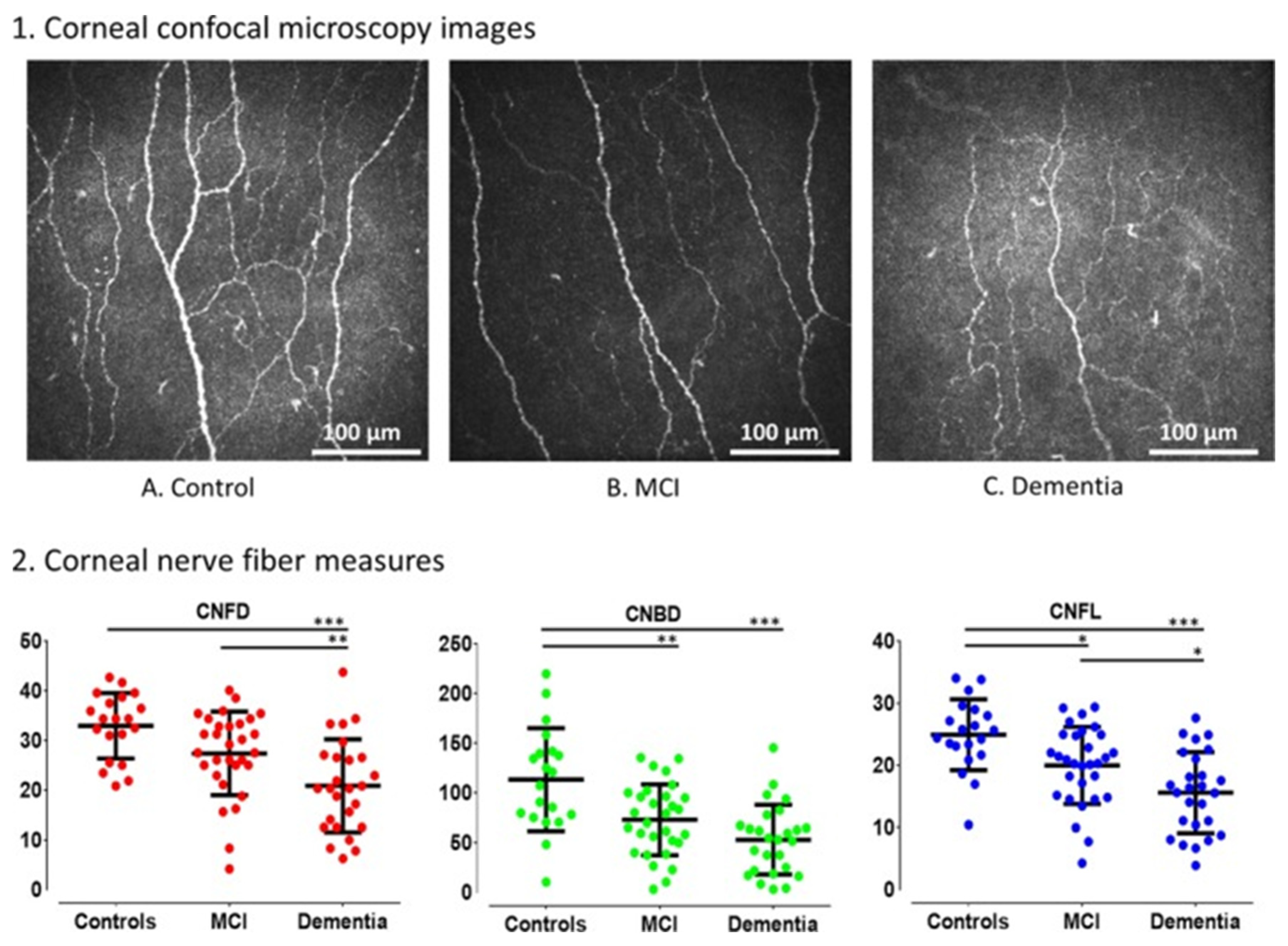
| Category | Disease | Cornea Manifestations | Imaging | Source |
|---|---|---|---|---|
| Endocrine and Metabolic Diseases | ||||
| Diabetes Mellitus | Decrease in corneal nerve fiber length Decrease in corneal nerve fiber density Increase in corneal epithelial cell density Lower sub-basal nerve density Increased corneal nerve tortuosity Increased central corneal thickness Reduced corneal optical density | IVCM Ultrasound Pachymeter | Utsunomiya et al. [72] Hashemi et al. [73] Medina et al. [74] Cuadrado et al. [75] Hossain et al. [76] Kallinikos et al. [77] Misra et al. [78] H.W. Su [79] Ramm et al. [80] Jiang et al. [81] | |
| Graves’ | Increased highest concavity Prolonged A2 time | Corvis ST | Soleymanzadeh et al. [5] | |
| Multiple Endocrine Neoplasia | Hyperreflective nerve plexus Corneal nerve thickening Disorganized nerve bundle | IVCM | Yin et al. [7] Kinoshita et al. [8] Javadi et al. [9] Petrie et al. [6] | |
| Neurofibromatosis Type 1 Syndrome | Increased corneal nerve branching Increased corneal endothelial cell density | IVCM | Moramarco [10] | |
| Hyperparathyroidism | Band keratopathy | Slit-lamp examination | Golan et al. [11] Abeysiri and Sinha [12] | |
| Polycystic Ovarian Syndrome | Increased central and peripheral corneal densitometry Increased central corneal thickness | Pentacam Non-contact specular biomicroscope Corneal pachymetry | Ozturk et al. [19] Puthiyedath et al. [17] Adiyeke et al. [18] | |
| Infectious Diseases | ||||
| SARS-CoV-2 | Reductions in corneal nerve fiber density, corneal nerve branch density, corneal nerve fiber length, and corneal nerve branch thickness | IVCM | Barros et al. [23] | |
| Neurological and Neuromuscular Disorders | ||||
| Alzheimer’s Disease | Decreased corneal sensitivity Reduction in corneal nerve fiber density Corneal nerve branch density Corneal nerve fiber length | Cochet–Bonnet esthesiometer IVCM | Ornek et al. [31] Al-Janahi et al. [33] Ponirakis et al. [32] | |
| Parkinson’s Disease | Reduced corneal nerve fiber density, corneal nerve branch density, corneal nerve fiber length, and CNBD/CNFD ratio Decreased corneal sensitivity | Cochet–Bonnet esthesiometer IVCM | Ornek et al. [31] Niu et al. [37] Lim et al. [36] Che et al. [35] | |
| Multiple Sclerosis | Decreased CNFD, CNFL, and CNBD Corneal sensitivity | Cochet–Bonnet esthesiometer IVCM | Ornek et al. [31] Mikolajczak et al. [82] Dericioglu et al. [83] | |
| Amyotrophic Lateral Sclerosis | Decreased CNFL Increased dendritic cell density Complex CBFD | IVCM | Fu et al. [41] | |
| Autoimmune and Rheumatologic Disorders | ||||
| Rheumatoid Arthritis | Increased K1, K2, and Km Decreased CCT, ACT, TCT, and CV | Pentacam HR Oculus | Ozkaya et al. [42] | |
| Sjogren’s Syndrome | Decreased CCT Higher dendritic cell density Patchy alterations and irregularities | IVCM | Villani et al. [47] Hao et al. [48] Tuominen et al. [84] | |
| Systemic Lupus Erythematosus | Lower corneal hysteresis Lower corneal resistance factor Lower CCT Higher peripheral corneal thickness | Reichert ocular response analyzer OCT Schiempflug imaging | Yazici et al. [50] Saldana-Garrido et al. [51] Eissa et al. [52] | |
| Gout | Increased total and higher order aberrations Lower corneal hysteresis | IVCM | Icoz et al. [53] | |
| Genetic Diseases | ||||
| Marfan Syndrome | Increased corneal thickness | Orbscan corneal topography system | Nehemet [55] | |
| Ehlers-Danlos | Thinner and steeper corneas Thinner stroma Lower keratocyte densities Increased endothelial hyperreflective dots | IVCM | Villani et al. [58] Gharbiya et al. [59] | |
| Wilson’s Disease | Intense hyperreflective band | AS-OCT | Sridhar [60] | |
| Fabry Disease | Increased corneal densitometry values A1 velocity, A2 velocity, deformation amplitude ratio, Corvis biomechanical index, tomographic and biomechanical index, and stiffness parameters Reduced corneal sensitivity Reduced corneal nerve fiber density Reduced nerve fiber length Increase in DC density | Pentacam HR Corvis ST IVCM Contact corneal esthesiometer | Cankurtaran et al. [62] Bitirgen et al. [24] Yang et al. [61] | |
| Down Syndrome | Increase in steepest keratometry Decrease in CCT | Corneal topography Corneal pachymetry | Alio et al. [63] | |
| Polycystic Kidney Disease | Increased corneal hysteresis | ORA | Serefoglu Cabuk et al. [64] | |
| Hematological | ||||
| Thalassemia | Decreased tear break-up time Corneal epithelial thickness Decreased branch density Corneal topographic parameters (K2, CV) Endothelial cell density | Corneal confocal microscopy Pentacam OCT Specular microscopy | Ebeid [69] Khan [70] Hanna [71] | |
| Leukemias (ALL, AML, and NHL, etc.) | Superficial punctate Corneal ulcers Conjunctival hemorrhage | Slit-lamp biomicroscope | Bouazza et al. [65] Hoehn et al. [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, R.; Kumar, R.; Weaver, A.; Kim, J.H.; Raza, A.; Ong, J.; Waisberg, E.; Pandit, R. Cornea Oculomics: A Clinical Blueprint for Extending Corneal Diagnostics and Artificial Intelligence in Systemic Health Insights. Diagnostics 2025, 15, 643. https://doi.org/10.3390/diagnostics15050643
Lee R, Kumar R, Weaver A, Kim JH, Raza A, Ong J, Waisberg E, Pandit R. Cornea Oculomics: A Clinical Blueprint for Extending Corneal Diagnostics and Artificial Intelligence in Systemic Health Insights. Diagnostics. 2025; 15(5):643. https://doi.org/10.3390/diagnostics15050643
Chicago/Turabian StyleLee, Ryung, Rahul Kumar, Alex Weaver, Ji Hyun Kim, Arriyan Raza, Joshua Ong, Ethan Waisberg, and Rahul Pandit. 2025. "Cornea Oculomics: A Clinical Blueprint for Extending Corneal Diagnostics and Artificial Intelligence in Systemic Health Insights" Diagnostics 15, no. 5: 643. https://doi.org/10.3390/diagnostics15050643
APA StyleLee, R., Kumar, R., Weaver, A., Kim, J. H., Raza, A., Ong, J., Waisberg, E., & Pandit, R. (2025). Cornea Oculomics: A Clinical Blueprint for Extending Corneal Diagnostics and Artificial Intelligence in Systemic Health Insights. Diagnostics, 15(5), 643. https://doi.org/10.3390/diagnostics15050643








