A Cross-Sectional Comparative Study: Could Asprosin and Peptide Tyrosine-Tyrosine Be Used in Schizophrenia to Define the Disease and Determine Its Phases?
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedures
2.2. Ethics and Consent
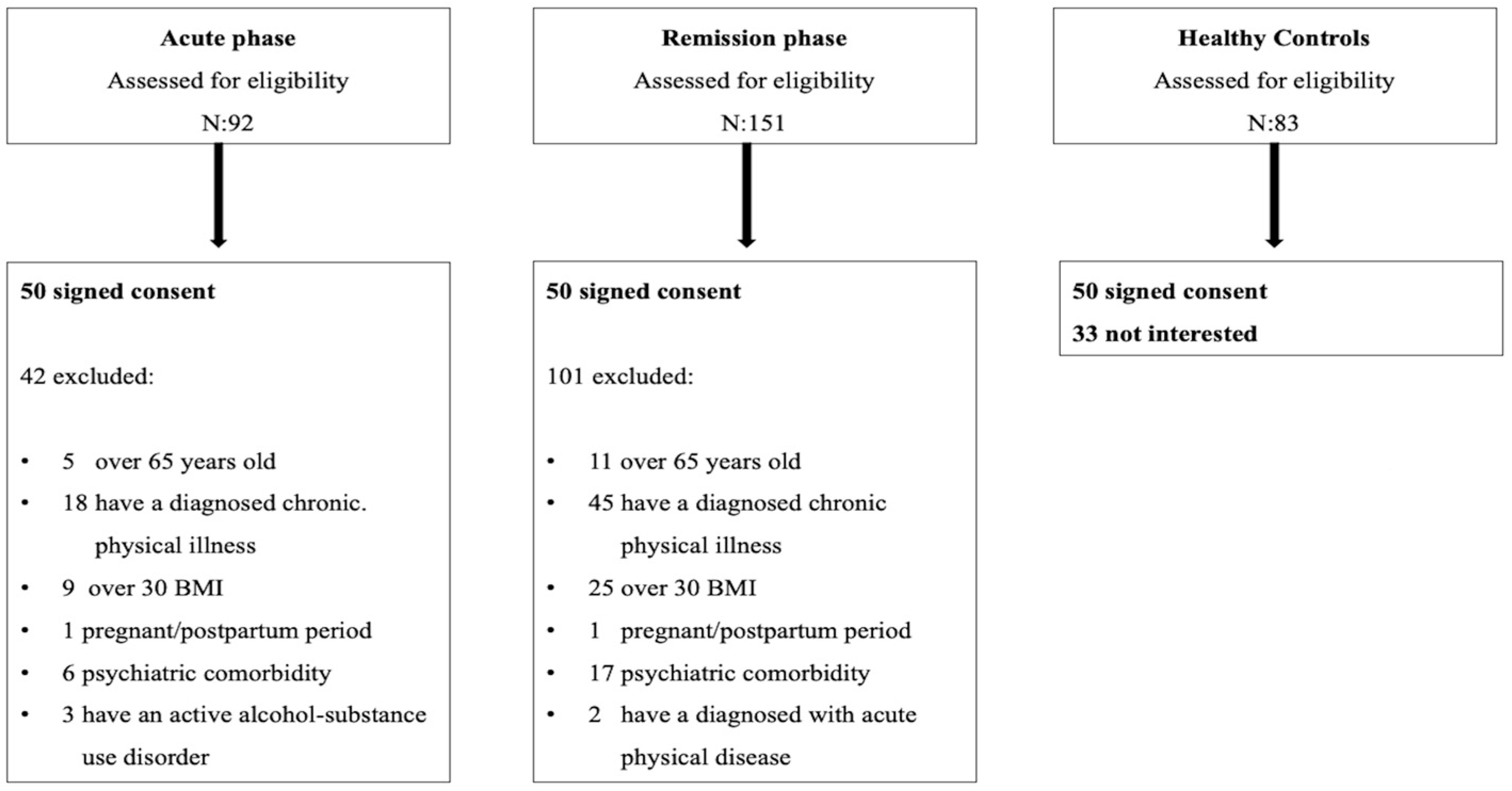
2.3. Research Sample
2.4. Data Collection Tools
2.4.1. Sociodemographic and Clinical Data Form
2.4.2. SCID-5-CV
2.4.3. Positive and Negative Syndrome Scale (PANSS)
2.5. Biochemical Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PYY | Peptide Tyrosine–Tyrosine |
| PANSS | Positive and Negative Syndrome Scale |
| ROC | Receiver operating characteristic |
| BMI | Body mass index |
| SCID-5/CV | Statistical Manual of Mental Disorders 5 clinician version |
| FBG | Fasting blood glucose |
| HbA1c | Glycated hemoglobin |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| TG | Triglyceride |
| LAI | Long-acting injectable |
References
- Pieczenik, S.R.; Neustadt, J. Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol. 2007, 83, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shachar, D. Mitochondrial dysfunction in schizophrenia: A possible linkage to dopamine. J. Neurochem. 2002, 83, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shachar, D.; Bonne, O.; Chisin, R.; Klein, E.; Lester, H.; Aharon-Peretz, J.; Yona, I.; Freedman, N. Cerebral glucose utilization and platelet mitochondrial complex I activity in schizophrenia: A FDG-PET study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shachar, D.; Zuk, R.; Gazawi, H.; Ljubuncic, P. Dopamine toxicity involves mitochondrial complex I inhibition: Implications to dopamine-related neuropsychiatric disorders. Biochem. Pharmacol. 2004, 67, 1965–1974. [Google Scholar] [CrossRef]
- Dror, N.; Klein, E.; Karry, R.; Sheinkman, A.; Kirsh, Z.; Mazor, M.; Tzukerman, M.; Ben-Shachar, D. State-dependent alterations in mitochondrial complex I activity in platelets: A potential peripheral marker for schizophrenia. Mol. Psychiatry 2002, 7, 995–1001. [Google Scholar] [CrossRef]
- Romaní-Pérez, M.; Bullich-Vilarrubias, C.; López-Almela, I.; Liébana-García, R.; Olivares, M.; Sanz, Y. The microbiota and the gut–brain axis in controlling food intake and energy homeostasis. Int. J. Mol. Sci. 2021, 22, 5830. [Google Scholar] [CrossRef]
- Montégut, L.; Lopez-Otin, C.; Magnan, C.; Kroemer, G. Old paradoxes and new opportunities for appetite control in obesity. Trends Endocrinol. Metab. 2021, 32, 264–294. [Google Scholar] [CrossRef]
- Yuan, M.; Li, W.; Zhu, Y.; Yu, B.; Wu, J. Asprosin: A Novel Player in Metabolic Diseases. Front. Endocrinol. 2020, 11, 64. [Google Scholar] [CrossRef]
- Hu, Y.; Kang, Q.; Chen, C.; Guo, L.; Chen, J. Increased plasma asprosin levels are associated with overeating and loss of control in drug-free bulimia nervosa. Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 2022, 27, 2783–2789. [Google Scholar] [CrossRef]
- Gürbüzer, N.; Zengil, S.; Laloğlu, E.; Kaşali, K. The Potential Impact of Agouti Related Peptide and Asprosin on Metabolic Parameters and Eating Behavior in Attention Deficit Hyperactivity Disorder. Arch. Neuropsychiatry 2024, 61, 30. [Google Scholar] [CrossRef]
- Birsöz, S. Şizofreni ve iki uçlu spektrum bozuklukları benzerlikler ve farklılıklar. J. Mood Disord. 2013, 3, S54–S55. [Google Scholar]
- Parlak, N.; Görgülü, Y.; Çinar, R.K.; Sönmez, M.B.; Parlak, E. Serum agouti-related protein (AgRP) levels in bipolar disorder: Could AgRP be a state marker for mania? Psychiatry Res. 2018, 260, 36–40. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Hatlebakk, J.G.; Hausken, T. Possible role of peptide YY (PYY) in the pathophysiology of irritable bowel syndrome (IBS). Neuropeptides 2020, 79, 101973. [Google Scholar] [CrossRef] [PubMed]
- Widerlöv, E.; Lindström, L.; Wahlestedt, C.; Ekman, R. Neuropeptide Y and peptide YY as possible cerebrospinal fluid markers for major depression and schizophrenia, respectively. J. Psychiatr. Res. 1988, 22, 69–79. [Google Scholar] [CrossRef]
- Wysokiński, A.; Kowalski, M.; Kłoszewska, I. Serum levels of PYY (1-36) peptide in patients with schizophrenia on clozapine monotherapy. Pharmacopsychiatry 2014, 47, 169–173. [Google Scholar] [CrossRef]
- van Os, J.; Burns, T.; Cavallaro, R.; Leucht, S.; Peuskens, J.; Helldin, L.; Bernardo, M.; Arango, C.; Fleischhacker, W.; Lachaux, B. Standardized remission criteria in schizophrenia. Acta Psychiatr. Scand. 2006, 113, 91–95. [Google Scholar] [CrossRef]
- Elbir, M.; Alp Topbaş, Ö.; Bayad, S.; Kocabaş, T.; Topak, O.Z.; Çetin, Ş.; Özdel, O.; Ateşçi, F.; Aydemir, Ö. DSM-5 bozuklukları için yapılandırılmış klinik görüşmenin klinisyen versiyonunun türkçeye uyarlanması ve güvenilirlik çalışması. Turk Psikiyatr. Derg. 2019, 30, 51–56. [Google Scholar] [CrossRef]
- Kostakoğlu, A.; Batur, S.; Tiryaki, A.; Göğüş, A. Reliability and Validity of the Turkish version of the Positive and Negative Syndrome Scale. Türk Psikol. Derg. 1999, 14, 23–34. [Google Scholar]
- George, D. SPSS for Windows Step by Step: A Simple Study Guide and Reference; 17.0 update, 10/e; Pearson Education: Noida, India, 2011. [Google Scholar]
- Gottschalk, M.G.; Wesseling, H.; Guest, P.C.; Bahn, S. Proteomic enrichment analysis of psychotic and affective disorders reveals common signatures in presynaptic glutamatergic signaling and energy metabolism. Int. J. Neuropsychopharmacol. 2015, 18, pyu019. [Google Scholar] [CrossRef]
- Dai, W.; Qiu, Y.; Teng, Z.; Li, S.; Huang, J.; Tang, H.; Wang, B.; Chen, J.; Wu, H. Gut microbial dysbiosis and cognitive impairment in bipolar disorder: Current evidence. Front. Pharmacol. 2022, 13, 893567. [Google Scholar] [CrossRef]
- Weise, C.M.; Thiyyagura, P.; Reiman, E.M.; Chen, K.; Krakoff, J. Postprandial plasma PYY concentrations are associated with increased regional gray matter volume and rCBF declines in caudate nuclei—A combined MRI and H215O PET study. Neuroimage 2012, 60, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Tamminga, C.A.; Thaker, G.K.; Buchanan, R.; Kirkpatrick, B.; Alphs, L.D.; Chase, T.N.; Carpenter, W.T. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch. Gen. Psychiatry 1992, 49, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Martins-de-Souza, D.; Harris, L.W.; Guest, P.C.; Bahn, S. The role of energy metabolism dysfunction and oxidative stress in schizophrenia revealed by proteomics. Antioxid. Redox Signal. 2011, 15, 2067–2079. [Google Scholar] [CrossRef]
- Kamiike, W.; Fujikawa, M.; Koseki, M.; Sumimura, J.; Miyata, M.; Kawashima, Y.; Wada, H.; Tagawa, K. Different patterns of leakage of cytosolic and mitochondrial enzymes. Clin. Chim. Acta 1989, 185, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Peet, M. Diet, diabetes and schizophrenia: Review and hypothesis. Br. J. Psychiatry 2004, 184, s102–s105. [Google Scholar] [CrossRef]
- Li, W.; Yue, L.; Sun, L.; Xiao, S. An increased aspartate to alanine aminotransferase ratio is associated with a higher risk of cognitive impairment. Front. Med. 2022, 9, 780174. [Google Scholar] [CrossRef]
- Limosin, F.; Gasquet, I.; Leguay, D.; Azorin, J.M.; Rouillon, F. Body mass index and prevalence of obesity in a French cohort of patients with schizophrenia. Acta Psychiatr. Scand. 2008, 118, 19–25. [Google Scholar] [CrossRef]
- Paton, C.; Esop, R.; Young, C.; Taylor, D. Obesity, dyslipidaemias and smoking in an inpatient population treated with antipsychotic drugs. Acta Psychiatr. Scand. 2004, 110, 299–305. [Google Scholar] [CrossRef]
- Saari, K.; Jokelainen, J.; Veijola, J.; Koponen, H.; Jones, P.; Savolainen, M.; Järvelin, M.R.; Lauren, L.; Isohanni, M.; Lindeman, S. Serum lipids in schizophrenia and other functional psychoses: A general population northern Finland 1966 birth cohort survey. Acta Psychiatr. Scand. 2004, 110, 279–285. [Google Scholar] [CrossRef]
- Solberg, D.K.; Bentsen, H.; Refsum, H.; Andreassen, O.A. Lipid profiles in schizophrenia associated with clinical traits: A five year follow-up study. BMC Psychiatry 2016, 16, 299. [Google Scholar] [CrossRef]
- Lakka, T.A.; Laaksonen, D.E. Physical activity in prevention and treatment of the metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 76–88. [Google Scholar] [CrossRef]

| Control N: 50 | Remission Phase N: 50 | Acute Phase N: 50 | p Value | ||
|---|---|---|---|---|---|
| Sex | women | 28 | 19 | 23 | 0.195 |
| men | 22 | 31 | 27 | ||
| Age(Year) | 39.08 ± 11.63 | 43.30 ± 11.58 | 39.76 ± 10.35 | 0.133 | |
| Marital Status | single | 12 | 21 | 28 | p < 0.001 |
| married | 37 | 15 | 19 | ||
| divorced, widowed | 1 | 14 | 3 | ||
| Education Time (Year) | 11.12 ± 2.46 | 7.44 ± 3.68 | 7.08 ± 2.60 | p < 0.001 | |
| Smoking | yes | 29 | 39 | 36 | 0.084 |
| no | 21 | 11 | 14 | ||
| Alcohol Use | yes | 7 | 1 | 0 | 0.003 |
| no | 43 | 49 | 50 | ||
| Substance Use History | yes | 0 | 2 | 10 | p < 0.001 |
| no | 50 | 48 | 40 | ||
| BMI (kg/m2) | 25.35 ± 3.36 | 27.13 ± 3.11 | 24.86 ± 3.00 | p < 0.001 | |
| Asprosin (ng/mL) | 28.26 ± 17.67 | 13.78 ± 12.34 | 7.37 ± 2.58 | p < 0.001 | |
| PYY (pg/mL) | 271.18 ± 104.02 | 116.70 ± 72.63 | 88.72 ± 25.68 | p < 0.001 | |
| ALT (U/L) | 25.68 ± 14.66 | 21.26 ± 10.47 | 18.88 ± 10.20 | 0.017 | |
| AST (U/L) | 14.26 ± 4.25 | 16.70 ± 8.28 | 23.44 ± 8.73 | p < 0.001 | |
| AST/ALT Ratio | 0.64 ± 0.23 | 0.83 ± 0.40 | 1.42 ± 0.53 | p < 0.001 | |
| TG (mg/dL) | 154.64 ± 126.88 | 155.70 ± 108.96 | 137.28 ± 67.59 | 0.612 | |
| HDL (mg/dL) | 41.68 ± 10.59 | 39.88 ± 10.48 | 42.06 ± 9.17 | 0.515 | |
| LDL (mg/dL) | 126.44 ± 34.71 | 121.56 ± 38.84 | 121.82 ± 34.92 | 0.751 | |
| Cholesterol (mg/dL) | 178.48 ± 34.73 | 164.02 ± 42.45 | 167.68 ± 33.98 | 0.134 | |
| FBG (mg/dL) | 82.38 ± 11.69 | 95.96 ± 18.43 | 112.26 ± 16.82 | p < 0.001 | |
| HbA1c (%) | 5.14 ± 0.64 | 5.04 ± 0.64 | 5.41 ± 0.49 | 0.006 | |
| Duration of illness (Years) | 16.44 ± 9.07 | 14.44 ± 8.53 | 0.259 | ||
| Number of exacerbations | 7.92 ± 5.75 | 9.42 ± 5.99 | 0.205 | ||
| PANSS positive | 11.78 ± 3.50 | 37.40 ± 5.75 | p < 0.001 | ||
| PANSS negative | 14.02 ± 4.67 | 35.34 ± 8.98 | p < 0.001 | ||
| PANSS general psychopathology | 31.66 ± 10.47 | 102 ± 5.14 | p < 0.001 | ||
| Drug use | |||||
| Paliperidone palmitate once monthly, LAI formulation | 19 | - | |||
| Paliperidone palmitate 3-monthly, LAI formulation | 7 | - | |||
| Aripiprazole once monthly, LAI formulation | 13 | - | |||
| Olanzapine | 8 | - | |||
| Risperidone | 5 | - | |||
| Quetiapin | 23 | - | |||
| Clozapine | 9 | - | |||
| Amisulpride | 5 | - | |||
| p Value | |||
|---|---|---|---|
| Control–Remission Phase | Control–Acute Phase | Acute Phase–Remission Phase | |
| Asprosin (ng/mL) | p < 0.001 | p < 0.001 | 0.002 |
| PYY (pg/mL) | p < 0.001 | p < 0.001 | 0.038 |
| ALT (U/L) | 0.237 | 0.025 | 0.582 |
| AST (U/L) | 0.19 | p < 0.001 | p < 0.001 |
| AST/ALT Ratio | 0.016 | p < 0.001 | p < 0.001 |
| FBG (mg/dL) | p < 0.001 | p < 0.001 | p < 0.001 |
| HbA1c (%) | 1 | 0.069 | 0.006 |
| BMI (kg/m2) | 0.017 | 1 | 0.001 |
| Remission Phase | Acute Phase | ||||
|---|---|---|---|---|---|
| Asprosin | PYY | Asprosin | PYY | ||
| Age (Year) | r | −0.164 | −0.214 | −0.119 | 0.290 * |
| p | 0.255 | 0.136 | 0.409 | 0.041 | |
| Duration of illness (years) | r | −0.369 ** | −0.367 ** | −0.448 ** | −0.207 |
| p | 0.008 | 0.009 | 0.001 | 0.149 | |
| Number of exacerbations | r | −0.254 | −0.266 | −0.476 ** | −0.450 ** |
| p | 0.075 | 0.062 | 0.000 | 0.001 | |
| PANSS positive | r | −0.318 * | −0.230 | −0.663 ** | −0.288 * |
| p | 0.025 | 0.107 | 0.000 | 0.042 | |
| PANSS negative | r | −0.670 ** | −0.658 ** | −0.857 ** | −0.315 * |
| p | 0.000 | 0.000 | 0.000 | 0.026 | |
| PANSS general psychopathology | r | −0.700 ** | −0.637 ** | −0.890 ** | −0.318 * |
| p | 0.000 | 0.000 | 0.000 | 0.025 | |
| ALT (U/L) | r | 0.341 * | 0.403 ** | −0.060 | 0.314 * |
| p | 0.015 | 0.004 | 0.681 | 0.026 | |
| AST (U/L) | r | 0.209 | 0.218 | −0.212 | −0.033 |
| p | 0.146 | 0.127 | 0.139 | 0.818 | |
| TG (mg/dL) | r | 0.093 | 0.118 | −0.289 * | −0.023 |
| p | 0.521 | 0.416 | 0.042 | 0.873 | |
| HDL (mg/dL) | r | −0.098 | −0.069 | 0.299 * | 0.215 |
| p | 0.497 | 0.636 | 0.035 | 0.134 | |
| LDL (mg/dL) | r | 0.228 | 0.148 | 0.155 | 0.455 ** |
| p | 0.111 | 0.304 | 0.283 | 0.001 | |
| Cholesterol (mg/dL) | r | 0.165 | 0.160 | 0.110 | 0.289 * |
| p | 0.252 | 0.268 | 0.445 | 0.042 | |
| FBG (mg/dL) | r | 0.078 | 0.026 | 0.124 | 0.119 |
| p | 0.590 | 0.859 | 0.390 | 0.412 | |
| HbA1c (%) | r | 0.133 | 0.188 | 0.187 | 0.216 |
| p | 0.357 | 0.191 | 0.194 | 0.132 | |
| 95% Confidence Interval | Model Summary | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dependent Variables | Group | Variables | B | SE | BETA | t | p | Lower Bound | Upper Bound | R | Durbin-Watson |
| PANSS total score | Remission Phase | (Constant) | 69.875 | 3.123 | 22.373 | 0.000 | 63.592 | 76.158 | 0.753 | 1.400 | |
| Asprosin | −1.005 | 0.311 | −0.806 | −3.228 | 0.002 | −1.632 | −0.379 | ||||
| PYY | 0.012 | 0.053 | 0.058 | 0.233 | 0.817 | −0.094 | 0.119 | ||||
| Acute Phase | (Constant) | 215.714 | 5.069 | 42.553 | 0.000 | 205.516 | 225.912 | 0.873 | 2.012 | ||
| Asprosin | −6.691 | 0.589 | −0.930 | −11.354 | 0.000 | −7.877 | −5.506 | ||||
| PYY | 0.094 | 0.059 | 0.130 | 1.593 | 0.118 | −0.025 | 0.214 | ||||
| PANSS positive | Remission Phase | (Constant) | 12.163 | 1.009 | 12.057 | 0.000 | 10.134 | 14.193 | 0.357 | 2.095 | |
| Asprosin | −0.202 | 0.101 | −0.711 | −2.004 | 0.051 | −0.404 | 0.001 | ||||
| PYY | 0.021 | 0.017 | 0.426 | 1.201 | 0.236 | −0.014 | 0.055 | ||||
| Acute Phase | (Constant) | 47.679 | 2.399 | 19.871 | 0.000 | 42.852 | 52.506 | 0.665 | 1.898 | ||
| Asprosin | −1.535 | 0.279 | −0.689 | −5.502 | 0.000 | −2.096 | −0.973 | ||||
| PYY | 0.012 | 0.028 | 0.052 | 0.418 | 0.678 | −0.045 | 0.068 | ||||
| PANSS negative | Remission Phase | (Constant) | 18.235 | 1.061 | 17.179 | 0.000 | 16.100 | 20.370 | 0.678 | 1.362 | |
| Asprosin | −0.161 | 0.106 | −0.426 | −1.525 | 0.134 | −0.0374 | 0.051 | ||||
| PYY | −0.017 | 0.018 | −0.265 | −0.948 | 0.348 | −0.053 | 0.019 | ||||
| Acute Phase | (Constant) | 54.669 | 2.505 | 21.820 | 0.000 | 49.629 | 59.709 | 0.866 | 2.002 | ||
| Asprosin | −3.227 | 0.291 | −0.928 | −11.078 | 0.000 | −3.813 | −2.641 | ||||
| PYY | 0.050 | 0.029 | 0.144 | 1.720 | 0.092 | −0.009 | 0.109 | ||||
| PANSS general psychopathology | Remission Phase | (Constant) | 39.477 | 2.306 | 17.118 | 0.000 | 34.837 | 44.116 | 0.700 | 1.663 | |
| Asprosin | −0.642 | 0.230 | −0.757 | −2.793 | 0.008 | −1.105 | −0.180 | ||||
| PYY | 0.009 | 0.039 | 0.061 | 0.227 | 0.822 | −0.070 | 0.087 | ||||
| Acute Phase | (Constant) | 113.365 | 1.246 | 91.002 | 0.000 | 110.859 | 115.872 | 0.901 | 2.213 | ||
| Asprosin | −1.930 | 0.145 | −0.970 | −13.326 | 0.000 | −2.221 | −1.639 | ||||
| PYY | 0.032 | 0.015 | 0.162 | 2.220 | 0.031 | 0.003 | 0.062 | ||||
| Group 1 (N: 43) | Group 2 (N: 57) | p Value | |
|---|---|---|---|
| Mean ± SD | |||
| PANSS positive | 32.42 ± 13.20 | 18.68 ± 10.93 | p < 0.001 |
| PANSS negative | 35.65 ± 10.78 | 16.40 ± 6.59 | p < 0.001 |
| PANSS general psychopathology | 88.49 ± 27.52 | 50.49 ± 33.59 | p < 0.001 |
| BMI (kg/m2) | 25.96 ± 3.55 | 26.02 ± 3.04 | 0.92 |
| ALT (U/L) | 19.91 ± 10.06 | 20.19 ± 10.66 | 0.892 |
| AST (U/L) | 23.30 ± 10.42 | 17.63 ± 7.17 | 0.002 |
| AST/ALT ratio | 1.26 | 1.03 | 0.035 |
| FBG (mg/dL) | 107.60 ± 19.58 | 101.47 ± 18.96 | 0.118 |
| HbA1c (%) | 5.30 ± 0.59 | 5.17 ± 0.60 | 0.295 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tozoğlu, E.Ö.; Gürbüzer, N.; Özkaya, A.L.; Akyıldırım, S. A Cross-Sectional Comparative Study: Could Asprosin and Peptide Tyrosine-Tyrosine Be Used in Schizophrenia to Define the Disease and Determine Its Phases? Diagnostics 2025, 15, 632. https://doi.org/10.3390/diagnostics15050632
Tozoğlu EÖ, Gürbüzer N, Özkaya AL, Akyıldırım S. A Cross-Sectional Comparative Study: Could Asprosin and Peptide Tyrosine-Tyrosine Be Used in Schizophrenia to Define the Disease and Determine Its Phases? Diagnostics. 2025; 15(5):632. https://doi.org/10.3390/diagnostics15050632
Chicago/Turabian StyleTozoğlu, Elif Özcan, Nilifer Gürbüzer, Alev Lazoğlu Özkaya, and Sümeyya Akyıldırım. 2025. "A Cross-Sectional Comparative Study: Could Asprosin and Peptide Tyrosine-Tyrosine Be Used in Schizophrenia to Define the Disease and Determine Its Phases?" Diagnostics 15, no. 5: 632. https://doi.org/10.3390/diagnostics15050632
APA StyleTozoğlu, E. Ö., Gürbüzer, N., Özkaya, A. L., & Akyıldırım, S. (2025). A Cross-Sectional Comparative Study: Could Asprosin and Peptide Tyrosine-Tyrosine Be Used in Schizophrenia to Define the Disease and Determine Its Phases? Diagnostics, 15(5), 632. https://doi.org/10.3390/diagnostics15050632






