Measurement of Fusion Control with Eye Tracking Device in Intermittent Exotropia
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Physical Examination
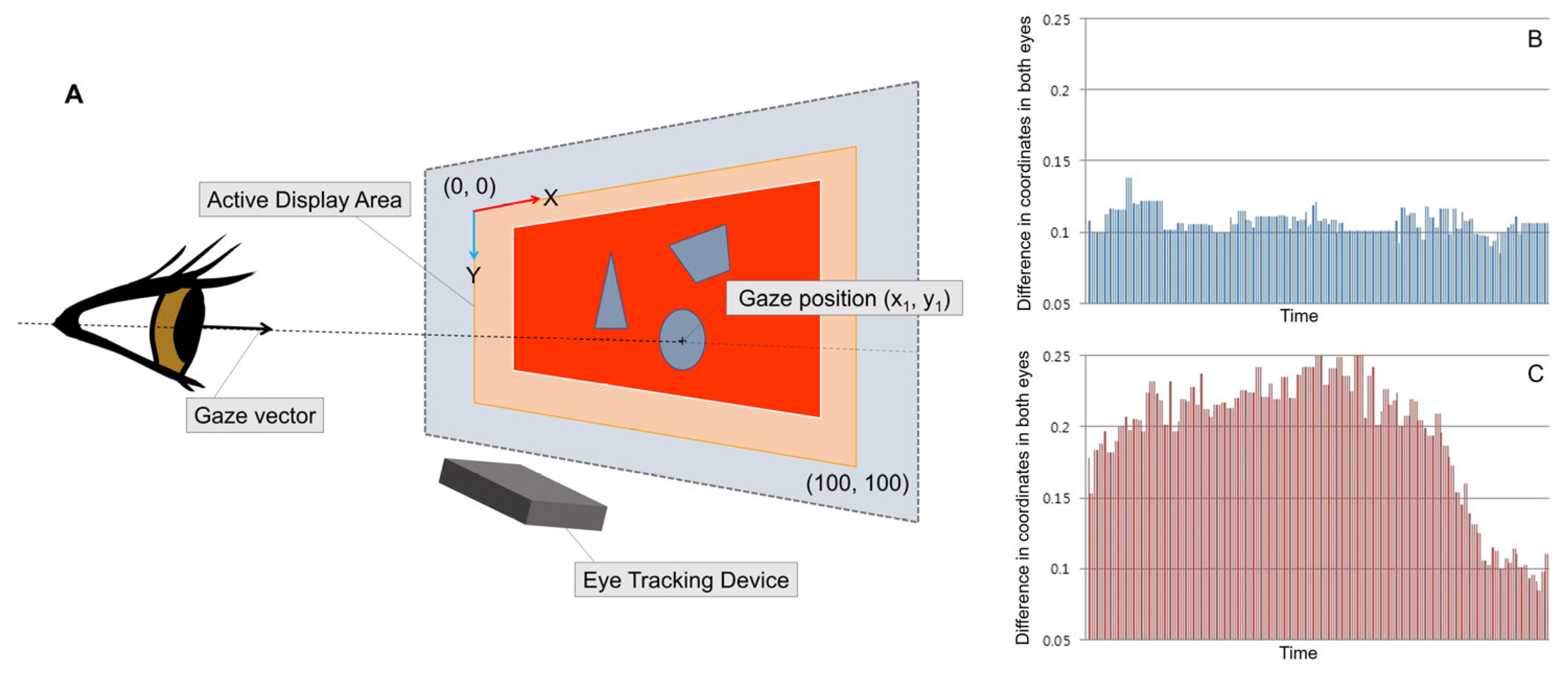
2.3. The Eye Tracking Procedure
2.4. Automated Fusion Control Assessment
2.5. The Conventional Fusion Control Assessment
2.6. Validity and Reliability
3. Results
3.1. Participant Characteristics
3.2. Assessment of Automated and Conventional Fusion Control
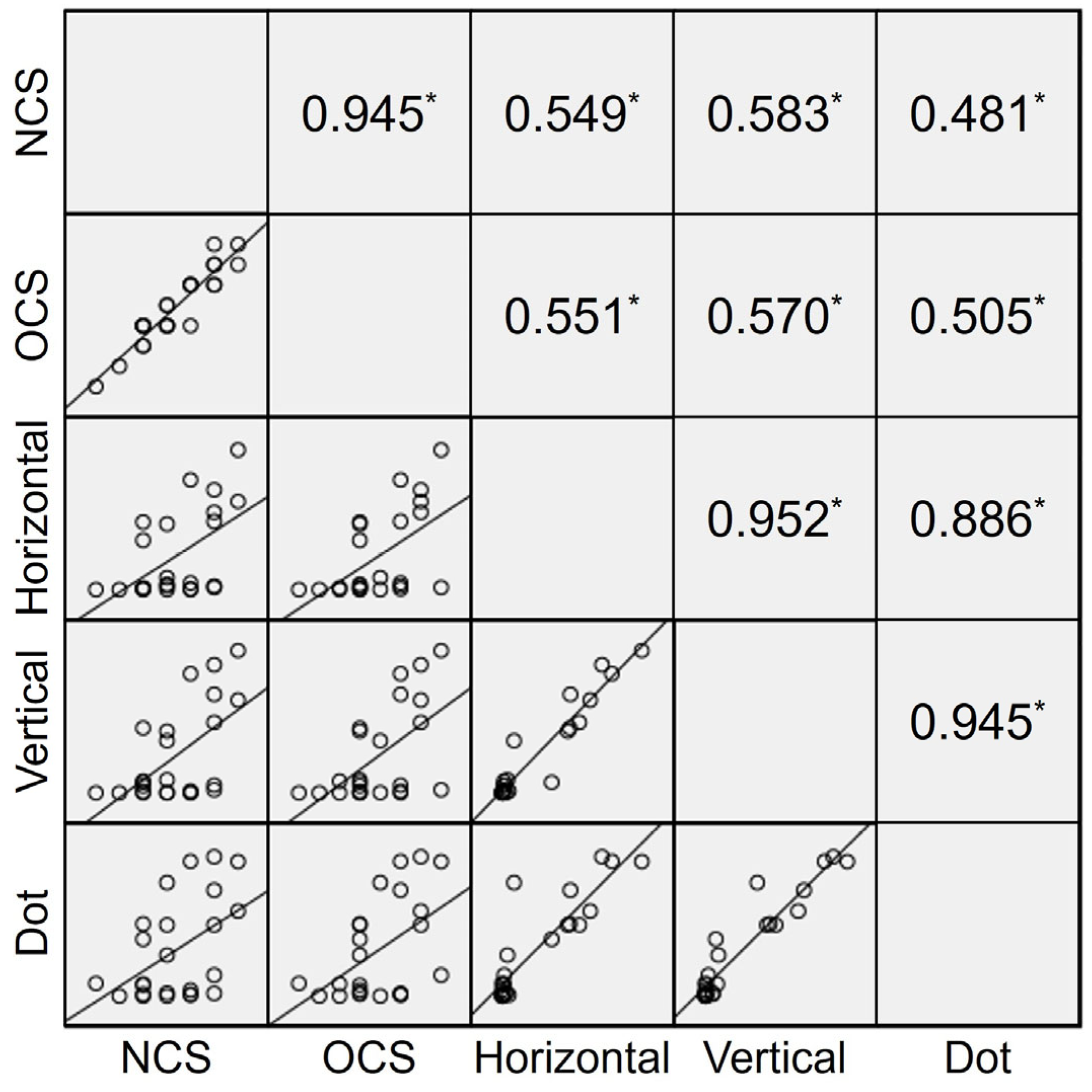
3.3. Validity of the Automated Fusion Control Assessment
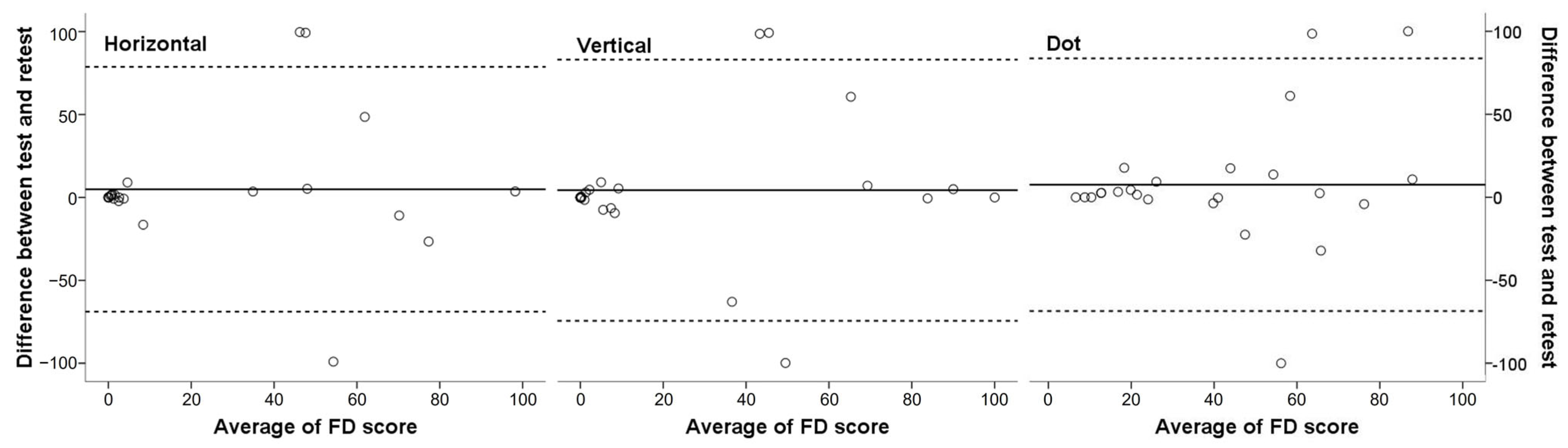
3.4. Reliability of the Automated Fusion Control Assessment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lambert, S.R.; Lyons, C.J. Taylor and Hoyt’s Pediatric Ophthalmology and Strabismus, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 892–902. [Google Scholar]
- Rutstein, R.P.; Corliss, D.A. The clinical course of intermittent exotropia. Optom. Vis. Sci. 2003, 80, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Nusz, K.J.; Mohney, B.G.; Diehl, N.N. The course of intermittent exotropia in a population-based cohort. Ophthalmology 2006, 113, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Pediatric Eye Disease Investigator Group; Writing Committee; Mohney, B.G.; Cotter, S.A.; Chandler, D.L.; Holmes, J.M.; Wallace, D.K.; Yamada, T.; Petersen, D.B.; Kraker, R.T.; et al. Three-year observation of children 3 to 10 years of age with untreated intermittent exotropia. Ophthalmology 2019, 126, 1249–1260. [Google Scholar] [CrossRef]
- Govindan, M.; Mohney, B.G.; Diehl, N.N.; Burke, J.P. Incidence and types of childhood exotropia: A population-based study. Ophthalmology 2005, 112, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Petrunak, J.L.; Rao, R. The evaluation of office control in intermittent exotropia: A systematic approach. Am. Orthopt. J. 2003, 53, 98–104. [Google Scholar] [CrossRef]
- Holmes, J.M.; Birch, E.E.; Leske, D.A.; Fu, V.L.; Mohney, B.G. New tests of distance stereoacuity and their role in evaluating intermittent exotropia. Ophthalmology 2007, 114, 1215–1220. [Google Scholar] [CrossRef]
- Pineles, S.L.; Ela-Dalman, N.; Zvansky, A.G.; Yu, F.; Rosenbaum, A.L. Long-term results of the surgical management of intermittent exotropia. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2010, 14, 298–304. [Google Scholar] [CrossRef]
- Kim, U.S.; Park, S.; Yoo, H.J.; Hwang, J.M. Psychosocial distress of part-time occlusion in children with intermittent exotropia. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 315–319. [Google Scholar] [CrossRef]
- Hatt, S.R.; Leske, D.A.; Mohney, B.G.; Brodsky, M.C.; Holmes, J.M. Fusional convergence in childhood intermittent exotropia. Am. J. Ophthalmol. 2011, 152, 314–319. [Google Scholar] [CrossRef]
- Haggerty, H.; Richardson, S.; Hrisos, S.; Strong, N.P.; Clarke, M.P. The Newcastle Control Score: A new method of grading the severity of intermittent distance exotropia. Br. J. Ophthalmol. 2004, 88, 233–235. [Google Scholar] [CrossRef]
- Buck, D.; Clarke, M.P.; Haggerty, H.; Hrisos, S.; Powell, C.; Sloper, J.; Strong, N.P. Grading the severity of intermittent distance exotropia: The revised Newcastle Control Score. Br. J. Ophthalmol. 2008, 92, 577. [Google Scholar] [CrossRef]
- Mohney, B.G.; Holmes, J.M. An office-based scale for assessing control in intermittent exotropia. Strabismus 2006, 14, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Hatt, S.R.; Liebermann, L.; Leske, D.A.; Mohney, B.G.; Holmes, J.M. Improved assessment of control in intermittent exotropia using multiple measures. Am. J. Ophthalmol. 2011, 152, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Hatt, S.R.; Leske, D.A.; Liebermann, L.; Holmes, J.M. Quantifying variability in the measurement of control in intermittent exotropia. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2015, 19, 33–37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, H.; Kim, D.H.; Ahn, H.; Lim, H.T. Proposing a new scoring system in intermittent exotropia: Towards a better assessment of control. Can. J. Ophthalmol. 2017, 52, 235–239. [Google Scholar] [CrossRef]
- Moon, Y.; Kim, H.; Kim, D.H.; Lim, H.T. LACTOSE control scoring helps predict surgical outcomes for childhood intermittent exotropia. Can. J. Ophthalmol. 2019, 54, 659–663. [Google Scholar] [CrossRef]
- Hashemi, H.; Pakbin, M.; Ali, B.; Yekta, A.; Ostadimoghaddam, H.; Asharlous, A.; Aghamirsalim, M.; Khabazkhoob, M. Near points of convergence and accommodation in a population of university students in Iran. J. Ophthalmic Vis. Res. 2019, 14, 306–314. [Google Scholar] [CrossRef]
- Gibaldi, A.; Vanegas, M.; Bex, P.J.; Maiello, G. Evaluation of the Tobii EyeX Eye tracking controller and Matlab toolkit for research. Behav. Res. Methods 2017, 49, 923–946. [Google Scholar] [CrossRef]
- Burian, H.M. Exodeviations: Their classification, diagnosis and treatment. Am. J. Ophthalmol. 1966, 62, 1161–1166. [Google Scholar] [CrossRef]
- Economides, J.R.; Dilbeck, M.D.; Gentry, T.N.; Horton, J.C. Ambulatory monitoring with eye tracking glasses to assess the severity of intermittent exotropia. Am. J. Ophthalmol. 2023, 250, 120–129. [Google Scholar] [CrossRef]
- Cicchetti, D. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instrument in psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Hatt, S.R.; Gnanaraj, L. Interventions for intermittent exotropia. Cochrane Database Syst. Rev. 2013, 5, CD003737. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, A.L.; Stathacopoulos, R.A. Subjective and objective criteria for recommending surgery in intermittent exotropia. Am. Orthopt. J. 1992, 42, 46–51. [Google Scholar] [CrossRef]
- Chia, A.; Seenyen, L.; Long, Q.B. A retrospective review of 287 consecutive children in Singapore presenting with intermittent exotropia. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2005, 9, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Buck, D.; Hatt, S.R.; Haggerty, H.; Hrisos, S.; Strong, N.P.; Steen, N.I.; Clarke, M.P. The use of the Newcastle Control Score in the management of intermittent exotropia. Br. J. Ophthalmol. 2007, 91, 215–218. [Google Scholar] [CrossRef]
- Lee, H.S.; Lew, H.; Choi, H.J. Clinical significance of refixation time in intermittent exotropia. J. Korean Ophthalmol. Soc. 2003, 44, 1567–1571. [Google Scholar]
- Kang, K.T.; Lee, S.Y. Relationship between control grade, stereoacuity and surgical success in basic intermittent exotropia. Korean J. Ophthalmol. 2015, 29, 173–177. [Google Scholar] [CrossRef][Green Version]
- Hatt, S.R.; Mohney, B.G.; Leske, D.A.; Holmes, J.M. Variability of control in intermittent exotropia. Ophthalmology 2008, 115, 371–376.e2. [Google Scholar] [CrossRef]
- Anderson, H.A.; Manny, R.E.; Cotter, S.A.; Mitchell, G.L.; Irani, J.A. Effect of examiner experience and technique on the alternate cover test. Optom. Vis. Sci. 2010, 87, 168–175. [Google Scholar] [CrossRef]
| Characteristics | IXT Group (n = 25) | Control Group (n = 25) | p Value |
|---|---|---|---|
| Gender (M:F) | 13:12 | 10:15 | 0.571 * |
| Age at examination (y) | 24.3 ± 6.6 (15.5~40.7) | 26.8 ± 2.5 (21.4~30.9) | 0.095 † |
| Distance best corrected visual acuity (LogMAR) | 0.00 ± 0.01 (0.00~0.02) | 0.00 ± 0.01 (0.00~0.05) | 0.428 ‡ |
| Near best corrected visual acuity (LogMAR) | 0.00 ± 0.01 (0.00~0.05) | 0.00 ± 0.00 (0.00~0.00) | 0.317 ‡ |
| Spherical equivalent refractive errors (D) | −3.19 ± 2.74 (−9.88~+1.81) | −3.00 ± 2.89 (−8.94~+0.69) | 0.814 † |
| Stereopsis (LogArcsec) | 1.95 ± 0.29 (1.51~2.60) | 1.58 ± 0.30 (1.10~2.00) | <0.001 † |
| Near point of convergence (cm) | 8.7 ± 3.0 (3.5~26.0) | 7.9 ± 2.0 (5.0~13.0) | 0.245 † |
| Near point of accommodation (cm) | 9.8 ± 3.7 (4.8~19.0) | 9.1 ± 2.0 (5.5~14.5) | 0.430 † |
| Distance deviation (PD) | 21.1± 13.0 (4~50) | 0.2 ± 0.9 (0~4) | <0.001 ‡ |
| Near deviation (PD) | 27.1 ± 10.0 (14~50) | 0.8 ± 1.4 (0~4) | <0.001 ‡ |
| Automated assessment | Score | ||||||||||
| Horizontal FD | 23.6 ± 31.0 (0–98.20) | ||||||||||
| Vertical FD | 26.0 ± 33.6 (0–100) | ||||||||||
| Dot FD | 25.1 ± 32.5 (0–96.70) | ||||||||||
| Conventional assessment | Score | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| NCS | 4.5 ± 1.7 (1–8) | 0 | 1 (4%) | 1 (4%) | 6 (24%) | 5 (20%) | 4 (16%) | 5 (20%) | 2 (8%) | 1 (4%) | 0 |
| OCS | 4.1 ± 2.0 (1–9) | 1 (4%) | 1 (4%) | 2 (8%) | 8 (32%) | 2 (8%) | 5 (20%) | 3 (12%) | 2 (8%) | 0 | 1 (4%) |
| Horizontal | Vertical | Dot | ||
|---|---|---|---|---|
| OCS | Correlation coefficient | 0.683 | 0.699 | 0.751 |
| p value | <0.001 | <0.001 | <0.001 |
| Score | ICC | 95% CI | p Value |
|---|---|---|---|
| Horizontal FD | 0.633 | 0.146–0.842 | 0.011 |
| Vertical FD | 0.656 | 0.197–0.852 | 0.008 |
| Dot FD | 0.697 | 0.307–0.869 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.H.; Yang, H.K.; Han, S.B.; Hwang, J.M. Measurement of Fusion Control with Eye Tracking Device in Intermittent Exotropia. Diagnostics 2025, 15, 361. https://doi.org/10.3390/diagnostics15030361
Kim DH, Yang HK, Han SB, Hwang JM. Measurement of Fusion Control with Eye Tracking Device in Intermittent Exotropia. Diagnostics. 2025; 15(3):361. https://doi.org/10.3390/diagnostics15030361
Chicago/Turabian StyleKim, Dong Hyun, Hee Kyung Yang, Sang Beom Han, and Jeong Min Hwang. 2025. "Measurement of Fusion Control with Eye Tracking Device in Intermittent Exotropia" Diagnostics 15, no. 3: 361. https://doi.org/10.3390/diagnostics15030361
APA StyleKim, D. H., Yang, H. K., Han, S. B., & Hwang, J. M. (2025). Measurement of Fusion Control with Eye Tracking Device in Intermittent Exotropia. Diagnostics, 15(3), 361. https://doi.org/10.3390/diagnostics15030361







