The Role of Nailfold Videocapillaroscopy in the Diagnosis and Monitoring of Interstitial Lung Disease Associated with Rheumatic Autoimmune Diseases
Abstract
1. Introduction
2. Normal Capillary Pattern
3. NVC in Systemic Sclerosis Associated Interstitial Lung Disease (SSc-ILD)
4. NVC in Mixed Connective Tissue Disease Associated with Interstitial Lung Disease (MCTD-ILD)
5. NVC in Idiopathic Inflammatory Myopathies Associated with Interstitial Lung Disease (IIM-ILD)
6. NVC in Systemic Lupus Erythematosus Associated with Interstitial Lung Disease (SLE-ILD)
7. NVC in Sjögren’s Syndrome (SS) Associated with ILD
8. Discussion
9. Future Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, V.; Distler, O.; Du Four, T.; Cutolo, M. Is there a role for nailfold videocapillaroscopy in interstitial lung disease? Rheumatology 2022, 61, 2217–2220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Venetsanopoulou, A.I.; Goules, A.V.; Vlachoyiannopoulos, P.G.; Drosos, A.A.; Tzioufas, A.G.; Voulgari, P.V. The Role of Nailfold Capillaroscopy in Evaluating Patients with Interstitial Lung Disease Related to Connective Tissue Disease. Mediterr. J. Rheumatol. 2023, 4, 588–591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, V.; Ickinger, C.; Hysa, E.; Snow, M.; Frech, T.; Sulli, A.; Cutolo, M. Nailfold capillaroscopy. Best. Pr. Res. Clin. Rheumatol. 2023, 37, 101849. [Google Scholar] [CrossRef] [PubMed]
- Umashankar, E.; Abdel-Shaheed, C.; Plit, M.; Girgis, L. Assessing the role for nailfold videocapillaroscopy in interstitial lung disease classfication: A systematic review and meta-analysis. Rheumatology 2022, 61, 2221–2234. [Google Scholar] [CrossRef]
- Lee, S.H.; Min, H.K.; Kim, S.H.; Kim, Y.W.; Yoo, K.H.; Kim, H.J.; Kim, I.A.; Kim, H.-R. Nailfold capillaroscopy findings of interstitial pneumonia with autoimmune features. Korean J. Intern. Med. 2023, 38, 903–911. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fischer, A.; Antoniou, K.M.; Brown, K.K.; Cadranel, J.; Corte, T.J.; du Bois, R.M.; Lee, J.S.; Leslie, K.O.; Lynch, D.A.; Matteson, E.L.; et al. An official European Respiratory Society/American Thoracic Society research statement: Interstitial pneumonia with autoimmune features. Eur. Respir. J. 2015, 46, 976–987. [Google Scholar] [CrossRef]
- Ruaro, B.; Confalonieri, M.; Salton, F.; Wade, B.; Baratella, E.; Geri, P.; Confalonieri, P.; Kodric, M.; Biolo, M.; Bruni, C. The Relationship between Pulmonary Damage and Peripheral Vascular Manifestations in Systemic Sclerosis Patients. Pharmaceuticals 2021, 14, 403. [Google Scholar] [CrossRef]
- Niklas, K.; Niklas, A.; Mularek-Kubzdela, T.; Puszczewicz, M.; Samborski, W. Relationship between changes observed in nailfold capillaroscopy and serological profile, lung fibrosis, and elevated risk of pulmonary hypertension in patients with systemic sclerosis and mixed connective tissue disease. Postep. Dermatol. Alergol. 2022, 39, 880–886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mostmans, Y.; Richert, B.; Badot, V.; Nagant, C.; Smith, V.; Michel, O. The importance of skin manifestations, serology and nailfold (video) capillaroscopy in morphea and systemic sclerosis: Current understanding and new insights. J. Eur. Acad. Dermatol. Venereol. 2020, 35, 597–606. [Google Scholar] [CrossRef]
- Smith, V.; Herrick, A.L.; Ingegnoli, F.; Damjanov, N.; De Angelis, R.; Denton, C.P.; Distler, O.; Espejo, K.; Foeldvari, I.; Frech, T.; et al. EULAR Study Group on Microcirculation in Rheumatic Diseases and the Scleroderma Clinical Trials Consortium Group on Capillaroscopy. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud’s phenomenon and systemic sclerosis. Autoimmun. Rev. 2020, 19, 102458. [Google Scholar] [CrossRef] [PubMed]
- Arvanitaki, A.; Giannakoulas, G.; Triantafyllidou, E.; Karvounis, H.; Garyfallos, A.; Kitas, G.; Dimitroulas, T. Nailfold videocapillaroscopy: A novel possible surrogate marker for the evaluation of peripheral microangiopathy in pulmonary arterial hypertension. Scand. J. Rheumatol. 2020, 50, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Lambova, S.N.; Muller-Ladner, U. Nailfold capillaroscopy in systemic sclerosis–state of the art: The evolving knowledge about capillaroscopic abnormalities in systemic sclerosis. J. Scleroderma Relat. Disord. 2019, 4, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Caetano, J.; Paula, F.S.; Amaral, M.; Oliveira, S.; Alves, J.D. Nailfold Videocapillaroscopy Changes Are Associated With the Presence and Severity of Systemic Sclerosis–Related Interstitial Lung Disease. JCR J. Clin. Rheumatol. 2018, 25, e12–e15. [Google Scholar] [CrossRef] [PubMed]
- Andracco, R.; Irace, R.; Zaccara, E.; Vettori, S.; Maglione, W.; Riccardi, A.; Pignataro, F.; Ferrara, R.; Sambataro, D.; Sambataro, G. The cumulative number of micro-haemorrhages and micro-thromboses in nailfold videocapillaroscopy is a good indicator of disease severity in systemic sclerosis: A validation study of the NEMO score. Arthritis Res. Ther. 2017, 19, 133. [Google Scholar] [CrossRef]
- Ji, L.L.; Wang, H.; Zhang, X.H.; Zhang, Z.L. Correlation between nailfold capillaroscopic findings and presence of interstitial lung disease in systemic sclerosis patients. Beijing Da Xue Xue Bao Yi Xue Ban. 2018, 50, 501–506. (In Chinese) [Google Scholar] [PubMed]
- Guillen-Del-Castillo, A.; Simeòn-Aznar, C.P.; Callejas-Moraga, E.L.; Tolosa-Vilella, C.; Alonso-Vila, S.; Fonollosa-Pla, V.; Selva-O’callaghan, A. Quantitative Videocapillaroscopy correlates with functional respiratory parameters: A clue for vasculopathy as a pathogenic mechanism for lung injury in systemic sclerosis. Arthritis Res. Ther. 2018, 20, 281. [Google Scholar] [CrossRef]
- Celińska-Löwenhoff, M.; Pastuszczak, M.; Pełka, K.; Stec-Polak, M.; Wojas-Pelc, A.; Musiał, J. Associations between nailfold capillaroscopy findings and interstitial lung disease in patients with mixed connective tissue disease. Arch. Med. Sci. 2019, 16, 297–301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ciancio, N.; Pavone, M.; Torrisi, S.E.; Vancheri, A.; Sambataro, D.; Palmucci, S.; Vancheri, C.; Di Marco, F.; Sambataro, G. Contribution of pulmonary function tests (PFTs) to the diagnosis and follow up of connective tissue diseases. Multidiscip. Respir. Med. 2019, 14, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lega, J.C.; Reynaud, Q.; Belot, A.; Fabien, N.; Durieu, I.; Cottin, V. Idiopathic inflammatory myopathies and the lung. Eur. Respir. Rev. 2015, 24, 216–238. [Google Scholar] [CrossRef]
- Moghadam-Kia, S.; Oddis, C.V.; Sato, S.; Kuwana, M.; Aggarwal, R. Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res. 2016, 68, 689–694. [Google Scholar] [CrossRef]
- Witt, L.J.; Curran, J.J.; Strek, M.E. The Diagnosis and Treatment of Antisynthetase Syndrome. Clin. Pulm. Med. 2016, 23, 218–226. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sambataro, D.; Sambataro, G.; Libra, A.; Vignigni, G.; Pino, F.; Fagone, E.; Fruciano, M.; Gili, E.; Pignataro, F.; Del Papa, N.; et al. Nailfold Videocapillaroscopy Is a Useful Tool to Recognize Definite Forms of Systemic Sclerosis and Idiopathic Inflammatory Myositis in Interstitial Lung Disease Patients. Diagnostics 2020, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, M.; Triantafyllias, K.; Manfredi, A.; González-Gay, M.A.; Palmou-Fontana, N.; Cassone, G.; Drott, U.; Delbrück, C.; Rojas-Serrano, J.; Bertolazzi, C.; et al. American and European Network of Antisynthetase Syndrome Collaborative Group. Nailfold Capillaroscopy Characteristics of Antisynthetase Syndrome and Possible Clinical Associations: Results of a Multicenter International Study. J. Rheumatol. 2019, 46, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Connors, G.R.; Christopher-Stine, L.; Oddis, C.V.; Danoff, S.K. Interstitial lung disease associated with the idiopathic inflammatory myopathies: What progress has been made in the past 35 years? Chest 2010, 138, 1464–1474. [Google Scholar] [CrossRef]
- Bertolazzi, C.; Cutolo, M.; Smith, V.; Gutierrez, M. State of the art on nailfold capillaroscopy in dermatomyositis and polymyositis. Semin. Arthritis Rheum. 2017, 47, 432–444. [Google Scholar] [CrossRef]
- Zhao, T.; Lin, F.A.; Chen, H.P. Pattern of Nailfold Capillaroscopy in Patients With Systemic Lupus Erythematosus. Arch. Rheumatol. 2020, 35, 568–574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Álvarez Barreneche, M.F.; Velásquez Franco, C.J.; Giraldo Cuartas, C.E.; Navas, M.A.M. Capillaroscopic abnormalities in systemic lupus erythematosus and its association with clinical manifestation. Rev. Colomb. Reumatol. 2023, 31, 304–310. [Google Scholar] [CrossRef]
- Çakmakçı Karadoğan, D.; Balkarlı, A.; Önal, Ö.; Altinişik, G.; Çobankara, V. The role of nailfold capillaroscopy in interstitial lung diseases—Can it differentiate idiopathic cases from collagen tissue disease associated interstitial lung diseases? Tuberk. Toraks. 2015, 63, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Melsens, K.; Leone, M.C.; Paolino, S.; Elewaut, D.; Gerli, R.; Vanhaecke, A.; Peene, I.; Cutolo, M.; Smith, V. Nailfold capillaroscopy in Sjögren’s syndrome: A systematic literature review and standardised interpretation. Clin. Exp. Rheumatol. 2020, 38 (Suppl. S126), 150–157. [Google Scholar] [CrossRef] [PubMed]
- Chopra, M.; Pandey, I.; Vasdev, V.B.; Shrinath, V.; Yadav, A. A Medical Record-based Pilot Study on the Diffusing Capacity of the Lungs for Carbon Monoxide versus Nailfold Capillaroscopy as an Early Marker for Lung Involvement in Connective Tissue Disease-related Interstitial Lung Diseases. J. Mar. Med. Soc. 2024, 26, 265–269. [Google Scholar] [CrossRef]
- Sambataro, D.; Sambataro, G.; Pignataro, F.; Zanframundo, G.; Codullo, V.; Fagone, E.; Martorana, E.; Ferro, F.; Orlandi, M.; Del Papa, N.; et al. Patients with Interstitial lung disease secondary to autoimmune diseases: How to recognize them? Diagnostics 2020, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, M.; Gandin, I.; Riccardo, P.; Hughes, M.; Lepidi, S.; Salton, F.; Confalonieri, P.; Confalonieri, M.; Tavano, S.; Ruaro, B. Correlation between Microvascular Damage and Internal Organ Involvement in Scleroderma: Focus on Lung Damage and Endothelial Dysfunction. Diagnostics 2022, 13, 55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hysa, E.; Pizzorni, C.; Sammorì, S.; Gotelli, E.; Cere, A.; Schenone, C.; Ferrari, G.; Campitiello, R.; Gerli, V.; Paolino, S.; et al. Microvascular damage in autoimmune connective tissue diseases: A capillaroscopic analysis from 20 years of experience in a EULAR training and research referral centre for imaging. RMD Open 2023, 9, e003071. [Google Scholar] [CrossRef] [PubMed]
- Ghizzoni, C.; Sebastiani, M.; Manfredi, A.; Campomori, F.; Colaci, M.; Giuggioli, D.; Ferri, C. Prevalence and evolution of sclera derma pattern at nailfold videocapillaroscopy in systemic sclerosis patients: Clinical and prognostic implications. Microvasc. Res. 2015, 99, 92–95. [Google Scholar] [CrossRef]
- Rudra, O.; Baisya, S.; Mallick, S.; Chatterjee, G. Nailfold Capillaroscopic Changes as a Marker of Interstitial Lung Disease in Systemic Sclerosis: A Cross-Sectional Study in a Tertiary Care Hospital in Eastern India. Indian. Dermatol. Online J. 2022, 13, 216–220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Graney, B.A.; Fischer, A. Interstitial Pneumonia with Autoimmune Features. Ann. Am. Thorac. Soc. 2019, 16, 525–533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Acemoğlu, Ş.Ş.Z.; Türk, İ.; Deniz, P.P.; Aşık, M.A.; Arslan, D.; Hanta, I.; Ünal, I. Relationship between nailfold capillaroscopy findings and the etiology and prognosis of interstitial lung disease. Clin. Rheumatol. 2024, 43, 2679–2687. [Google Scholar] [CrossRef]
- Abdelwahab, H.W.; Shalabi, N.M.; Rashad Ghoneim, M.M.; Farrag, N.S.; Hamdy, F.; Elhoseiny, F.; Ali, R.E. Screening for Subclinical Interstitial Lung Disease in Rheumatoid Arthritis Patients: Functional and Radiological Methods. Turk. Thorac. J. 2022, 23, 261–267. [Google Scholar] [CrossRef]
- Distler, O.; Assassi, S.; Cottin, V.; Cutolo, M.; Danoff, S.K.; Denton, C.P.; Distler, J.H.; Hoffmann-Vold, A.-M.; Johnson, S.R.; Ladner, U.M.; et al. Predictors of progression in systemic sclerosis patients with interstitial lung disease. Eur. Respir. J. 2020, 55, 1902026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giacomelli, R.; Afeltra, A.; Alunno, A.; Bartoloni-Bocci, E.; Berardicurti, O.; Bombardieri, M.; Bortoluzzi, A.; Caporali, R.; Caso, F.; Cervera, R.; et al. Guidelines for biomarkers in autoimmune rheumatic diseases—Evidence based analysis. Autoimmun. Rev. 2019, 18, 93–106. [Google Scholar] [CrossRef]
- Weigold, F.; Günther, J.; Pfeiffenberger, M.; Cabral-Marques, O.; Siegert, E.; Dragun, D.; Philippe, A.; Regensburger, A.-K.; Recke, A.; Yu, X.; et al. Antibodies against chemokine receptors CXCR3 and CXCR4 predict progressive deterioration of lung function in patients with systemic sclerosis. Arthritis Res. Ther. 2018, 20, 52. [Google Scholar] [CrossRef] [PubMed]
- Barratt, S.; Millar, A. Vascular remodelling in the pathogenesis of idiopathic pulmonary fibrosis. QJM 2014, 107, 515–519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Acemoğlu, Ş.Ş.Z.; Türk, İ.; Aşık, M.A.; Bircan, A.; De Niz, P.P.; Arslan, D.; Hanta, I.; Ünal, I. Microvascular damage evaluation based on nailfold videocapillarosopy in sarcoidosis. Clin. Rheumatol. 2023, 42, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Castelino, F.V.; Varga, J. Interstitial lung disease in connective tissue diseases: Evolving concepts of pathogenesis and management. Arthritis Res. Ther. 2010, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Soulaidopoulos, S.; Triantafyllidou, E.; Garyfallos, A.; Kitas, G.D.; Dimitroulas, T. The role of nailfold capillaroscopy in the assessment of internal organ involvement in systemic sclerosis: A critical review. Autoimmun. Rev. 2017, 16, 787–795. [Google Scholar] [CrossRef]
- Ferrara, A.L.; Bova, M.; Petraroli, A.; Veszeli, N.; Galdiero, M.R.; Braile, M.; Marone, G.; Cristinziano, L.; Marcella, S.; Modestino, L.; et al. Hereditary angioedema attack: What happens to vasoactive mediators? Int. Immunopharmacol. 2020, 78, 106079. [Google Scholar] [CrossRef]
- Ferrara, A.L.; Cristinziano, L.; Petraroli, A.; Bova, M.; Gigliotti, M.C.; Marcella, S.; Modestino, L.; Varricchi, G.; Braile, M.; Galdiero, M.R.; et al. Roles of Immune Cells in Hereditary Angioedema. Clin. Rev. Allergy Immunol. 2021, 60, 369–382. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, S.Y.; Kim, D.S.; Kim, Y.W.; Chung, M.P.; Uh, S.T.; Park, C.S.; Jeong, S.H.; Park, Y.B.; Lee, H.L.; et al. Predicting survival of patients with idiopathic pulmonary fibrosis using GAP score: A nationwide cohort study. Respir. Res. 2016, 17, 131. [Google Scholar] [CrossRef]
- Anghel, D.; Sîrbu, C.A.; Petrache, O.G.; Opriș-Belinski, D.; Negru, M.M.; Bojincă, V.-C.; Pleșa, C.F.; Radu, F.I. Nailfold Videocapillaroscopy in Patients with Rheumatoid Arthritis and Psoriatic Arthropathy on ANTI-TNF-ALPHA Therapy. Diagnostics 2023, 13, 2079. [Google Scholar] [CrossRef]
- Smith, V.; Distler, O.; Cutolo, M. Might nailfold capillaroscopy be a “proxy” for lung involvement in connective tissue diseases? J. Rheumatol. 2019, 46, 1061–1063. [Google Scholar] [CrossRef]
- Markusse, I.M.; Meijs, J.; de Boer, B.; Bakker, J.A.; Schippers, H.P.C.; Schouffoer, A.A.; Marsan, N.A.; Kroft, L.J.M.; Ninaber, M.K.; Huizinga, T.W.J.; et al. Predicting cardiopulmonary involvement in patients with systemic sclerosis: Complementary value of nailfold videocapillaroscopy patterns and disease-specific autoantibodies. Rheumatology 2017, 56, 1081–1088. [Google Scholar] [CrossRef][Green Version]
- Tardella, M.; Di Carlo, M.; Carotti, M.; Filippucci, E.; Grassi, W.; Salaffi, F. Ultrasound B-lines in the evaluation of interstitial lung disease in patients with systemic sclerosis: Cut-off point definition for the presence of significant pulmonary fibrosis. Medicine 2018, 97, e0566. [Google Scholar] [CrossRef]
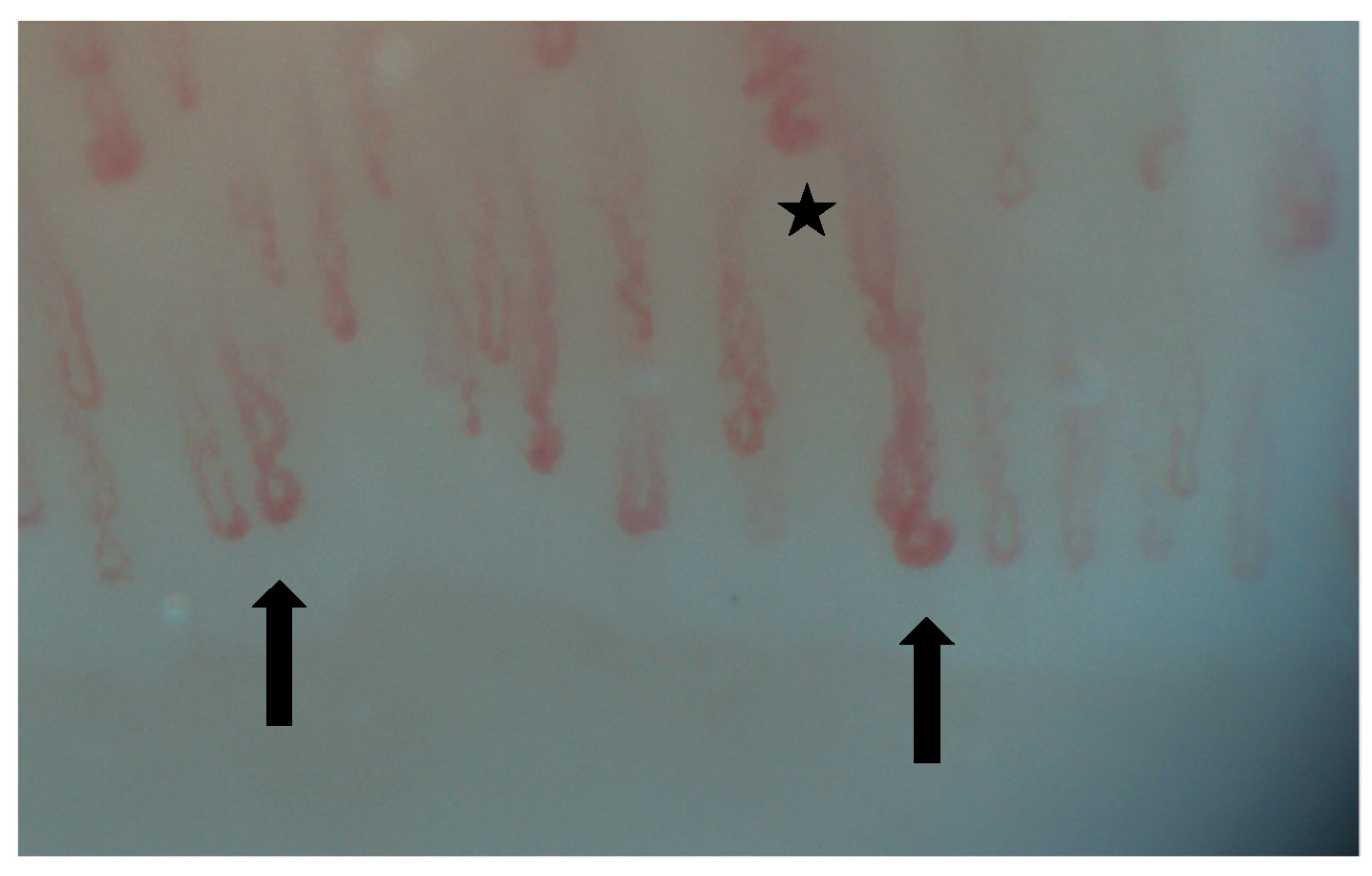
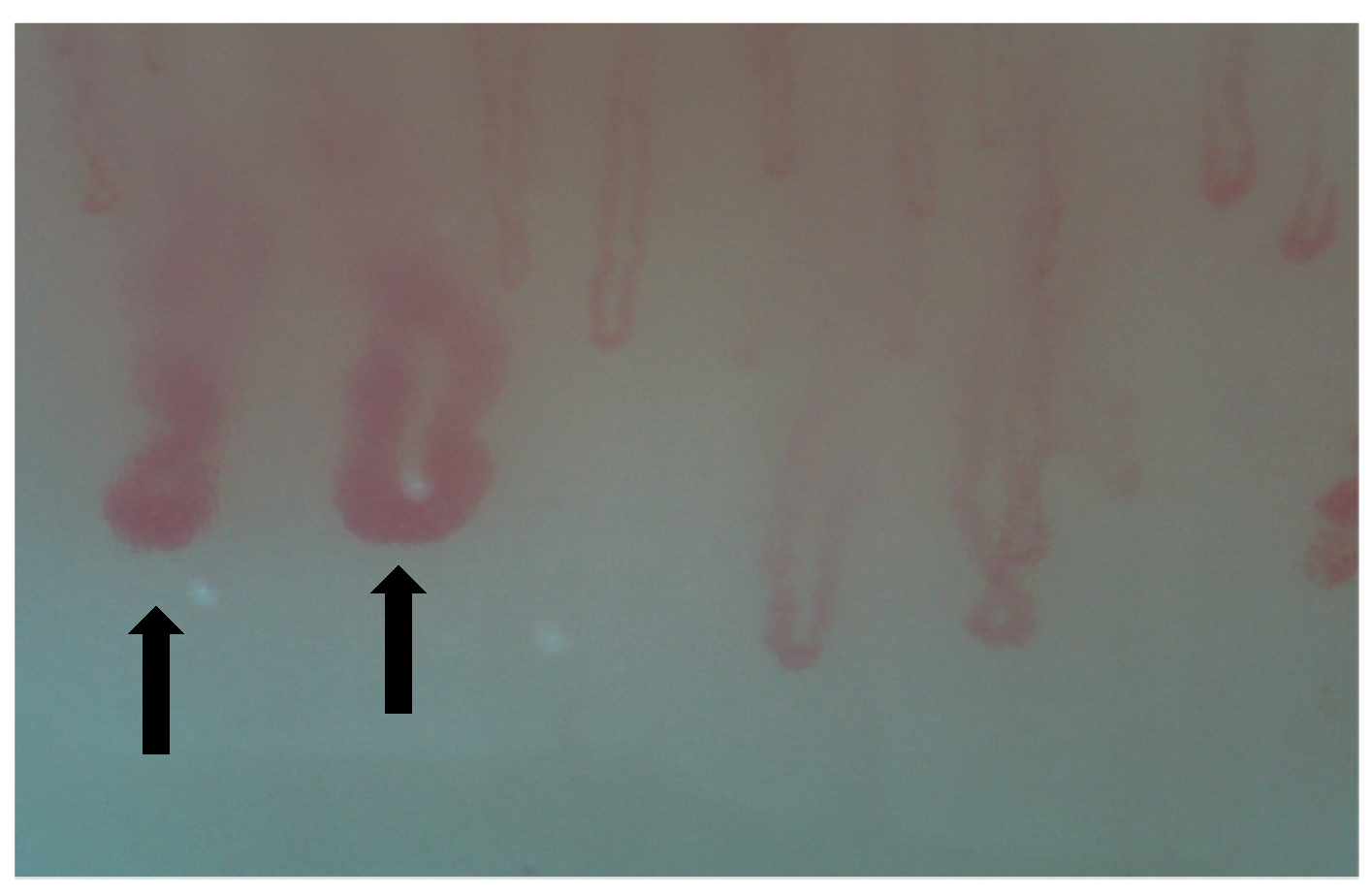
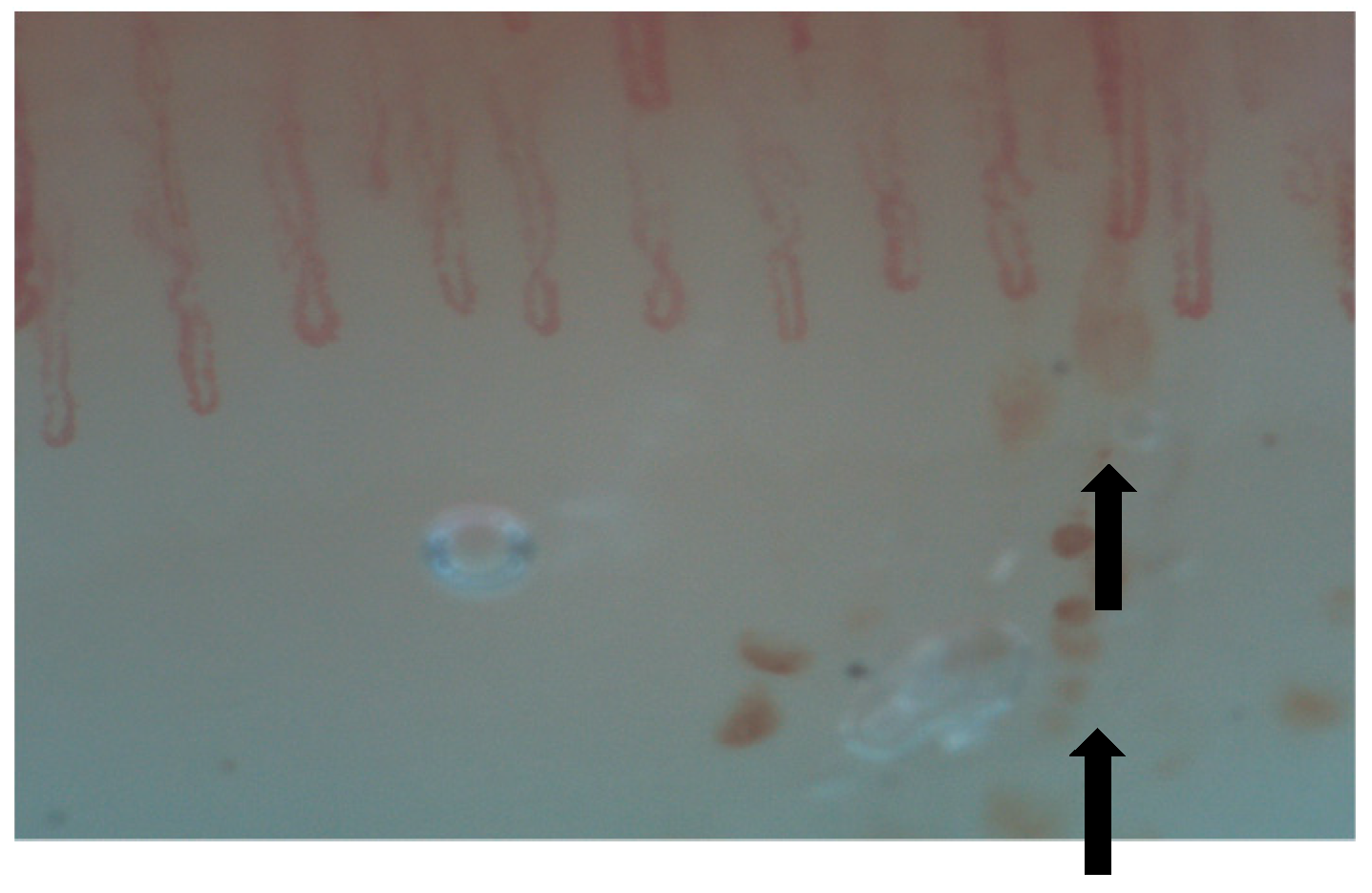
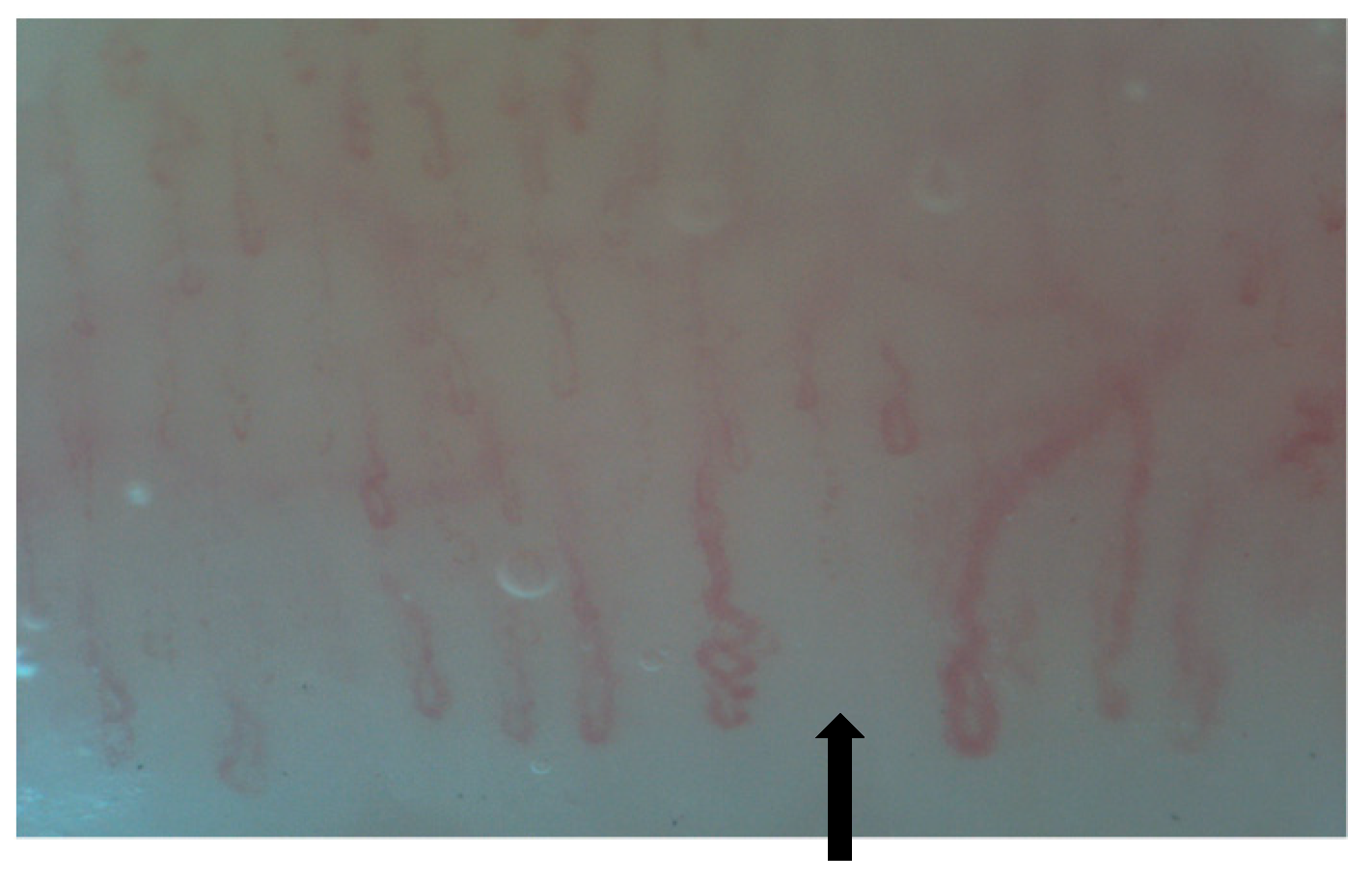
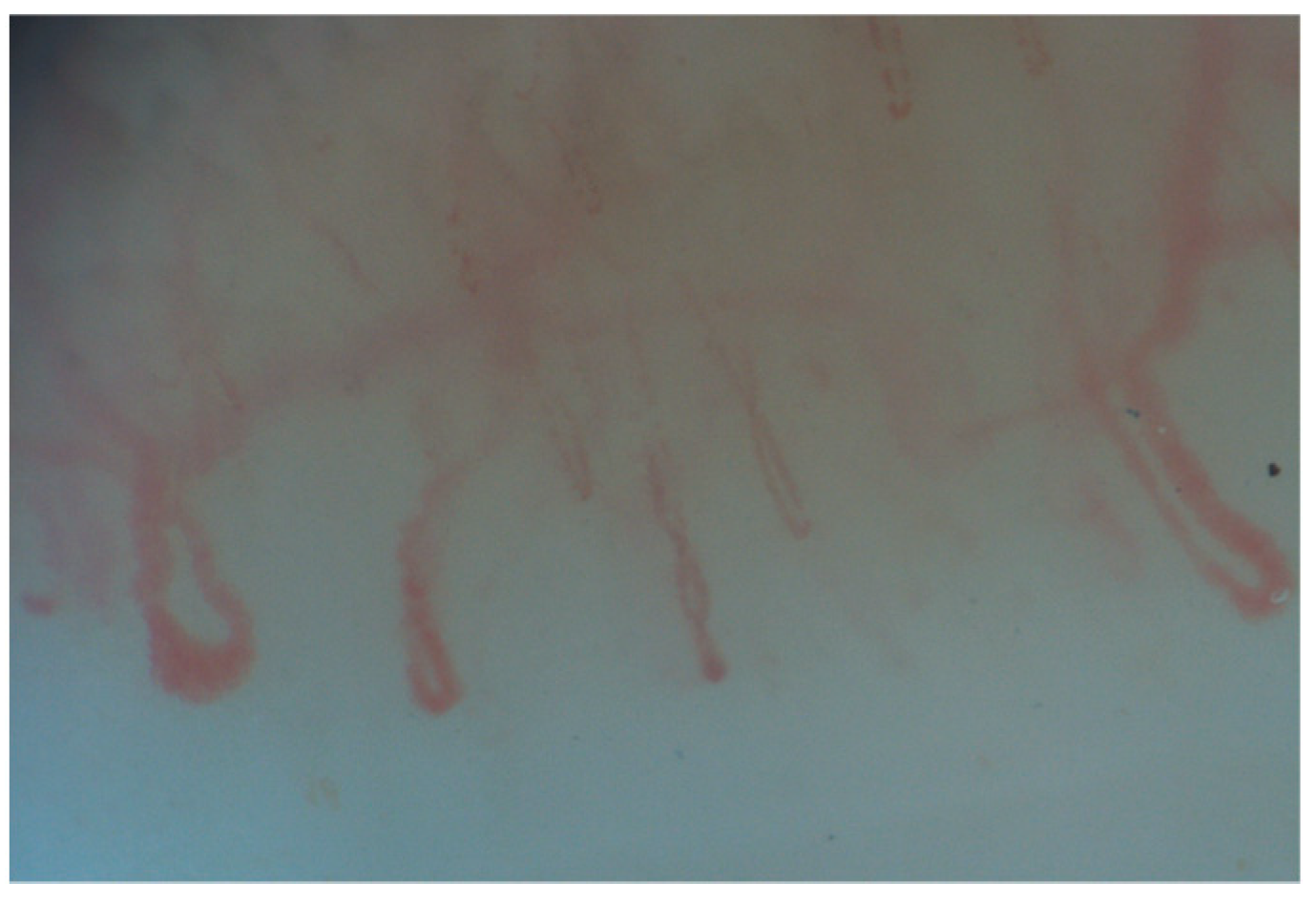
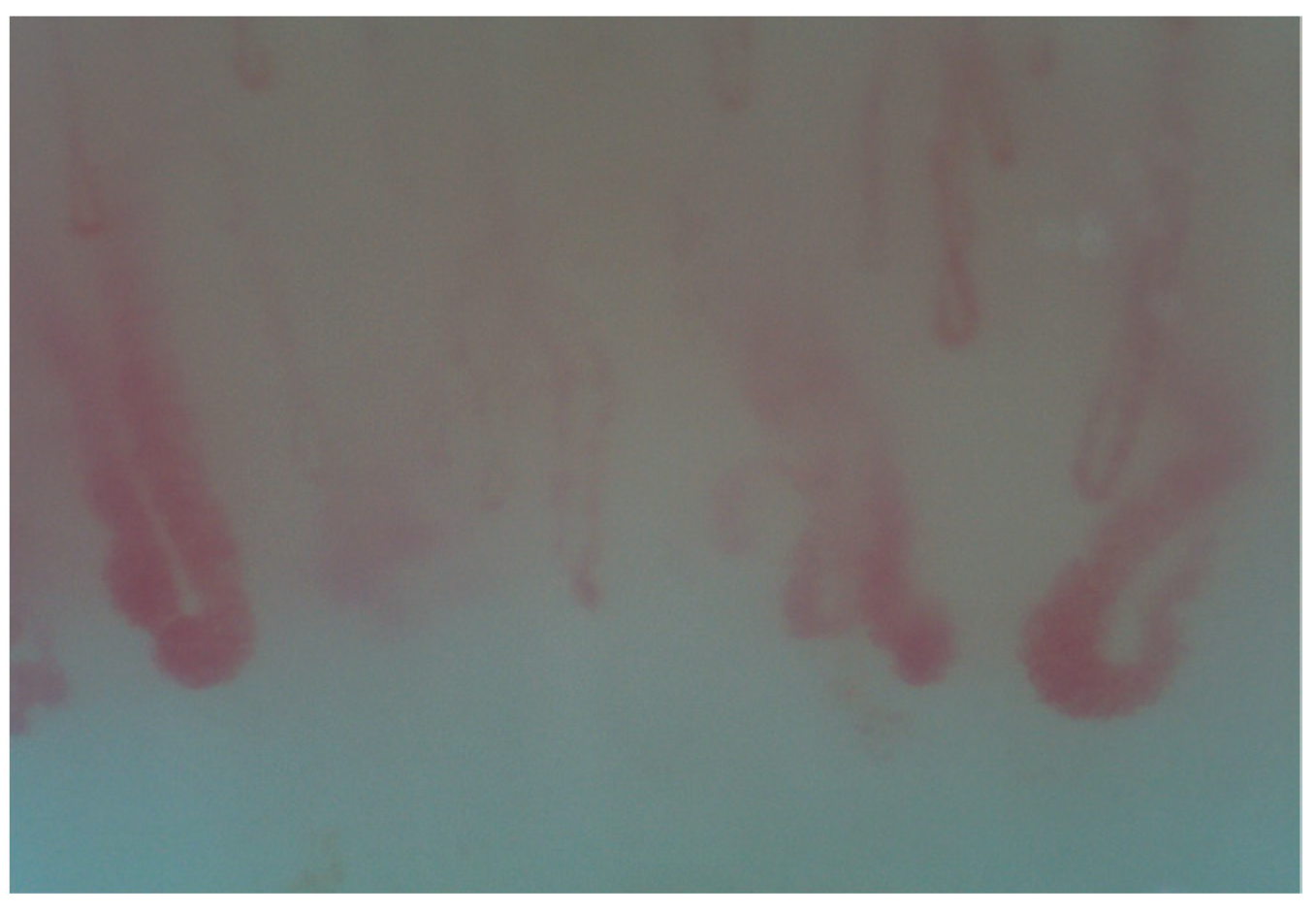
| SSc-ILD | MCTD-ILD | IIM-ILD | SLE-ILD | SS-ILD | |
|---|---|---|---|---|---|
| NVC abnormalities |
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anghel, D.; Prioteasă, O.-G.; Nicolau, I.-N.; Bucurică, S.; Belinski, D.-O.; Popescu, G.-G.; Ghinescu, M.C.; Bobircă, A.; Groșeanu, M.-L.; Bojincă, V.-C. The Role of Nailfold Videocapillaroscopy in the Diagnosis and Monitoring of Interstitial Lung Disease Associated with Rheumatic Autoimmune Diseases. Diagnostics 2025, 15, 362. https://doi.org/10.3390/diagnostics15030362
Anghel D, Prioteasă O-G, Nicolau I-N, Bucurică S, Belinski D-O, Popescu G-G, Ghinescu MC, Bobircă A, Groșeanu M-L, Bojincă V-C. The Role of Nailfold Videocapillaroscopy in the Diagnosis and Monitoring of Interstitial Lung Disease Associated with Rheumatic Autoimmune Diseases. Diagnostics. 2025; 15(3):362. https://doi.org/10.3390/diagnostics15030362
Chicago/Turabian StyleAnghel, Daniela, Oana-Georgiana Prioteasă, Iulia-Nadine Nicolau, Săndica Bucurică, Daniela-Opriș Belinski, Gilda-Georgeta Popescu, Minerva Claudia Ghinescu, Anca Bobircă, Maria-Laura Groșeanu, and Violeta-Claudia Bojincă. 2025. "The Role of Nailfold Videocapillaroscopy in the Diagnosis and Monitoring of Interstitial Lung Disease Associated with Rheumatic Autoimmune Diseases" Diagnostics 15, no. 3: 362. https://doi.org/10.3390/diagnostics15030362
APA StyleAnghel, D., Prioteasă, O.-G., Nicolau, I.-N., Bucurică, S., Belinski, D.-O., Popescu, G.-G., Ghinescu, M. C., Bobircă, A., Groșeanu, M.-L., & Bojincă, V.-C. (2025). The Role of Nailfold Videocapillaroscopy in the Diagnosis and Monitoring of Interstitial Lung Disease Associated with Rheumatic Autoimmune Diseases. Diagnostics, 15(3), 362. https://doi.org/10.3390/diagnostics15030362








