Association of [68Ga]Ga-PSMA-11 PET/CT Metrics with PSA Persistence Following Radical Prostatectomy in Patients with Intermediate- and High-Risk Prostate Cancer
Abstract
1. Introduction
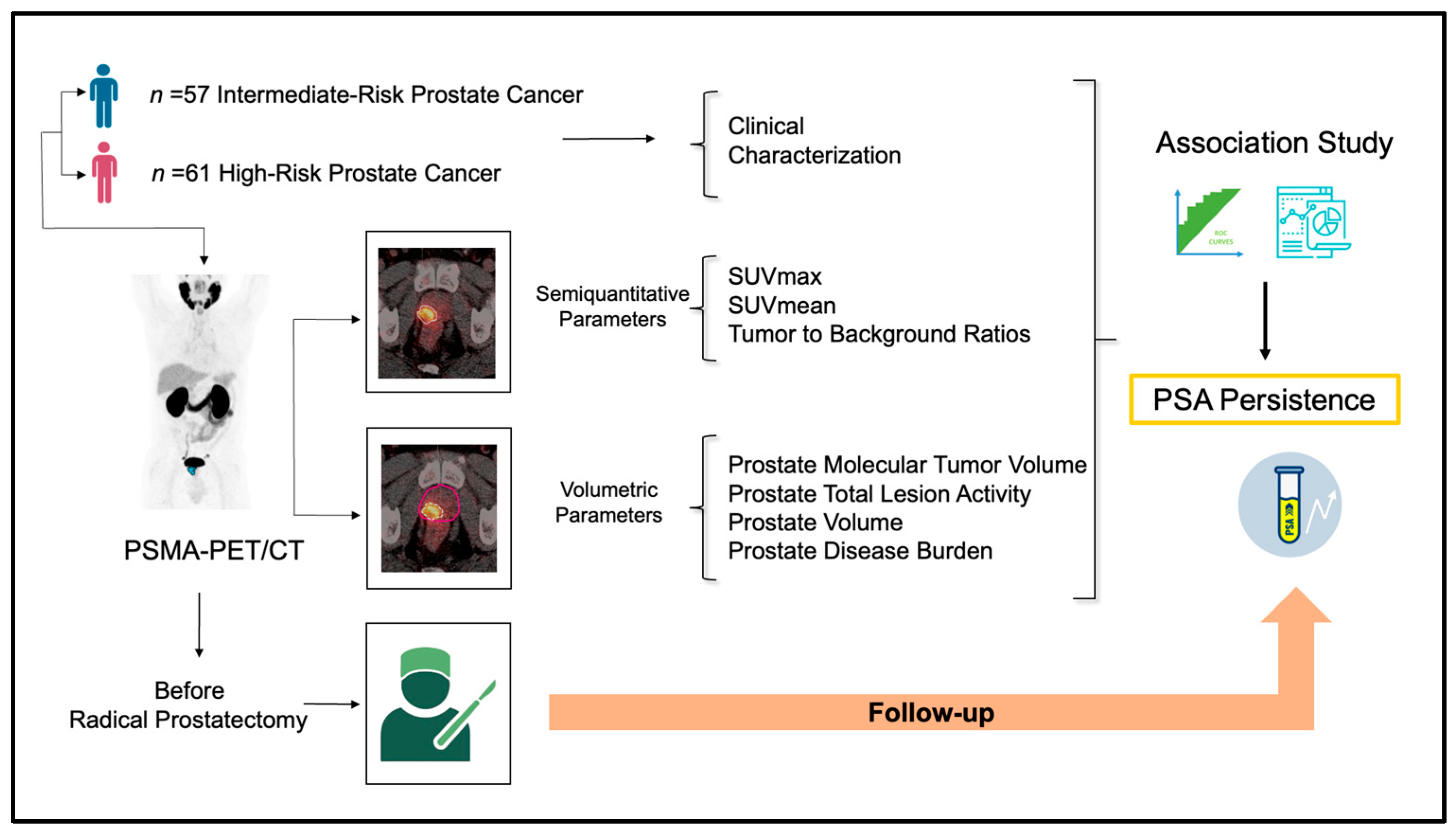
2. Materials and Methods
2.1. Study Design and Participants
2.2. [68Ga]Ga-PSMA-11 PET/CT Protocol
2.3. Image Analysis
2.4. Outcome
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Prostate PSMA-PET Metrics by Baseline Risk Classification (Intermediate- vs. High-Risk)
3.3. Prostate PSMA-PET Metrics by Final ISUP Grade (ISUP 1–2 vs. ISUP 3–5)
3.4. Prostate PSMA-PET Metrics by Seminal Vesicles Infiltration
3.5. Prostate PSMA-PET Metrics by Node Infiltration Status
3.6. Prostate PSMA-PET Metrics by PSA Status Following RP (Undetectable vs. Persistent)
3.7. ROC Analysis and Logistic Regression of PSMA-PET Metrics as Predictors of PSA Persistence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| PSMA-PET Metrics | pN1–TP miN1 (n = 16) Median (IQR) | pN0-TN miN0 (n = 102) Median (IQR) | FP miN1 (n = 13) Median (IQR) | p-Value * |
|---|---|---|---|---|
| pMTV (cc) | 10.4 (5.1–14.5) | 3.8 (2.2–5.9) | 3.1 (2.3–6.7) | 0.023 |
| pTLA | 48.6 (34.1–114.8) | 20.1 (12.9–29.5) | 19.4 (12.5–25.6) | 0.001 |
| pDB (%) | 16.6 (8.4–26.4) | 7.4 (3.9–11.2) | 7.5 (3.6–11.6) | 0.028 |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Eggener, S.E.; Scardino, P.T.; Walsh, P.C.; Han, M.; Partin, A.W.; Trock, B.J.; Feng, Z.; Wood, D.P.; Eastham, J.A.; Yossepowitch, O.; et al. Predicting 15-Year Prostate Cancer Specific Mortality After Radical Prostatectomy. J. Urol. 2011, 185, 869–875. [Google Scholar] [CrossRef]
- Zumsteg, Z.S.; Spratt, D.E.; Romesser, P.B.; Pei, X.; Zhang, Z.; Polkinghorn, W.; McBride, S.; Kollmeier, M.; Yamada, Y.; Zelefsky, M.J. The Natural History and Predictors of Outcome Following Biochemical Relapse in the Dose Escalation Era for Prostate Cancer Patients Undergoing Definitive External Beam Radiotherapy. Eur. Urol. 2015, 67, 1009–1016. [Google Scholar] [CrossRef]
- Kupelian, P.A.; Buchsbaum, J.C.; Elshaikh, M.; Reddy, C.A.; Zippe, C.; Klein, E.A. Factors Affecting Recurrence Rates after Prostatectomy or Radiotherapy in Localized Prostate Carcinoma Patients with Biopsy Gleason Score 8 or Above. Cancer 2002, 95, 2302–2307. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Humphreys, E.B.; Mangold, L.A.; Eisenberger, M.; Dorey, F.J.; Walsh, P.C.; Partin, A.W. Risk of Prostate Cancer–Specific Mortality Following Biochemical Recurrence After Radical Prostatectomy. JAMA 2005, 294, 433. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lopez, R.; Tunariu, N.; Padhani, A.R.; Oyen, W.J.G.; Fanti, S.; Vargas, H.A.; Omlin, A.; Morris, M.J.; de Bono, J.; Koh, D.-M. Imaging Diagnosis and Follow-up of Advanced Prostate Cancer: Clinical Perspectives and State of the Art. Radiology 2019, 292, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Briganti, A.; Abdollah, F.; Nini, A.; Suardi, N.; Gallina, A.; Capitanio, U.; Bianchi, M.; Tutolo, M.; Passoni, N.M.; Salonia, A.; et al. Performance Characteristics of Computed Tomography in Detecting Lymph Node Metastases in Contemporary Patients with Prostate Cancer Treated with Extended Pelvic Lymph Node Dissection. Eur. Urol. 2012, 61, 1132–1138. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-Specific Membrane Antigen PET-CT in Patients with High-Risk Prostate Cancer before Curative-Intent Surgery or Radiotherapy (ProPSMA): A Prospective, Randomised, Multicentre Study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Dondi, F.; Albano, D.; Bertagna, F.; Treglia, G. Bone Scintigraphy versus PSMA-Targeted PET/CT or PET/MRI in Prostate Cancer: Lessons Learned from Recent Systematic Reviews and Meta-Analyses. Cancers 2022, 14, 4470. [Google Scholar] [CrossRef]
- Zacho, H.D.; Nielsen, J.B.; Dettmann, K.; Haberkorn, U.; Langkilde, N.C.; Jensen, J.B.; Petersen, L.J. 68Ga-PSMA PET/CT in Patients With Biochemical Recurrence of Prostate Cancer. Clin. Nucl. Med. 2018, 43, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Tang, R.; Nandurkar, R.; Hruby, G.; Roach, P.; Watts, J.A.; Cusick, T.; Kneebone, A.; Ho, B.; Chan, L.; et al. 3-Year Freedom from Progression After 68 Ga-PSMA PET/CT–Triaged Management in Men with Biochemical Recurrence After Radical Prostatectomy: Results of a Prospective Multicenter Trial. J. Nucl. Med. 2020, 61, 866–872. [Google Scholar] [CrossRef]
- EAU. EAU Guidelines. In Proceedings of the EAU Annual Congress Paris, Paris, France, 5–8 April 2024; EAU: Arnhem, The Netherlands, 2024. ISBN 978-94-92671-23-3. [Google Scholar]
- Cooperberg, M.R.; Hilton, J.F.; Carroll, P.R. The CAPRA-S Score: A Straightforward Tool for Improved Prediction of Outcomes after Radical Prostatectomy. Cancer 2011, 117, 5039–5046. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.V.; Whittington, R.; Kaplan, I.; Beard, C.; Schultz, D.; Malkowicz, S.B.; Wein, A.; Tomaszewski, J.E.; Coleman, C.N. Calculated Prostate Carcinoma Volume: The Optimal Predictor of 3-Year Prostate Specific Antigen (PSA) Failure Free Survival after Surgery or Radiation Therapy of Patients with Pretreatment PSA Levels of 4-20 Nanograms per Milliliter. Cancer 1998, 82, 334–341. [Google Scholar] [CrossRef]
- Karpinski, M.J.; Hüsing, J.; Claassen, K.; Möller, L.; Kajüter, H.; Oesterling, F.; Grünwald, V.; Umutlu, L.; Kleesiek, J.; Telli, T.; et al. Combining PSMA-PET and PROMISE to Re-Define Disease Stage and Risk in Patients with Prostate Cancer: A Multicentre Retrospective Study. Lancet Oncol. 2024, 25, 1188–1201. [Google Scholar] [CrossRef]
- Roberts, M.J.; Morton, A.; Donato, P.; Kyle, S.; Pattison, D.A.; Thomas, P.; Coughlin, G.; Esler, R.; Dunglison, N.; Gardiner, R.A.; et al. 68Ga-PSMA PET/CT Tumour Intensity Pre-Operatively Predicts Adverse Pathological Outcomes and Progression-Free Survival in Localised Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Amiel, T.; Würnschimmel, C.; Langbein, T.; Steiger, K.; Rauscher, I.; Horn, T.; Maurer, T.; Weber, W.; Wester, H.-J.; et al. PSMA-Ligand Uptake Can Serve as a Novel Biomarker in Primary Prostate Cancer to Predict Outcome after Radical Prostatectomy. EJNMMI Res. 2021, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- de Bie, K.C.C.; Veerman, H.; Bodar, Y.J.L.; Meijer, D.; van Leeuwen, P.J.; van der Poel, H.G.; Donswijk, M.L.; Vis, A.N.; Oprea-Lager, D.E. Higher Preoperative Maximum Standardised Uptake Values (SUVmax) Are Associated with Higher Biochemical Recurrence Rates after Robot-Assisted Radical Prostatectomy for [68Ga]Ga-PSMA-11 and [18F] DCFPyL Positron Emission Tomography/Computed Tomography. Diagnostics 2023, 13, 2343. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, T.; Zhu, Z.; Zhang, L.; Qian, S.; Fu, H.; Cao, Q.; Kang, J. 18F-DCFPyL PET/CT in Newly Diagnosed Prostate Cancer: Diagnostic Value of Intraprostatic PSMA Uptake in Risk Classification of Prostate Cancer. Front. Oncol. 2022, 12, 800904. [Google Scholar] [CrossRef] [PubMed]
- Koerber, S.A.; Utzinger, M.T.; Kratochwil, C.; Kesch, C.; Haefner, M.F.; Katayama, S.; Mier, W.; Iagaru, A.H.; Herfarth, K.; Haberkorn, U.; et al. 68 Ga-PSMA-11 PET/CT in Newly Diagnosed Carcinoma of the Prostate: Correlation of Intraprostatic PSMA Uptake with Several Clinical Parameters. J. Nucl. Med. 2017, 58, 1943–1948. [Google Scholar] [CrossRef]
- Seifert, R.; Kessel, K.; Schlack, K.; Weber, M.; Herrmann, K.; Spanke, M.; Fendler, W.P.; Hadaschik, B.; Kleesiek, J.; Schäfers, M.; et al. PSMA PET Total Tumor Volume Predicts Outcome of Patients with Advanced Prostate Cancer Receiving [177Lu]Lu-PSMA-617 Radioligand Therapy in a Bicentric Analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1200–1210. [Google Scholar] [CrossRef]
- Kuo, P.; Hesterman, J.; Rahbar, K.; Kendi, A.T.; Wei, X.X.; Fang, B.; Adra, N.; Armstrong, A.J.; Garje, R.; Michalski, J.M.; et al. [68Ga] Ga-PSMA-11 PET Baseline Imaging as a Prognostic Tool for Clinical Outcomes to [177Lu] Lu-PSMA-617 in Patients with MCRPC: A VISION Substudy. J. Clin. Oncol. 2022, 40, 5002. [Google Scholar] [CrossRef]
- Eiber, M.; Herrmann, K.; Calais, J.; Hadaschik, B.; Giesel, F.L.; Hartenbach, M.; Hope, T.; Reiter, R.; Maurer, T.; Weber, W.A.; et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed MiTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J. Nucl. Med. 2018, 59, 469–478. [Google Scholar] [CrossRef]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Calais, J.; Ceci, F.; Cho, S.Y.; Fanti, S.; Giesel, F.L.; Goffin, K.; et al. PSMA PET/CT: Joint EANM Procedure Guideline/SNMMI Procedure Standard for Prostate Cancer Imaging 2.0. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Ceci, F.; Oprea-Lager, D.E.; Emmett, L.; Adam, J.A.; Bomanji, J.; Czernin, J.; Eiber, M.; Haberkorn, U.; Hofman, M.S.; Hope, T.A.; et al. E-PSMA: The EANM Standardized Reporting Guidelines v1.0 for PSMA-PET. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1626–1638. [Google Scholar] [CrossRef] [PubMed]
- Mihatsch, P.W.; Beissert, M.; Pomper, M.G.; Bley, T.A.; Seitz, A.K.; Kübler, H.; Buck, A.K.; Rowe, S.P.; Serfling, S.E.; Hartrampf, P.E.; et al. Changing Threshold-Based Segmentation Has No Relevant Impact on Semi-Quantification in the Context of Structured Reporting for PSMA-PET/CT. Cancers 2022, 14, 270. [Google Scholar] [CrossRef]
- Schmidkonz, C.; Cordes, M.; Goetz, T.I.; Prante, O.; Kuwert, T.; Ritt, P.; Uder, M.; Wullich, B.; Goebell, P.; Bäuerle, T. 68Ga-PSMA-11 PET/CT Derived Quantitative Volumetric Tumor Parameters for Classification and Evaluation of Therapeutic Response of Bone Metastases in Prostate Cancer Patients. Ann. Nucl. Med. 2019, 33, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 1067–1096. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Pasta, D.J.; Elkin, E.P.; Litwin, M.S.; Latini, D.M.; Du Chane, J.; Carroll, P.R. The University of California, San Francisco Cancer of the Prostate Risk Assessment Score: A Straightforward and Reliable Preoperative Predictor of Disease Recurrence After Radical Prostatectomy. J. Urol. 2005, 173, 1938–1942. [Google Scholar] [CrossRef] [PubMed]
- Gnanapragasam, V.J.; Lophatananon, A.; Wright, K.A.; Muir, K.R.; Gavin, A.; Greenberg, D.C. Improving Clinical Risk Stratification at Diagnosis in Primary Prostate Cancer: A Prognostic Modelling Study. PLoS Med. 2016, 13, e1002063. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.L.; Scardino, P.T.; Kattan, M.W. A Catalog of Prostate Cancer Nomograms. J. Urol. 2001, 165, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- King, C.R. Patterns of Prostate Cancer Biopsy Grading: Trends and Clinical Implications. Int. J. Cancer 2000, 90, 305–311. [Google Scholar] [CrossRef]
- Singh, V.; Sharma, K.; Singh, M.; Tripathi, S.S.; Bhirud, D.P.; Jena, R.; Navriya, S.C.; Choudhary, G.R.; Sandhu, A.S. Discrepancies in Gleason Score Between Needle Core Biopsy and Radical Prostatectomy Specimens with Correlation Between Clinical and Pathological Staging. Urol. J. 2024, 91, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Incesu, R.-B.; Preisser, F.; Nohe, F.; Maurer, T.; Graefen, M.; Tilki, D. Negative PSMA PET Can Be Used to Avoid Unnecessary Pelvic Lymph Node Dissection in Intermediate Risk Prostate Cancer. Prostate Cancer Prostatic Dis. 2025, 1–5. [Google Scholar] [CrossRef]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-Specific Membrane Antigen Expression in Normal and Malignant Human Tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Perner, S.; Hofer, M.D.; Kim, R.; Shah, R.B.; Li, H.; Möller, P.; Hautmann, R.E.; Gschwend, J.E.; Kuefer, R.; Rubin, M.A. Prostate-Specific Membrane Antigen Expression as a Predictor of Prostate Cancer Progression. Hum. Pathol. 2007, 38, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Kinahan, P.E.; Fletcher, J.W. Positron Emission Tomography-Computed Tomography Standardized Uptake Values in Clinical Practice and Assessing Response to Therapy. Semin. Ultrasound CT MRI 2010, 31, 496–505. [Google Scholar] [CrossRef]
- Boellaard, R.; Krak, N.C.; Hoekstra, O.S.; Lammertsma, A.A. Effects of Noise, Image Resolution, and ROI Definition on the Accuracy of Standard Uptake Values: A Simulation Study. J. Nucl. Med. 2004, 45, 1519–1527. [Google Scholar] [PubMed]
- Rogic, I.; Golubic, A.T.; Zuvic, M.; Smitran, T.; Jukic, N.; Gamulin, M.; Kastelan, Z.; Huic, D. Clinical Utility of [68Ga]Ga-PSMA-11 PET/CT in Initial Staging of Patients with Prostate Cancer and Importance of Intraprostatic SUVmax Values. Nucl. Med. Rev. 2024, 27, 6–12. [Google Scholar] [CrossRef]
- Bodar, Y.J.L.; Veerman, H.; Meijer, D.; de Bie, K.; van Leeuwen, P.J.; Donswijk, M.L.; van Moorselaar, R.J.A.; Hendrikse, N.H.; Boellaard, R.; Oprea-Lager, D.E.; et al. Standardised Uptake Values as Determined on Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography Is Associated with Oncological Outcomes in Patients with Prostate Cancer. BJU Int. 2022, 129, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Schmuck, S.; von Klot, C.A.; Henkenberens, C.; Sohns, J.M.; Christiansen, H.; Wester, H.-J.; Ross, T.L.; Bengel, F.M.; Derlin, T. Initial Experience with Volumetric 68 Ga-PSMA I&T PET/CT for Assessment of Whole-Body Tumor Burden as a Quantitative Imaging Biomarker in Patients with Prostate Cancer. J. Nucl. Med. 2017, 58, 1962–1968. [Google Scholar] [CrossRef]
- Chandekar, K.R.; Satapathy, S.; Singh, H.; Kumar, R.; Kumar, S.; Kakkar, N.; Mittal, B.R.; Singh, S.K. Utility of PSMA-PET Derived Volumetric Parameters in Initial Risk Stratification and Prediction of Prostate Cancer Metastasis—A Head-to-Head Comparison of the Radiotracers 18F-PSMA-1007 and 68Ga-PSMA-11. Nucl. Med. Commun. 2024, 45, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Uhlman, M.A.; Sun, L.; Stackhouse, D.A.; Caire, A.A.; Polascik, T.J.; Robertson, C.N.; Madden, J.; Vollmer, R.; Albala, D.M.; Moul, J.W. Tumor Volume, Tumor Percentage Involvement, or Prostate Volume: Which Is Predictive of Prostate-Specific Antigen Recurrence? Urology 2010, 75, 460–466. [Google Scholar] [CrossRef] [PubMed]
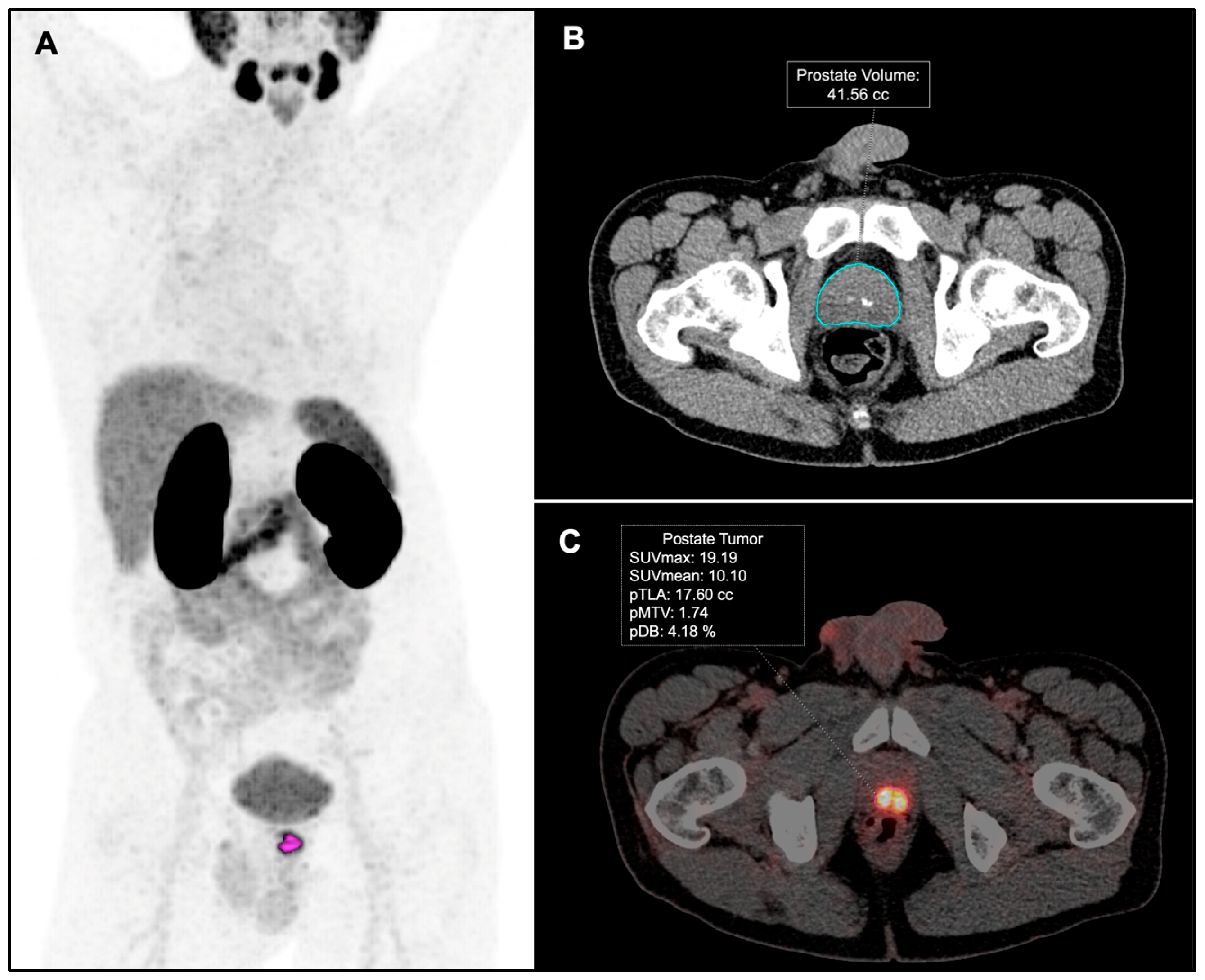


| Characteristics | n (%)—Median (IQR) |
|---|---|
| EAU Risk Classification | |
| Intermediate-Risk | 57 (48.3) |
| High-Risk | 61 (51.7) |
| Age (years) | 65 years (61–70) |
| Biopsy ISUP Grade Group (Gleason Score) | |
| Group 1 (3 + 3) | 24 (20.3) |
| Group 2 (3 + 4) | 28 (23.7) |
| Group 3 (4 + 3) | 21 (17.8) |
| Group 4 (8) | 29 (24.6) |
| Group 5 (9–10) | 16 (13.6) |
| PSA at PET (ng/mL) | 10.8 ng/mL (7.1–16.9) |
| Time between biopsy and PSMA-PET | 34 days (23–63) |
| PSMA-PET Metrics | Intermediate Risk Median (IQR) | High Risk Median (IQR) | p * |
|---|---|---|---|
| SUVmax | 9.4 (5.8–12.8) | 10.1 (6.3–18.0) | 0.195 |
| SUVmean | 5.9 (3.6–7.6) | 5.8 (3.7–9.0) | 0.503 |
| Liver SUVmean | 4.6 (3.7–5.4) | 4.6 (3.8–5.7) | 0.650 |
| TBR-liver | 1.5 (1.0–2.1) | 1.6 (1.0–2.8) | 0.482 |
| TBR-spleen | 1.3 (0.7–2.0) | 1.1 (0.7–2.0) | 0.959 |
| TBR-blood pool | 6.7 (4.3–9.2) | 6.7 (4.5–11.1) | 0.275 |
| TBR-salivary glands | 0.5 (0.3–0.7) | 0.6 (0.3–1.1) | 0.320 |
| pMTV (cc) | 3.6 (1.9–6.0) | 4.6 (3.0–6.6) | 0.022 |
| pTLA | 19.1 (11.8–25.4) | 29.4 (15.3–54.4) | 0.001 |
| pDB (%) | 6.8 (3.4–9.6) | 9.2 (5.6–14.5) | 0.007 |
| pV (cc) | 52.9 (41.2–70.5) | 51.3 (40.1–6.9) | 0.504 |
| PSMA-PET Metrics | Undetectable PSA After RP Median (IQR) | PSA Persistence Median (IQR) | p-Value * |
|---|---|---|---|
| Tumor SUVmax | 9.4 (5.8–13.1) | 11.6 (8.7–22.8) | 0.071 |
| Tumor SUVmean | 5.2 (3.7–7.6) | 6.33 (5.4–12.3) | 0.062 |
| Liver SUVmean | 4.6 (3.8–5.6) | 4.4 (3.5–5.1) | 0.211 |
| TBR-liver | 1.5 (1.0–2.1) | 2.2 (1.2–2.8) | 0.083 |
| TBR-spleen | 1.1 (0.7–2.0) | 1.58 (1.2–2.8) | 0.065 |
| TBR-blood pool | 6.4 (4.5–9.2) | 9.7 (5.2–11.3) | 0.090 |
| TBR-salivary glands | 0.5 (0.3–0.8) | 0.71 (0.4–1.2) | 0.050 |
| pMTV | 3.6 (2.1–5.8) | 8.6 (4.1–14.1) | <0.001 |
| pTLA | 19.3 (11.8–27.7) | 44.2 (30.8–74.5) | <0.001 |
| pDB | 6.9 (3.9–10.1) | 13.2 (8.7–22.3) | 0.001 |
| pV | 51.4 (40.5–69.0) | 54.00 (43.8–72.8) | 0.750 |
| PET/CT Metrics | Cut-Off | Sensitivity % (95% CI) | Specificity % (95% CI) | YI | AUC | p-Value * |
|---|---|---|---|---|---|---|
| pMTV | 4.7 | 69.6 (47.1–86.8) | 64.2 (53.7–73.8) | 0.338 | 0.754 (0.643–0.865) | <0.001 |
| pTLA | 25.1 | 91.3 (71.9–98.9) | 66.3 (55.9–75.7) | 0.576 | 0.831 (0.743–0.918) | <0.001 |
| pDB | 9.1 | 73.9 (51.6–89.7) | 66.3 (55.9–75.7) | 0.402 | 0.758 (0.646–0.869) | <0.001 |
| Parameters (n) | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value * | |
| pN1 (16) | 39.8 (9.6–164.1) | <0.001 | 15.6 (3.3–73.6) | 0.001 |
| pMTV > 4.7 (50) | 4.1 (1.5–10.9) | 0.005 | 0.8 (0.21–3.5) | 0.842 |
| pTLA > 25.1 (53) | 20.6 (4.5–93.7) | <0.001 | 7.4 (1.4–39.1) | 0.017 |
| pDB > 9.1 (49) | 5.5 (2–15.2) | <0.001 | 2.64 (0.58–12) | 0.209 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosales, J.J.; Betech-Antar, V.; Mínguez, F.; Guillén, E.F.; Prieto, E.; Quincoces, G.; Beorlegui, C.; Fenor de la Maza, M.D.; Díez-Caballero, F.; Miñana, B.; et al. Association of [68Ga]Ga-PSMA-11 PET/CT Metrics with PSA Persistence Following Radical Prostatectomy in Patients with Intermediate- and High-Risk Prostate Cancer. Diagnostics 2025, 15, 301. https://doi.org/10.3390/diagnostics15030301
Rosales JJ, Betech-Antar V, Mínguez F, Guillén EF, Prieto E, Quincoces G, Beorlegui C, Fenor de la Maza MD, Díez-Caballero F, Miñana B, et al. Association of [68Ga]Ga-PSMA-11 PET/CT Metrics with PSA Persistence Following Radical Prostatectomy in Patients with Intermediate- and High-Risk Prostate Cancer. Diagnostics. 2025; 15(3):301. https://doi.org/10.3390/diagnostics15030301
Chicago/Turabian StyleRosales, Juan J., Vicky Betech-Antar, Fernando Mínguez, Edgar F. Guillén, Elena Prieto, Gemma Quincoces, Carmen Beorlegui, María Dolores Fenor de la Maza, Fernando Díez-Caballero, Bernardino Miñana, and et al. 2025. "Association of [68Ga]Ga-PSMA-11 PET/CT Metrics with PSA Persistence Following Radical Prostatectomy in Patients with Intermediate- and High-Risk Prostate Cancer" Diagnostics 15, no. 3: 301. https://doi.org/10.3390/diagnostics15030301
APA StyleRosales, J. J., Betech-Antar, V., Mínguez, F., Guillén, E. F., Prieto, E., Quincoces, G., Beorlegui, C., Fenor de la Maza, M. D., Díez-Caballero, F., Miñana, B., Pérez-Gracia, J. L., & Rodríguez-Fraile, M. (2025). Association of [68Ga]Ga-PSMA-11 PET/CT Metrics with PSA Persistence Following Radical Prostatectomy in Patients with Intermediate- and High-Risk Prostate Cancer. Diagnostics, 15(3), 301. https://doi.org/10.3390/diagnostics15030301







