Claudin-4 Overexpression Predicts Poor Survival and Platinum Resistance in Epithelial Ovarian Cancer: A Potential Biomarker for Clinical Decision-Making †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Pat3ient Selection
2.2. Definitions and Endpoints
2.3. Sample Preparation and Immunohistochemistry
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Survival Outcomes
3.3. Platinum Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EOC | Epithelial ovarian cancer |
| ADCs | Antibody–drug conjugates |
| FRα | Folate receptor alpha |
| OS | Overall survival |
| DFS | Disease-free survival |
| SD | Standard deviation |
| IQR | Interquartile range |
| HR | Hazard ratio |
| CI | Confidence intervals |
| OR | Odds ratio |
| FIGO | International Federation of Gynecology and Obstetrics |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Renz, M.; Friedlander, M.; Berek, J.S. Cancer of the ovary, fallopian tube, and peritoneum: 2025 update. Int. J. Gynecol. Obstet. 2025, 171, 6–35. [Google Scholar] [CrossRef]
- Ledermann, J.; Matias-Guiu, X.; Amant, F.; Concin, N.; Davidson, B.; Fotopoulou, C.; González-Martin, A.; Gourley, C.; Leary, A.; Lorusso, D. ESGO–ESMO–ESP consensus conference recommendations on ovarian cancer: Pathology and molecular biology and early, advanced and recurrent disease. Ann. Oncol. 2024, 35, 248–266. [Google Scholar] [CrossRef]
- Karam, A.; Ledermann, J.; Kim, J.-W.; Sehouli, J.; Lu, K.; Gourley, C.; Katsumata, N.; Burger, R.; Nam, B.-H.; Bacon, M. Fifth ovarian cancer consensus conference of the gynecologic cancer intergroup: First-line interventions. Ann. Oncol. 2017, 28, 711–717. [Google Scholar] [CrossRef]
- Perez-Fidalgo, J.A.; Gálvez-Montosa, F.; Guerra, E.M.; Madariaga, A.; Manzano, A.; Martin-Lorente, C.; Gaba, L. SEOM–GEICO Clinical Guideline on Epithelial Ovarian Cancer (2023). Clin. Transl. Oncol. 2024, 26, 2758–2770. [Google Scholar] [CrossRef]
- Morand, S.; Devanaboyina, M.; Staats, H.; Stanbery, L.; Nemunaitis, J. Ovarian Cancer Immunotherapy and Personalized Medicine. Int. J. Mol. Sci. 2021, 22, 6532. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Zhu, X.; Zhong, L.; Jiang, Q.; Wang, Y.; Zou, D. Drug Resistance in Ovarian Cancer: From Mechanism to Clinical Trial. Mol. Cancer 2024, 23, 66. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Leary, A.; Pignata, S.; Cropet, C.; González-Martín, A.; Marth, C.; Harter, P. Olaparib plus Bevacizumab First-Line Maintenance in Ovarian Cancer: Final Overall Survival Results from the PAOLA-1/ENGOT-ov25 Trial. Ann. Oncol. 2023, 34, 681–692. [Google Scholar] [CrossRef]
- Moore, K.N.; Angelergues, A.; Konecny, G.E.; García, Y.; Banerjee, S.; Lorusso, D.; Lee, J.-Y.; Moroney, J.W.; Colombo, N.; Roszak, A. Mirvetuximab soravtansine in FRα-positive, platinum-resistant ovarian cancer. N. Engl. J. Med. 2023, 389, 2162–2174. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Lorusso, D.; Oaknin, A.; Pignata, S.; Dean, A.; Denys, H.; Coleman, R.L. Efficacy and Safety of Mirvetuximab Soravtansine in Patients with Platinum-Resistant Ovarian Cancer with High Folate Receptor Alpha Expression: Results from the SORAYA Study. J. Clin. Oncol. 2023, 41, 2436–2445. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I. ESMO–ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int. J. Gynecol. Cancer 2019, 29, 728–760. [Google Scholar] [CrossRef]
- Niessen, C.M. Tight junctions/adherens junctions: Basic structure and function. J. Investig. Dermatol. 2007, 127, 2525–2532. [Google Scholar] [CrossRef]
- Jeon, H.; Sterpi, M.; Mo, C.; Bteich, F. Claudins: From Gatekeepers of Epithelial Integrity to Potential Targets in Hepato-Pancreato-Biliary Cancers. Front. Oncol. 2024, 14, 1454882. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, M. The Role of Claudin-4 in the Development of Gastric Cancer. Scand. J. Gastroenterol. 2020, 55, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Miao, H.; Jing, B.; Pan, Q.; Zhang, H.; Chen, Y.; Li, M. Claudin-4 Controls the Proliferation, Apoptosis, Migration and In Vivo Growth of MCF-7 Breast Cancer Cells. Oncol. Rep. 2015, 34, 681–690. [Google Scholar] [CrossRef]
- Hough, C.D.; Sherman-Baust, C.A.; Pizer, E.S.; Montz, F.; Im, D.D.; Rosenshein, N.B.; Cho, K.R.; Riggins, G.J.; Morin, P.J. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000, 60, 6281–6287. [Google Scholar]
- Hashimoto, Y.; Okada, Y.; Shirakura, K.; Tachibana, K.; Sawada, M.; Yagi, K.; Doi, T.; Kondoh, M. Anti-claudin antibodies as a concept for development of claudin-directed drugs. J. Pharmacol. Exp. Ther. 2019, 368, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bitler, B.G.; Lang, J.; Nunez-Avellaneda, D.; Behbakht, K.; Davidson, N.R.; Woodruff, E.R.; Villagomez, F.R. Claudin-4 as a Dual Regulator of Genome Stability and Immune Evasion in High-Grade Serous Ovarian Cancer. Sci. Rep. 2025, 15, 39257. [Google Scholar] [CrossRef]
- Bhat, A.A.; Uppada, S.; Achkar, I.W.; Hashem, S.; Yadav, S.K.; Shanmugakonar, M.; Uddin, S. Tight Junction Proteins and Signaling Pathways in Cancer and Inflammation: A Functional Crosstalk. Front. Physiol. 2019, 9, 194. [Google Scholar] [CrossRef]
- Hicks, D.A.; Galimanis, C.E.; Webb, P.G.; Spillman, M.A.; Behbakht, K.; Neville, M.C.; Baumgartner, H.K. Claudin-4 activity in ovarian tumor cell apoptosis resistance and migration. BMC Cancer 2016, 16, 788. [Google Scholar] [CrossRef] [PubMed]
- Rangel, L.B.; Agarwal, R.; D’Souza, T.; Pizer, E.S.; Alo, P.L.; Lancaster, W.D.; Gregoire, L.; Schwartz, D.R.; Cho, K.R.; Morin, P.J. Tight junction proteins claudin-3 and claudin-4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas. Clin. Cancer Res. 2003, 9, 2567–2575. [Google Scholar] [PubMed]
- Hagen, S.J. Non-canonical functions of claudin proteins: Beyond the regulation of cell-cell adhesions. Tissue Barriers 2017, 5, e1327839. [Google Scholar] [CrossRef]
- Yoshida, H.; Sumi, T.; Zhi, X.; Yasui, T.; Honda, K.-I.; Ishiko, O. Claudin-4: A potential therapeutic target in chemotherapy-resistant ovarian cancer. Anticancer Res. 2011, 31, 1271–1277. [Google Scholar]
- Breed, C.; Hicks, D.A.; Webb, P.G.; Galimanis, C.E.; Bitler, B.G.; Behbakht, K.; Baumgartner, H.K. Ovarian tumor cell expression of claudin-4 reduces apoptotic response to paclitaxel. Mol. Cancer Res. 2019, 17, 741–750. [Google Scholar] [CrossRef]
- Hegab, H.M.; Malis, M.E.S.; Mostafa, M.F.; El-Saaba, B.M.; El-Agwany, A.S.; Tawfik, R.T. Claudin-4 in Ovarian Cancer and Its Relation to Platinum Compounds Resistance. Progr. Obstet. Ginecol. 2015, 58, 269–274. [Google Scholar] [CrossRef]
- Li, J.; Chigurupati, S.; Agarwal, R.; Mughal, M.R.; Mattson, M.P.; Becker, K.G.; Wood, I.; William, H.; Zhang, Y.; Morin, P.J. Possible angiogenic roles for claudin-4 in ovarian cancer. Cancer Biol. Ther. 2009, 8, 1806–1814. [Google Scholar] [CrossRef]
- Shitara, K.; Lordick, F.; Bang, Y.-J.; Enzinger, P.; Ilson, D.; Shah, M.A.; Van Cutsem, E.; Xu, R.-H.; Aprile, G.; Xu, J. Zolbetuximab plus mFOLFOX6 in patients with CLDN18. 2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2023, 401, 1655–1668. [Google Scholar] [CrossRef]
- Villagomez, F.R.; Lang, J.; Nunez-Avellaneda, D.; Behbakht, K.; Dimmick, H.L.; Webb, P.G.; Nephew, K.P.; Neville, M.; Woodruff, E.R.; Bitler, B.G. Claudin-4 Stabilizes the Genome via Nuclear and Cell-Cycle Remodeling to Support Ovarian Cancer Cell Survival. Cancer Res. Commun. 2025, 5, 39–53. [Google Scholar] [CrossRef]
- Naimi, A.; Zare, N.; Amjadi, E.; Soltan, M. High claudin-4 antigen expression in triple-negative breast cancer by the immunohistochemistry method. J. Res. Med. Sci. 2022, 27, 20. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, F.; Cocco, E.; Bellone, S.; Richter, C.E.; Bellone, M.; Todeschini, P.; Siegel, E.; Varughese, J.; Arin-Silasi, D.; Azodi, M. Eradication of chemotherapy-resistant CD44+ human ovarian cancer stem cells in mice by intraperitoneal administration of clostridium perfringens enterotoxin. Cancer 2011, 117, 5519–5528. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, X.; McClane, B.; Zeng, Q.; Litkouhi, B.; Welch, W.R.; Berkowitz, R.S.; Mok, S.C.; Garner, E.I. C terminus of Clostridium perfringens enterotoxin downregulates CLDN4 and sensitizes ovarian cancer cells to Taxol and Carboplatin. Clin. Cancer Res. 2011, 17, 1065–1074. [Google Scholar] [CrossRef]
- Yamamoto, T.M.; Webb, P.G.; Davis, D.M.; Baumgartner, H.K.; Woodruff, E.R.; Guntupalli, S.R.; Neville, M.; Behbakht, K.; Bitler, B.G. Loss of claudin-4 reduces DNA damage repair and increases sensitivity to PARP inhibitors. Mol. Cancer Ther. 2022, 21, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Lei, L.; Wang, Y.; Tian, X.; Wei, X.; Jiang, N.; Liu, L. CLDN4 as a novel diagnostic and prognostic biomarker and its association with immune infiltrates in ovarian cancer. Mediat. Inflamm. 2023, 2023, 1075265. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.; White, J.T.; Yan, X.; Collins, S.; Drescher, C.W.; Urban, N.D.; Hood, L.; Lin, B. Proteins associated with Cisplatin resistance in ovarian cancer cells identified by quantitative proteomic technology and integrated with mRNA expression levels. Mol. Cell. Proteom. 2006, 5, 433–443. [Google Scholar] [CrossRef]
- Litkouhi, B.; Kwong, J.; Lot, C.-M.; Smedley, J.G., III; McClane, B.A.; Aponte, M.; Gao, Z.; Sarno, J.L.; Hinners, J.; Welch, W.R. Claudin-4 overexpression in epithelial ovarian cancer is associated with hypomethylation and is a potential target for modulation of tight junction barrier function using a C-terminal fragment of Clostridium perfringens enterotoxin. Neoplasia 2007, 9, 304–314. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, L.M.; Malander, S.; Hartman, L.; Jönsson, J.-M.; Ebbesson, A.; Nilbert, M.; Måsbäck, A.; Hedenfalk, I. Claudin-4 expression is associated with survival in ovarian cancer but not with chemotherapy response. Int. J. Gynecol. Pathol. 2018, 37, 101–109. [Google Scholar] [CrossRef]
- Ondič, O.; Šidlová, H.; Alaghehbandan, R. Claudin-4 expression is associated with survival in ovarian cancer but not with chemotherapy response. Int. J. Gynecol. Pathol. 2020, 39, e1. [Google Scholar] [CrossRef]
- Fujiwara-Tani, R.; Mori, S.; Ogata, R.; Sasaki, R.; Ikemoto, A.; Kishi, S.; Kondoh, M.; Kuniyasu, H. Claudin-4: A new molecular target for epithelial cancer therapy. Int. J. Mol. Sci. 2023, 24, 5494. [Google Scholar] [CrossRef]
- Kuwada, M.; Chihara, Y.; Luo, Y.; Li, X.; Nishiguchi, Y.; Fujiwara, R.; Sasaki, T.; Fujii, K.; Ohmori, H.; Fujimoto, K. Pro-chemotherapeutic effects of antibody against extracellular domain of claudin-4 in bladder cancer. Cancer Lett. 2015, 369, 212–221. [Google Scholar] [CrossRef]
- Kato-Nakano, M.; Suzuki, M.; Kawamoto, S.; Furuya, A.; Ohta, S.; Nakamura, K.; Ando, H. Characterization and evaluation of the antitumour activity of a dual-targeting monoclonal antibody against claudin-3 and claudin-4. Anticancer Res. 2010, 30, 4555–4562. [Google Scholar] [PubMed]
- Fujiwara-Tani, R.; Sasaki, T.; Luo, Y.I.; Goto, K.; Kawahara, I.; Nishiguchi, Y.; Kuniyasu, H. Anti-Claudin-4 Extracellular Domain Antibody Enhances the Antitumoral Effects of Chemotherapeutic and Antibody Drugs in Colorectal Cancer. Oncotarget 2018, 9, 37367–37378. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, I.; Qi, C.; Chen, Y.; Nakamura, Y.; Shen, L.; Shitara, K. Claudin 18.2 as a novel therapeutic target. Nat. Rev. Clin. Oncol. 2024, 21, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Greer, A.; Gockley, A.; Manning-Geist, B.; Melamed, A.; Sisodia, R.C.; Berkowitz, R.; Horowitz, N.; Del Carmen, M.; Growdon, W.B.; Worley, M., Jr. Impact of residual disease at interval debulking surgery on platinum resistance and patterns of recurrence for advanced-stage ovarian cancer. Int. J. Gynecol. Cancer 2021, 31, 1341–1347. [Google Scholar] [CrossRef]
- Kutlu, O.; Ozdemir, O.; Ayaz, D.; Akdemir, C.; Sanci, M. 1140P Claudin-4 expression in epithelial ovarian cancer: A prognostic biomarker with possible predictive role in platinum resistance. Ann. Oncol. 2025, 36 (Suppl. S2), S756. [Google Scholar] [CrossRef]
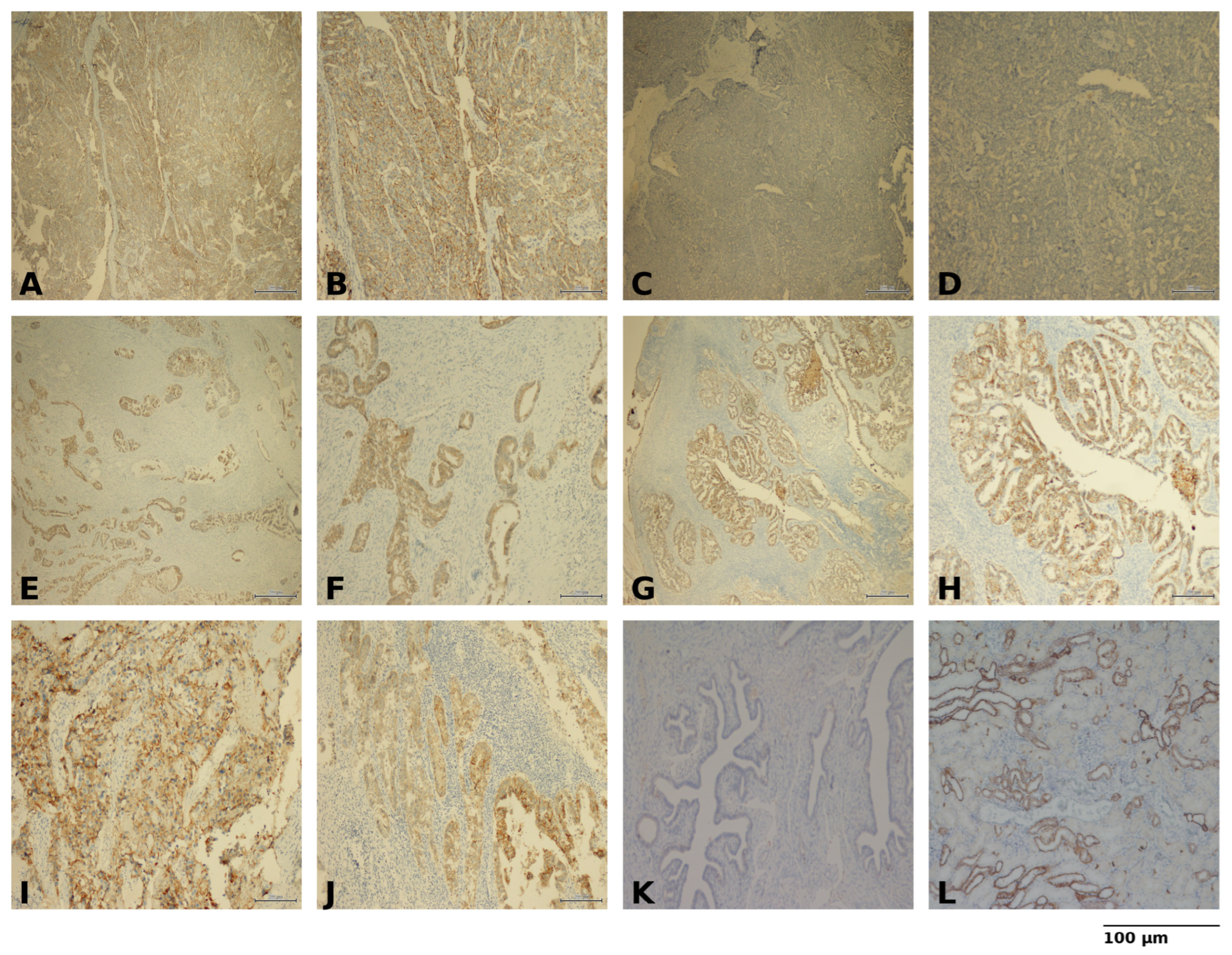

| Variables | Total (N = 83) | Low Claudin-4 (N = 37) | High Claudin-4 (N = 46) | p-Value a |
|---|---|---|---|---|
| Age (years) | ||||
| Mean ± SD | 54.24 ± 10.30 | 52.75 ± 8.58 | 55.43 ± 11.45 | 0.242 b |
| <60 | 58 (69.9%) | 29 (78.4%) | 29 (63.0%) | 0.130 |
| ≥60 | 25 (30.1%) | 8 (21.6%) | 17 (37.0%) | |
| Histology | ||||
| Serous | 72 (86.7%) | 33 (89.2%) | 39 (84.8%) | 0.747 |
| Others * | 11 (13.3%) | 4 (10.8%) | 7 (15.2%) | |
| Stage (FIGO) | ||||
| I–II | 27 (32.5%) | 15 (40.5%) | 12 (26.1%) | 0.162 |
| III–IV | 56 (67.5%) | 22 (59.5%) | 34 (73.9%) | |
| gBRCA mutation † | ||||
| Negative | 32 (38.6%) | 13 (35.1%) | 19 (41.3%) | 0.696 |
| Positive | 15 (18.1%) | 7 (18.9%) | 8 (17.4%) | |
| Timing of Surgery | ||||
| Primary | 55 (66.3%) | 26 (70.3%) | 29 (63.0%) | 0.489 |
| Interval | 28 (33.7%) | 11 (29.7%) | 17 (37.0%) | |
| Residual Disease | ||||
| R0 | 68 (81.9%) | 31 (83.8%) | 37 (80.4%) | 0.693 |
| R1 | 15 (18.1%) | 6 (16.2%) | 9 (19.6%) | |
| Platinum Resistance ‡ | ||||
| No | 72 (86.7%) | 36 (97.3%) | 36 (78.3%) | 0.019 |
| Yes | 11 (13.3%) | 1 (2.7%) | 10 (21.7%) | |
| Recurrence | ||||
| No | 35 (42.2%) | 22 (59.5%) | 13 (28.3%) | 0.004 |
| Yes | 48 (57.8%) | 15 (40.5%) | 33 (71.7%) | |
| Mortality | ||||
| No | 64 (77.1%) | 34 (91.9%) | 30 (65.2%) | 0.004 |
| Yes | 19 (22.9%) | 3 (8.1%) | 16 (34.8%) |
| Univariate | ||||||
|---|---|---|---|---|---|---|
| Variables | N | DFS HR (95% CI) | p-Value | OS HR (95% CI) | p-Value | |
| Age (years) | <60 | 58 | Reference | — | Reference | — |
| ≥60 | 25 | 1.55 (0.86–2.76) | 0.151 | 1.02 (0.36–2.70) | 0.961 | |
| Histology | Serous | 72 | Reference | — | Reference | — |
| Others * | 11 | 0.25 (0.04–1.04) | 0.056 | 0.45 (0.02–2.18) | 0.377 | |
| Stage (FIGO) | I–II | 27 | Reference | — | Reference | — |
| III–IV | 56 | 4.30 (1.90–9.76) | <0.001 | 4.08 (1.17–25.75) | 0.025 | |
| gBRCA mutation † | Negative | 32 | Reference | — | Reference | — |
| Positive | 15 | 0.51 (0.21–1.14) | 0.103 | 0.46 (0.02–5.10) | 0.526 | |
| Timing of Surgery | Primary | 55 | Reference | — | Reference | — |
| Interval | 28 | 3.38 (1.91–6.00) | <0.001 | 2.86 (1.15–7.42) | 0.024 | |
| Platinum Resistance ‡ | No | 72 | Reference | — | Reference | — |
| Yes | 11 | 11.88 (5.17–26.53) | <0.001 | 8.89 (3.17–23.63) | <0.001 | |
| Claudin-4 Expression | Low | 37 | Reference | — | Reference | — |
| High | 46 | 3.03 (1.63–5.64) | <0.001 | 5.47 (1.78–23.89) | 0.002 | |
| Residual Disease | R0 | 68 | Reference | — | Reference | |
| R1 | 15 | 1.38 (0.68–2.77) | 0.369 | 0.56 (0.09–1.96) | 0.405 | |
| Variables | N | Multivariate | ||||
|---|---|---|---|---|---|---|
| DFS | OS | |||||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |||
| Stage (FIGO) | I–II | 27 | Reference | — | Reference | — |
| III–IV | 56 | 2.25 (0.93–6.04) | 0.072 | 1.81 (0.4–12.72) | 0.464 | |
| Timing of Surgery | Primary | 55 | Reference | — | Reference | — |
| Interval | 28 | 2.07 (1.02–4.25) | 0.043 | 1.53 (0.50–5.0) | 0.456 | |
| Platinum resistance ‡ | No | 72 | Reference | — | Reference | — |
| Yes | 11 | 4.99 (2.06–12.0) | 0.0005 | 4.27 (1.36–13.53) | 0.014 | |
| Claudin-4 Expression | Low | 37 | Reference | — | Reference | — |
| High | 46 | 2.46 (1.25–4.98) | 0.009 | 3.59 (1.02–17.26) | 0.046 | |
| Variables | Univariate OR (95% CI) | p-Value | Multivariate OR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | <60 | Reference | — | — | — |
| ≥60 | 0.85 (0.17–3.27) | 0.824 | — | — | |
| Histology | Serous | Reference | — | — | — |
| Others * | 0.62 (0.03–3.81) | 0.648 | — | — | |
| Stage (FIGO) | I–II | Reference | — | — | — |
| III–IV | 2.39 (0.56–16.5) | 0.254 | — | — | |
| Timing of Surgery | Primary | Reference | — | Reference | — |
| Interval | 4.25 (1.16–17.7) | 0.029 | 4.19 (1.08–18.4) | 0.038 | |
| Residual Disease | R0 | Reference | — | — | — |
| R1 | 1.01 (0.14–4.52) | 0.992 | — | — | |
| Claudin-4 Expression | Low | Reference | — | Reference | — |
| High | 10.0 (1.78–188.3) | 0.006 | 9.88 (1.70–188.8) | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutlu, Ö.; Günenç, D.; Ayaz, D.; Özdemir, Ö.; Taşkaynatan, H.; Akdemir, C.; Sancı, M. Claudin-4 Overexpression Predicts Poor Survival and Platinum Resistance in Epithelial Ovarian Cancer: A Potential Biomarker for Clinical Decision-Making. Diagnostics 2025, 15, 3163. https://doi.org/10.3390/diagnostics15243163
Kutlu Ö, Günenç D, Ayaz D, Özdemir Ö, Taşkaynatan H, Akdemir C, Sancı M. Claudin-4 Overexpression Predicts Poor Survival and Platinum Resistance in Epithelial Ovarian Cancer: A Potential Biomarker for Clinical Decision-Making. Diagnostics. 2025; 15(24):3163. https://doi.org/10.3390/diagnostics15243163
Chicago/Turabian StyleKutlu, Özlem, Damla Günenç, Duygu Ayaz, Özlem Özdemir, Halil Taşkaynatan, Celal Akdemir, and Muzaffer Sancı. 2025. "Claudin-4 Overexpression Predicts Poor Survival and Platinum Resistance in Epithelial Ovarian Cancer: A Potential Biomarker for Clinical Decision-Making" Diagnostics 15, no. 24: 3163. https://doi.org/10.3390/diagnostics15243163
APA StyleKutlu, Ö., Günenç, D., Ayaz, D., Özdemir, Ö., Taşkaynatan, H., Akdemir, C., & Sancı, M. (2025). Claudin-4 Overexpression Predicts Poor Survival and Platinum Resistance in Epithelial Ovarian Cancer: A Potential Biomarker for Clinical Decision-Making. Diagnostics, 15(24), 3163. https://doi.org/10.3390/diagnostics15243163






