The Evolving Role of Cine MRI in Crohn’s Disease: From Functional Motility Analysis to Precision Management: A Review of the Last 10 Years
Abstract
1. Introduction:
2. Motility Scan Procedures
3. Methods
3.1. Databases and Search Strategy
3.2. Eligibility Criteria
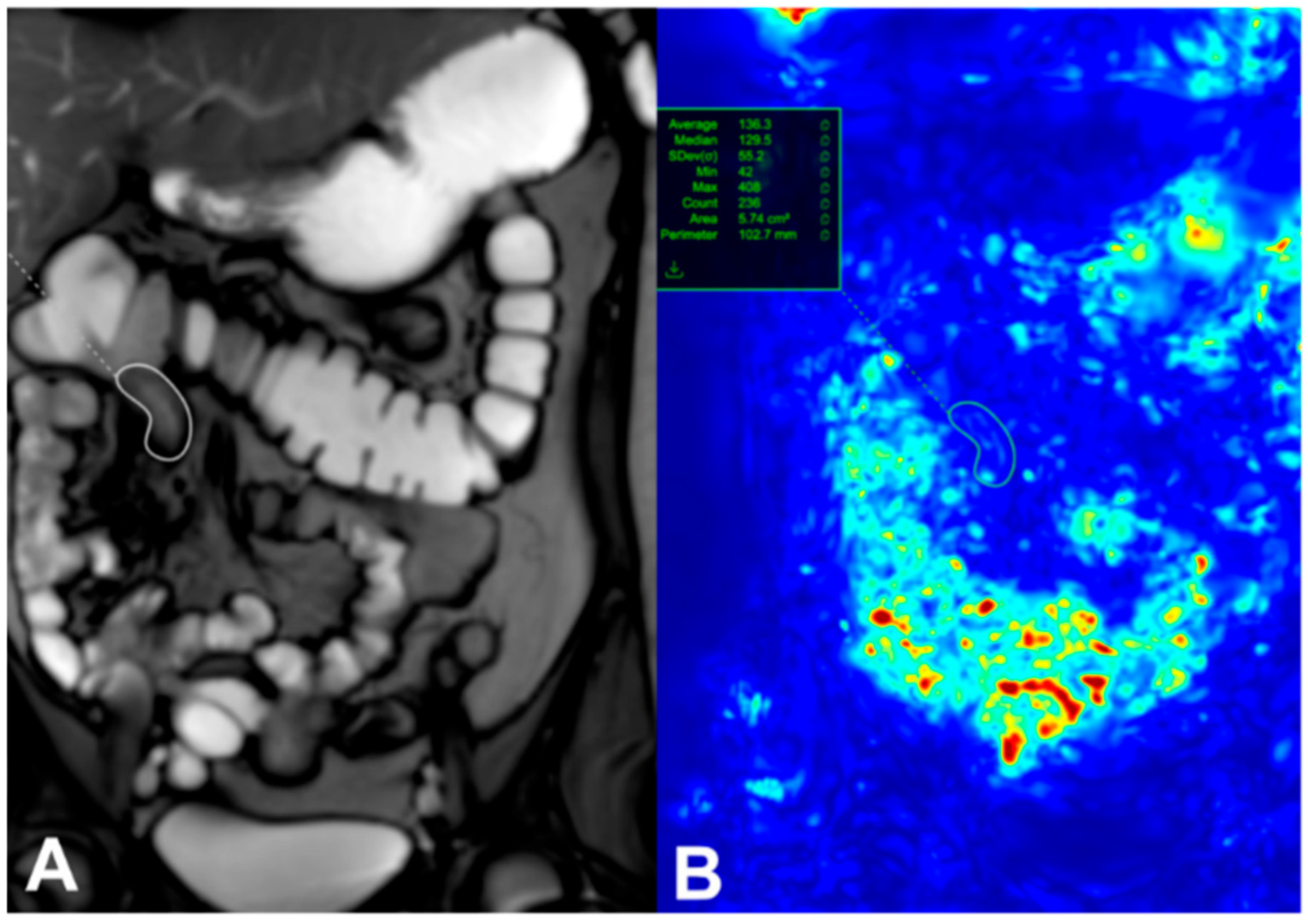
4. Using Cine MRI in CD Motility in Adults and Pediatric
4.1. In Adults
4.2. In Pediatrics
5. Using Cine MRI in CD Treatment in Adults and Pediatric Patients
5.1. In Adults
5.2. In Pediatrics
6. Role of Cine MRI in Differentiating Mural Fibrosis and Active Disease in CD
7. Discussion
8. Future Directions
9. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cockburn, E.; Kamal, S.; Chan, A.; Rao, V.; Liu, T.; Huang, J.Y.; Segal, J.P. Crohn’s disease: An update. Clin. Med. 2023, 23, 549–557. [Google Scholar] [CrossRef]
- Rieder, F.; Fiocchi, C.; Rogler, G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology 2017, 152, 340–350.e6. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Gower–Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794.e4. [Google Scholar] [CrossRef]
- Malagelada, C.; Malagelada, J.R. Small bowel motility. Curr. Gastroenterol. Rep. 2017, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Greenup, A.-J.; Bressler, B.; Rosenfeld, G. Medical imaging in small bowel Crohn’s disease—Computer tomography enterography, magnetic resonance enterography, and ultrasound: “which one is the best for what?”. Inflamm. Bowel Dis. 2016, 22, 1246–1261. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn’s Colitis 2019, 13, 144–164K. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, C.; Dal Buono, A.; Levi, R.; Gabbiadini, R.; Reca, C.; Bezzio, C.; Francone, M.; Armuzzi, A.; Balzarini, L. Reporting of Magnetic Resonance Enterography in Inflammatory Bowel Disease: Results of an Italian Survey. J. Clin. Med. 2024, 13, 3953. [Google Scholar] [CrossRef]
- Rimola, J.; Torres, J.; Kumar, S.; Taylor, S.A.; Kucharzik, T. Recent advances in clinical practice: Advances in cross-sectional imaging in inflammatory bowel disease. Gut 2022, 71, 2587–2597. [Google Scholar] [CrossRef]
- Bassotti, G.; Bologna, S.; Ottaviani, L.; Russo, M.; Dore, M.P. Intestinal manometry: Who needs it? Gastroenterol. Hepatol. Bed Bench 2015, 8, 246. [Google Scholar]
- Rao, S.S.C.; Camilleri, M.; Hasler, W.L.; Maurer, A.H.; Parkman, H.P.; Saad, R.; Scott, M.; Simren, M.; Soffer, E.; Szarka, L. Evaluation of gastrointestinal transit in clinical practice: Position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol. Motil. 2011, 23, 8–23. [Google Scholar] [CrossRef]
- Keller, J.; Bassotti, G.; Clarke, J.; Dinning, P.; Fox, M.; Grover, M.; Hellström, P.M.; Ke, M.; Layer, P.; Malagelada, C. Advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 291–308. [Google Scholar] [CrossRef]
- Sturm, A.; Maaser, C.; Calabrese, E.; Annese, V.; Fiorino, G.; Kucharzik, T.; Vavricka, S.; Verstockt, B.; van Rheenen, P.; Tolan, D. ECCO-ESGAR guideline for diagnostic assessment in inflammatory bowel disease. J. Crohn’s Colitis 2019, 13, 273–284. [Google Scholar] [CrossRef]
- Åkerman, A.; Månsson, S.; Fork, F.T.; Leander, P.; Ekberg, O.; Taylor, S.; Menys, A.; Ohlsson, B. Computational postprocessing quantification of small bowel motility using magnetic resonance images in clinical practice: An initial experience. J. Magn. Reson. Imaging 2016, 44, 277–287. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, C.S.; Smout, A.J.; Nederveen, A.J.; Stoker, J. Evaluation of gastrointestinal motility with MRI: Advances, challenges and opportunities. Neurogastroenterol. Motil. 2018, 30, e13257. [Google Scholar] [CrossRef] [PubMed]
- Odille, F.; Menys, A.; Ahmed, A.; Punwani, S.; Taylor, S.A.; Atkinson, D. Quantitative assessment of small bowel motility by nonrigid registration of dynamic MR images. Magn. Reson. Med. 2012, 68, 783–793. [Google Scholar] [CrossRef]
- Ohkubo, H.; Kessoku, T.; Fuyuki, A.; Iida, H.; Inamori, M.; Fujii, T.; Kawamura, H.; Hata, Y.; Manabe, N.; Chiba, T. Assessment of small bowel motility in patients with chronic intestinal pseudo-obstruction using cine-MRI. Off. J. Am. Coll. Gastroenterol. ACG 2013, 108, 1130–1139. [Google Scholar] [CrossRef]
- Vriesman, M.; de Jonge, C.; Kuizenga-Wessel, S.; Adler, B.; Menys, A.; Nederveen, A.; Stoker, J.; Benninga, M.; Di Lorenzo, C. Simultaneous assessment of colon motility in children with functional constipation by cine-MRI and colonic manometry: A feasibility study. Eur. Radiol. Exp. 2021, 5, 8. [Google Scholar] [CrossRef]
- Fuyuki, A.; Ohkubo, H.; Higurashi, T.; Iida, H.; Inoh, Y.; Inamori, M.; Nakajima, A. Clinical importance of cine-MRI assessment of small bowel motility in patients with chronic intestinal pseudo-obstruction: A retrospective study of 33 patients. J. Gastroenterol. 2017, 52, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Gollifer, R.M.; Menys, A.; Makanyanga, J.; Puylaert, C.A.; Vos, F.M.; Stoker, J.; Atkinson, D.; Taylor, S.A. Relationship between MRI quantified small bowel motility and abdominal symptoms in Crohn’s disease patients—A validation study. Br. J. Radiol. 2018, 91, 20170914. [Google Scholar] [CrossRef]
- Menys, A.; Puylaert, C.; Tutein Nolthenius, C.E.; Plumb, A.A.; Makanyanga, J.; Tielbeek, J.A.; Pendse, D.; Brosens, L.A.; Rodriguez-Justo, M.; Atkinson, D. Quantified terminal ileal motility during MR enterography as a biomarker of Crohn disease activity: Prospective multi-institution study. Radiology 2018, 289, 428–435. [Google Scholar] [CrossRef]
- Wnorowski, A.M.; Guglielmo, F.F.; Mitchell, D.G. How to perform and interpret cine MR enterography. J. Magn. Reson. Imaging 2015, 42, 1180–1189. [Google Scholar] [CrossRef]
- Courtier, J.; Ohliger, M.; Rhee, S.J.; Terreblanche, O.; Heyman, M.B.; MacKenzie, J.D. Shooting a moving target: Use of real-time cine magnetic resonance imaging in assessment of the small bowel. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 426–431. [Google Scholar] [CrossRef]
- Bieri, O.; Scheffler, K. Fundamentals of balanced steady state free precession MRI. J. Magn. Reson. Imaging 2013, 38, 2–11. [Google Scholar] [CrossRef]
- Taylor, S.; Avni, F.; Cronin, C.; Hoeffel, C.; Kim, S.; Laghi, A.; Napolitano, M.; Petit, P.; Rimola, J.; Tolan, D. The first joint ESGAR/ESPR consensus statement on the technical performance of cross-sectional small bowel and colonic imaging. Eur. Radiol. 2017, 27, 2570–2582. [Google Scholar] [CrossRef]
- Anupindi, S.A.; Grossman, A.B.; Nimkin, K.; Mamula, P.; Gee, M.S. Imaging in the evaluation of the young patient with inflammatory bowel disease: What the gastroenterologist needs to know. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 429–439. [Google Scholar] [CrossRef]
- Moy, M.P.; Sauk, J.; Gee, M.S. The role of MR enterography in assessing Crohn’s disease activity and treatment response. Gastroenterol. Res. Pract. 2016, 2016, 8168695. [Google Scholar] [CrossRef]
- Lambrou, T.; Chaudhry, N.A.; Grajo, J.R.; Moser, P.; Riverso, M.; Mramba, L.K.; Zimmermann, E.M. Small bowel stricture is associated with abnormal motility on the cine MRI sequence in patients with Crohn’s disease. Eur. J. Radiol. 2019, 118, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Hahnemann, M.; Nensa, F.; Kinner, S.; Köhler, J.; Gerken, G.; Umutlu, L.; Lauenstein, T. Quantitative assessment of small bowel motility in patients with Crohn’s disease using dynamic MRI. Neurogastroenterol. Motil. 2015, 27, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Menys, A.; Makanyanga, J.; Plumb, A.; Bhatnagar, G.; Atkinson, D.; Emmanuel, A.; Taylor, S.A. Aberrant motility in unaffected small bowel is linked to inflammatory burden and patient symptoms in Crohn’s disease. Inflamm. Bowel Dis. 2016, 22, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Dillman, J.R.; Tkach, J.A.; Imbus, R.; Towbin, A.J.; Denson, L.A. MRI-based characterization of intestinal motility in children and young adults with newly diagnosed ileal Crohn disease treated by biologic therapy: A controlled prospective study. Am. J. Roentgenol. 2022, 219, 655–664. [Google Scholar] [CrossRef]
- Plumb, A.A.; Menys, A.; Russo, E.; Prezzi, D.; Bhatnagar, G.; Vega, R.; Halligan, S.; Orchard, T.R.; Taylor, S.A. Magnetic resonance imaging-quantified small bowel motility is a sensitive marker of response to medical therapy in Crohn’s disease. Aliment. Pharmacol. Ther. 2015, 42, 343–355. [Google Scholar] [CrossRef]
- Plumb, A.A.; Moran, G.; Chowdhury, K.; Ahmed, N.; Philpott, S.; Ahmad, T.; Bloom, S.; Hart, A.; Jacobs, I.; Menys, A. Small bowel motility quantified by cine MRI to predict longer-term response in patients with Crohn’s disease commencing biological therapy: The motility study. Inflamm. Bowel Dis. 2025, 31, 2438–2450. [Google Scholar] [CrossRef] [PubMed]
- Dreja, J.; Ekberg, O.; Leander, P.; Månsson, S.; Ohlsson, B. Volumetric analysis of small bowel motility in an unselected cohort of patients with Crohn’s disease. Neurogastroenterol. Motil. 2020, 32, e13909. [Google Scholar] [CrossRef] [PubMed]
- Beek, K.J.; van Rijn, K.L.; de Jonge, C.S.; de Voogd, F.A.; Buskens, C.J.; van der Bilt, J.; Bemelman, W.; D’Haens, G.; Mookhoek, A.; Neefjes-Borst, E.A. Quantified motility in Crohn’s disease to evaluate stricture composition using cine-MRI. Br. J. Radiol. 2025, 98, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- van Harten, L.D.; de Jonge, C.S.; Struik, F.; Stoker, J.; Išgum, I. Quantitative analysis of small intestinal motility in 3D cine-MRI using centerline-aware motion estimation. J. Magn. Reson. Imaging 2025, 61, 1956–1966. [Google Scholar] [CrossRef]
- Cococcioni, L.; Fitzke, H.; Menys, A.; Gaunt, T.; Kumar, S.; Kiparissi, F.; Rampling, D.; Palm, L.; Taylor, S.A.; Watson, T.A. Quantitative assessment of terminal ileum motility on MR enterography in Crohn disease: A feasibility study in children. Eur. Radiol. 2021, 31, 775–784. [Google Scholar] [CrossRef]
- Meshaka, R.; Fitzke, H.E.; Barber, J.; Jones, K.; Taylor, S.A.; Watson, T.A. Quantified small bowel motility assessment on magnetic resonance enterography in paediatric inflammatory bowel disease–does it reflect clinical response? Pediatr. Radiol. 2024, 54, 2210–2219. [Google Scholar] [CrossRef]
- McSorley, B.; Plunk, M.; Challa, S.A.; Pan, A.Y.; Noe, J. Comparing magnetic resonance enterography and endoscopy findings to the motility of magnetic resonance imaging in pediatric Crohn’s disease. Pediatr. Radiol. 2025, 55, 857–864. [Google Scholar] [CrossRef]
- Hameed, M.; Plumb, A.A.; Chowdhury, K.; Ahmed, N.; Rahman, S.; Bhatnagar, G.; Thomson, E.; Mohsin, M.; Holmes, J.; Halligan, S. Inter-and intra-observer variability of software quantified bowel motility measurements of small bowel Crohn’s disease: Findings from the MOTILITY trial. Insights Into Imaging 2025, 16, 111. [Google Scholar] [CrossRef]
- Peña-Trujillo, V.; Gallo-Bernal, S.; Moran, C.J.; Cortes Albornoz, N.S.; Menys, A.; Gee, M.S. Magnetic resonance imaging-based ileal motility quantification predicts stricture response to biologic therapy in Crohn’s disease. Pediatr. Radiol. 2025, 1–9. [Google Scholar] [CrossRef]
- Kumar, S.; De Kock, I.; Blad, W.; Hare, R.; Pollok, R.; Taylor, S.A. Magnetic resonance enterography and intestinal ultrasound for the assessment and monitoring of Crohn’s disease. J. Crohn’s Colitis 2024, 18, 1450–1463. [Google Scholar] [CrossRef]
- Bruining, D.H.; Zimmermann, E.M.; Loftus, E.V., Jr.; Sandborn, W.J.; Sauer, C.G.; Strong, S.A.; Society of Abdominal Radiology Crohn’s Disease-Focused Panel. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Radiology 2018, 286, 776–799. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, K.L.; Stoker, J.; Menys, A.; de Jonge, C.S. Impact of bowel dilation on small bowel motility measurements with cine-MRI: Assessment of two quantification techniques. BJR Open 2022, 4, 20210049. [Google Scholar] [CrossRef] [PubMed]
- Rimola, J.; Beek, K.J.; Ordás, I.; Gecse, K.B.; Cuatrecasas, M.; Stoker, J. Contemporary imaging assessment of strictures and fibrosis in Crohn disease, with focus on quantitative biomarkers: From the AJR special series on imaging of fibrosis. Am. J. Roentgenol. 2024, 222, e2329693. [Google Scholar] [CrossRef] [PubMed]
- Alfredsson, J.; Wick, M.J. Mechanism of fibrosis and stricture formation in Crohn’s disease. Scand. J. Immunol. 2020, 92, e12990. [Google Scholar] [CrossRef]
- Chen, W.; Lu, C.; Hirota, C.; Iacucci, M.; Ghosh, S.; Gui, X. Smooth muscle hyperplasia/hypertrophy is the most prominent histological change in Crohn’s fibrostenosing bowel strictures: A semiquantitative analysis by using a novel histological grading scheme. J. Crohn’s Colitis 2017, 11, 92–104. [Google Scholar] [CrossRef]
- D’Alessio, S.; Ungaro, F.; Noviello, D.; Lovisa, S.; Peyrin-Biroulet, L.; Danese, S. Revisiting fibrosis in inflammatory bowel disease: The gut thickens. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 169–184. [Google Scholar] [CrossRef]
- Engel, T.; Ben-Horin, S.; Beer-Gabel, M. Autonomic dysfunction correlates with clinical and inflammatory activity in patients with Crohn’s disease. Inflamm. Bowel Dis. 2015, 21, 2320–2326. [Google Scholar]
- de Bruyn, J.R.; Becker, M.A.; Steenkamer, J.; Wildenberg, M.E.; Meijer, S.L.; Buskens, C.J.; Bemelman, W.A.; Löwenberg, M.; Ponsioen, C.Y.; van den Brink, G.R. Intestinal fibrosis is associated with lack of response to Infliximab therapy in Crohn’s disease. PLoS ONE 2018, 13, e0190999. [Google Scholar] [CrossRef]
| Pros | Cons |
|---|---|
|
|
| Population No. Study Type Scanner Ref. | Aim | Reference Standard | Acquisition | Findings |
|---|---|---|---|---|
| Adults | ||||
| CD:53 Adults Prospective 1.5 T or 3 T [29] | Evaluated the correlation between cine MRI quantified small bowel motility, patient symptom burden, and inflammatory activity | fC for inflammatory burden; HBI for subjective symptom burden | Cine, breath-hold MRE; Sequence: TrueFISP (1.5 T) or BTFE (3 T) with temporal resolution: 1 image/s over a 20 s breath hold | A significant negative correlation was found between global motility variance and fc (rho = −0.33, p = 0.015). A highly significant negative correlation was found between global motility variance and total HBI (rho = −0.45, p < 0.001). For each 0.01 unit reduction in global motility variance, the odds of a patient reporting a worse HBI well-being score increased by 0.19 (95% CI, 0.06−0.50; p < 0.001). Se, Sp, or AUC not reported. |
| CD: 82 Adults Prospective/Multi-institutional study 3 T [20] | Assessed the association between the terminal ileal motility and CD activity assessed with both the histopathologic score (eAIS) and endoscopic severity index (CDEIS) | Endoscopic CDEIS Histopathologic eAIS | Cine, breath-hold MRE Sequence (2D coronal TrueFISP/BTFE) acquired during a 22 s breath hold. The temporal resolution of the dynamic images was 1.1 s per section with a section thickness of 10 mm focused on the terminal ileum. | Reduced motility (0.30 au) was highly sensitive for both endoscopically and histologically (92% and 92%, respectively) defined active inflammation, but had moderate Sp (61% and 71%, respectively). MaRIA had 75% Sn and 74% Sp. Terminal ileal motility had a negative correlation with CDEIS (r = −0.59; 95% confidence interval [CI]: 0.7, −0.4) and eAIS (r = −0.61; 95% CI: 0.7, −0.5). TI motility: 0.86, 0.87 (against CDEIS and eAIS, respectively). Terminal ileal motility, evaluated by an automatic algorithm, has a higher Sn and similar specificity to MaRIA. |
| CD: 59 Adults Retrospective 1.5 T and 3 T [27] | To verify the association between intestinal inflammation and reduced motility within the study participants | CD: 59 Adults Retrospective | Coronal cine 2D breath steady state free precession (SSFP) FIESTA (Fast Imaging Employing Steady State Acquisition)/True FISP (Fast Imaging with Steady Precession) sequences and the cine temporal resolution was 1 s per image | A significant association was observed between the presence of a stricture and reduced small bowel motility (OR 0.40, 95% CI 0.17–0.95, p = 0.038). The study reported that in on cine MRI sequences, small bowel strictures in CD patients are associated with decreased small bowel motility. |
| CD:134 HC: 22 Adults Prospective 1.5 T [33] | Assessed value of motility indices derived from MRE images as a biomarker for CD in clinical practice | Signs of inflammation on MRE, endoscopy, and/or elevated lab markers (e.g., CRP, fC) | Free-breathing coronal TrueFISP images were acquired | Motility index of terminal ileum (active CD vs. HCs): AUC: 0.736 (95% CI: 0.625–0.848), p = 0.002. while (active CD vs. inactive CD): AUC: 0.682 (95% CI: 0.579–0.785), p = 0.001. In patients with active CD, the motility index in the terminal ileum and ileum was significantly lower compared to HCs and inversely correlated with mural thickness in the terminal ileum. However, there were no differences in the motility index between HCs and inactive CD. Motility index has poor to acceptable discrimination ability for CD activity. |
| CD:10 HC: 14 Adults Prospective 3 T [35] | To develop a technique for quantifying and characterizing small intestinal motility in 3D cine MRI, to differentiate motile, non-motile, and peristaltic motion patterns | Consensus of two blinded raters | 3D coronal dynamic balanced fast field echo (bFFE) during a breath hold | The mean (range) AUC of 0.81 (0.783–0.819) for the detection of peristalsis and AUC across all folds was 0.97 (0.975–0.979) for the detection of <50% motile segments. Absence of motility was significantly more common in CD compared to HC. Discernible peristalsis was significantly more common in HC compared to CD. The mean absolute velocity of intestinal content was significantly lower in CD (median [IQR] 1.06 [0.61, 1.56] mm/s) compared to HCs (median [IQR] 1.84 [1.37, 2.43] mm/s) (p < 0.001). |
| CD: 28 adults Prospective 3 T [34] | To evaluate the feasibility of distinguishing inflammatory (i.e., inflammatory and mixed) strictures from chronic (i.e., non-inflammatory) strictures in stricturing CD through the use of cine MRI | Histopathology | Coronal dynamic single slice 2D bFFE cine MRI (20 s breath-hold) | AUC for chronic stricture detection (based on pre-stricture dilatation motility): 0.93 (95% CI, 0.78–1.0, p = 0.011). No difference in motility was found between strictures. Motility in the pre-stricture dilatation was significantly higher for chronic (non-inflammatory) strictures (median: 289.5 AU [IQR 188.0–362.9]) compared to inflammatory strictures (median: 113.1 AU [IQR 83.6–142.4]) (p = 0.004). Quantified motility of pre-stricture dilatations showed high accuracy (AUC 0.93) in distinguishing chronic from inflammatory strictures. |
| Pediatric | ||||
| CD: 25 Pediatric Retrospective 1.5 T and 3 T [36] | Examine the association between quantified terminal ileal motility and fC, Crohn Disease MRI Index (CDMI), histopathological activity grading | Primary: Active disease defined as eAIS > 0; Secondary: Crohn Disease MRI Index (CDMI) and fC levels | Coronal bSSFP | Motility index was higher in normal bowel (median: 0.21) compared to inflamed bowel (median: 0.12) (p = 0.02). Motility index has an AUC of 79.2%. Agreement between the two readers was high with an intraclass correlation coefficient of 0.98 (CI 0.95–0.99, p < 0.001). A negative correlation was found between disease activity and terminal ileum motility, indicating that bowel motility decreases as intestinal inflammation intensifies. In active diseases, the motility index decreases and can be a diagnostic marker with medium accuracy. |
| Study, Population, Follow-Up, and Ref. | Reference Standard for Response | Acquisition and Motility Metric | Threshold for Prediction | Outcomes (Se, Sp, AUC, and OR) and Key Findings |
|---|---|---|---|---|
| CD Adults (n = 46) single-center follow-up: Median 55 weeks (retrospective) and after median 12 weeks (prospective) [31] | Primary: Physician global assessment (retro) or ≥3-point HBI drop + no surgery/switch (Prospect). Secondary: CRP normalization, MaRIA < 11. | Acquisition: Coronal Cine (TrueFISP/BTFE), multiple breath-holds. Metric: Change in segmental motility. Scanner: 1.5 T or 3 T. | Any increase in motility from baseline to follow-up. | For Anti-TNFα response: • Se: 93.1% (95% CI: 78.0–98.1%); • Sp: 76.5% (95% CI: 52.7–90.4%); • AUC: 91.5% (82.3–100.0%); • OR (per 0.01 AU increase): 1.24 (1.08–1.43); p = 0.0027. For CRP normalization: greater motility increase in normalized group (73.4% vs. 5.1%, p = 0.0035). For MaRIA < 11: greater motility increase in responders (94.7% vs. 15.2%, p = 0.017). Responders to anti-TNFα treatment showed significantly higher improvements in motility (median = 73.4% increase from baseline) compared to non-responders (median = 25% reduction, p < 0.001). Good agreement between two readers at both baseline and follow-up Cine MRI for small bowel CD (ICC = 0.65, p < 0.001 and ICC = 0.71, p < 0.001, respectively). AUROC: change in motility: 0.93 |
| CD adults (n = 86) prospective, multicenter follow-up: 1 year (Visit 3) [32] | Endoscopic response (≥50% drop in SES-CD or London Index) at 1 year. | Acquisition: Cine MRI. Metric: GIQuant score change (stable/improved). Scanner: 1.5 T or greater. | Stable or improved motility from baseline (V1) to 12–30 weeks (V2). | Cine MRI vs. CRP for predicting 1-yr response: • Se: 71.0% (52.0–85.8%) vs. CRP 45.2%; • Sp: 30.9% (19.1–44.8%) vs. CRP 67.3%; • AUC: 0.48 vs. CRP 0.53 (p = 0.65). Variations in cine MRI, fC, and CRP were unable to predict remission or response reliably at 1 year. Stable bowel motility assessed through cine MRI had high Sn but lower Sp for indicating response or remission. Moderate negative correlation between cine MRI scores and disease activity as quantified by morphological MRE parameters. |
| CD adults (n = 86) prospective, multicenter (agreement study) follow-up: 1 year (Visit 3) [39] | Endoscopic response (≥50% drop in London Index or SES-CD) at 12 months. | Acquisition: Cine MRI. Metric: GIQuant score. Scanner: 1.5 T or greater. | Not Applicable | Agreement: • Inter-observer ICC: 0.59 (95% CI: 0.51–0.66) to 0.70 (95% CI: 0.61–0.78); • Intra-observer ICC: 0.70 (95% CI: 0.44–0.86) and 0.71 (95% CI: 0.44–0.86). |
| Population, Study Type, Follow-Up, and Ref. | Reference Standard for Response | Acquisition and Motility Metric | Threshold for Prediction | Outcomes (Se, Sp, AUC, and OR) and Main Findings |
|---|---|---|---|---|
| CD pediatric/young adult (n = 35); prospective, controlled. Follow-up: baseline, 6 weeks, and 6 months post-treatment. [30] | Diagnosis: CD vs. HCs. Response: Stringent composite clinical/biological remission at 6 months. | Acquisition: Cine MRI. Metric: GIQuant score (FDA-cleared). Scanner: 1.5 T. | For diagnosis (CD vs. HC): normalized score ≤ 0.46. | For diagnosis (CD vs. HC): • Se: 80.0% (95% CI: 56.3–94.3%); • Sp: 93.3% (95% CI: 68.1–99.8%); • AUC: 0.88 (95% CI: 0.72–0.96). For predicting 6-month remission: • Neither baseline nor 6-week change predicted remission (AUC ~0.50–0.71, p > 0.05). • Cine MRI motility increased significantly with treatment (p = 0.03–0.04). • Cine MRI motility correlated negatively with CRP (r = −0.30 to −0.33), ESR (r = −0.35) and wPCDAI (r = −0.36 to −0.43). Excellent: ICC = 0.89 (95% CI: 0.83–0.93). CD patients showed increases in intestinal motility between baseline and 6 weeks after initiation of biologic treatment, while the entire study cohort showed increases between baseline and 6 months after treatment initiation. However, intestinal motility scores in CD patients did not significantly change between six weeks and six months following the start of treatment. |
| CD Pediatric/young adult (n = 40); retrospective. Follow-up: 6 months post-treatment. [40] | Response was defined as sustained symptom improvement with or without imaging improvement, without escalation of medical therapy, with need for discontinuation of anti-TNF agents within 6 months of initiating or adjusting treatment. | Acquisition: Cine MRI. Metric: Mean Motility Score (AU) via GIQuant. Scanner: 1.5 T or 3.0 T. | General: <150 AU (reduced), >300 AU (normal). Analysis: Continuous mean value. | Stricture motility in responders vs. non-responders: Mean: 176.03 ± 128.63 vs. 67.83 ± 33.88 (p = 0.006) • AUC: 0.81 (Overall); 0.75 (Subgroup ≤ 21 years); • OR (for mean): 1.020 (1.00–1.04). Estimated standard deviation for stricture motility also exhibited significant variation, being 72.06 ± 55.55 for responders compared to 30.27 ± 25.79 for non-responders (p = 0.02). Sn and Sp were not explicitly reported. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alyami, A.S. The Evolving Role of Cine MRI in Crohn’s Disease: From Functional Motility Analysis to Precision Management: A Review of the Last 10 Years. Diagnostics 2025, 15, 3078. https://doi.org/10.3390/diagnostics15233078
Alyami AS. The Evolving Role of Cine MRI in Crohn’s Disease: From Functional Motility Analysis to Precision Management: A Review of the Last 10 Years. Diagnostics. 2025; 15(23):3078. https://doi.org/10.3390/diagnostics15233078
Chicago/Turabian StyleAlyami, Ali S. 2025. "The Evolving Role of Cine MRI in Crohn’s Disease: From Functional Motility Analysis to Precision Management: A Review of the Last 10 Years" Diagnostics 15, no. 23: 3078. https://doi.org/10.3390/diagnostics15233078
APA StyleAlyami, A. S. (2025). The Evolving Role of Cine MRI in Crohn’s Disease: From Functional Motility Analysis to Precision Management: A Review of the Last 10 Years. Diagnostics, 15(23), 3078. https://doi.org/10.3390/diagnostics15233078






