STUMP Swiftly Followed by Large Adenomyoma in a Young Nulliparous Patient
Abstract
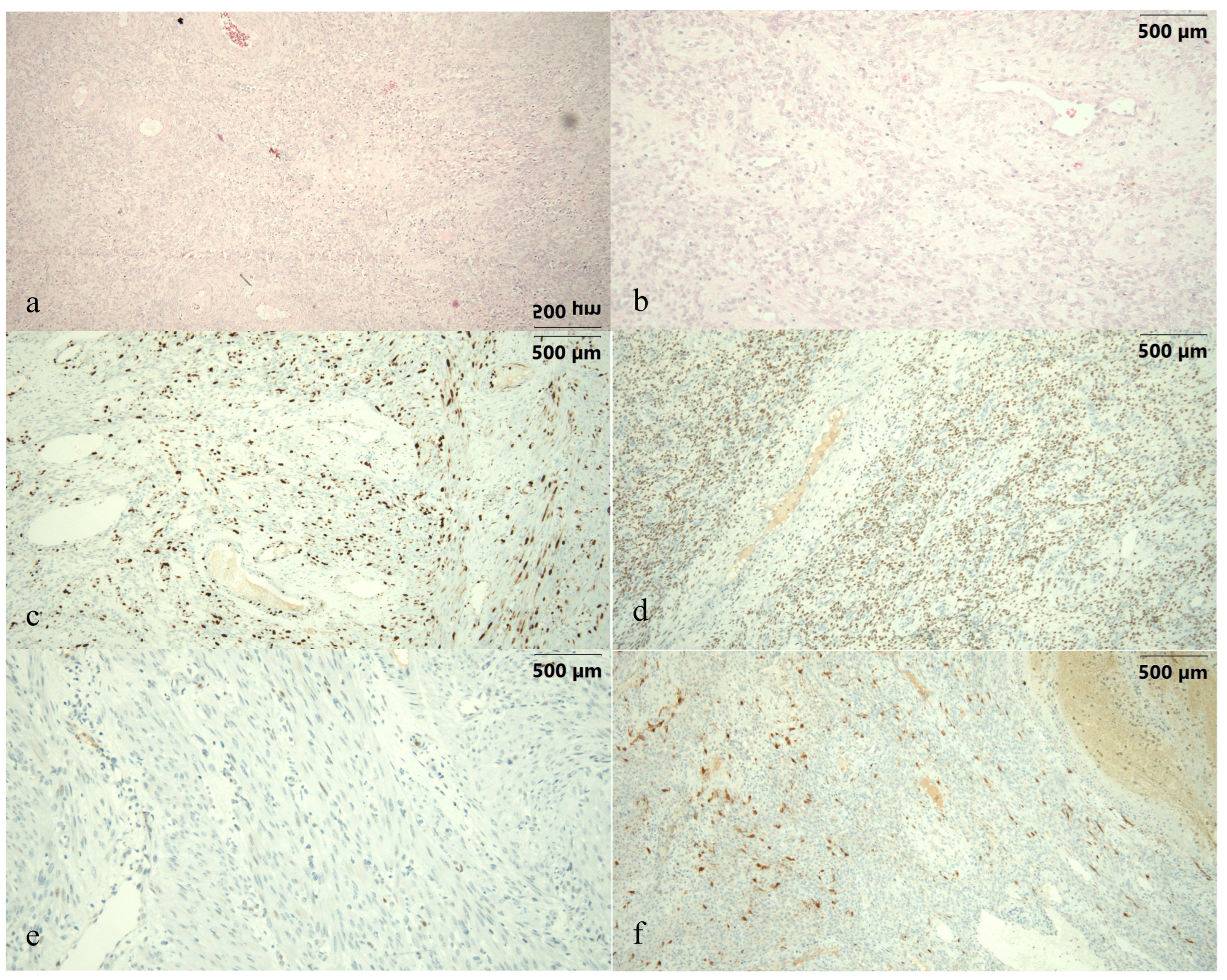
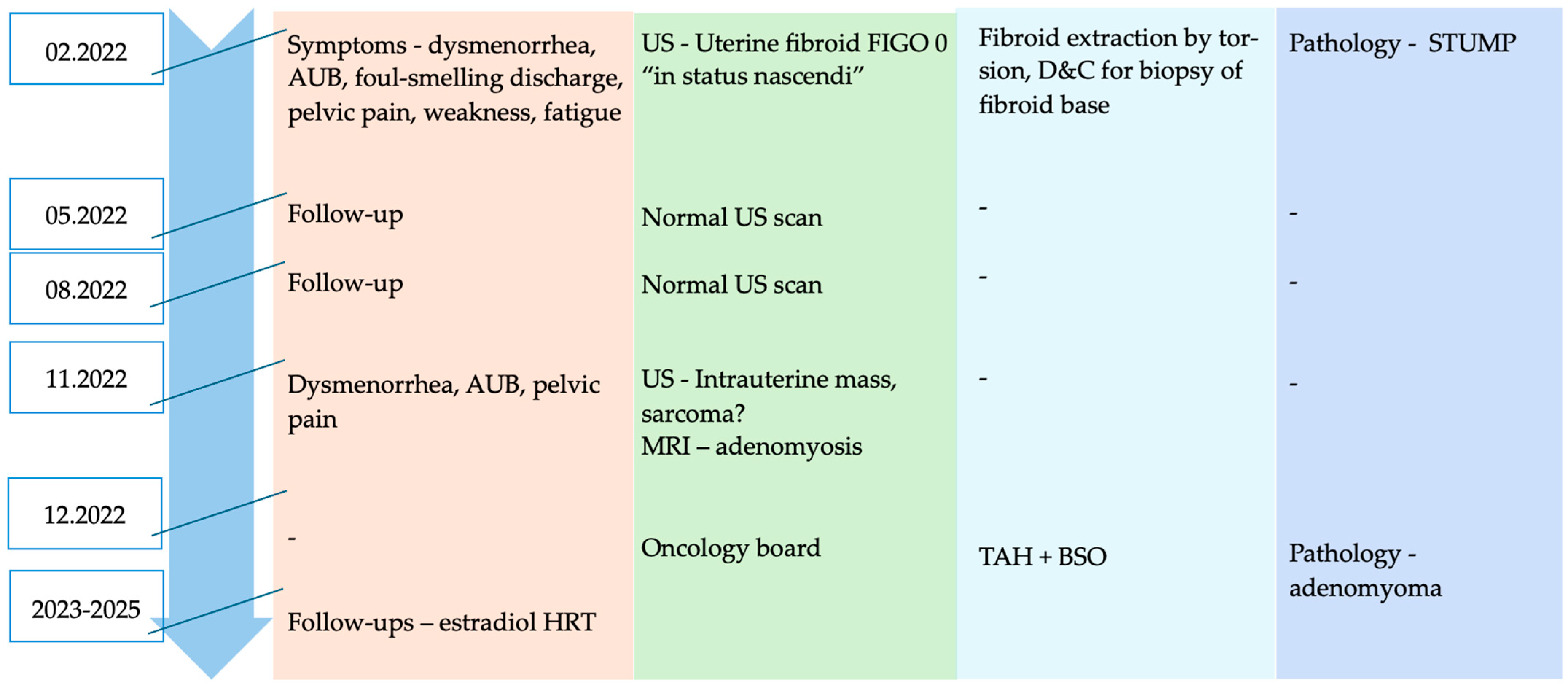
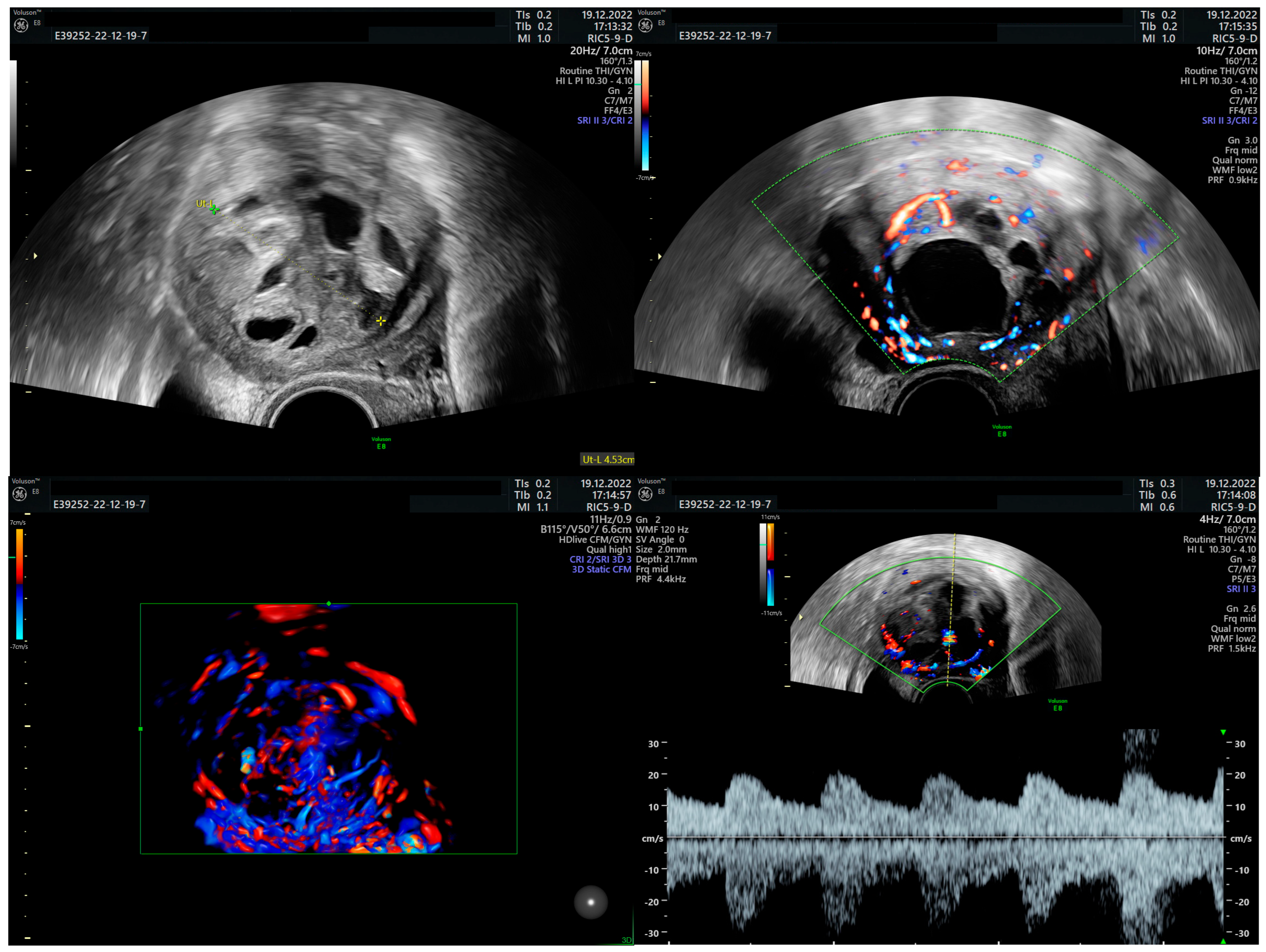
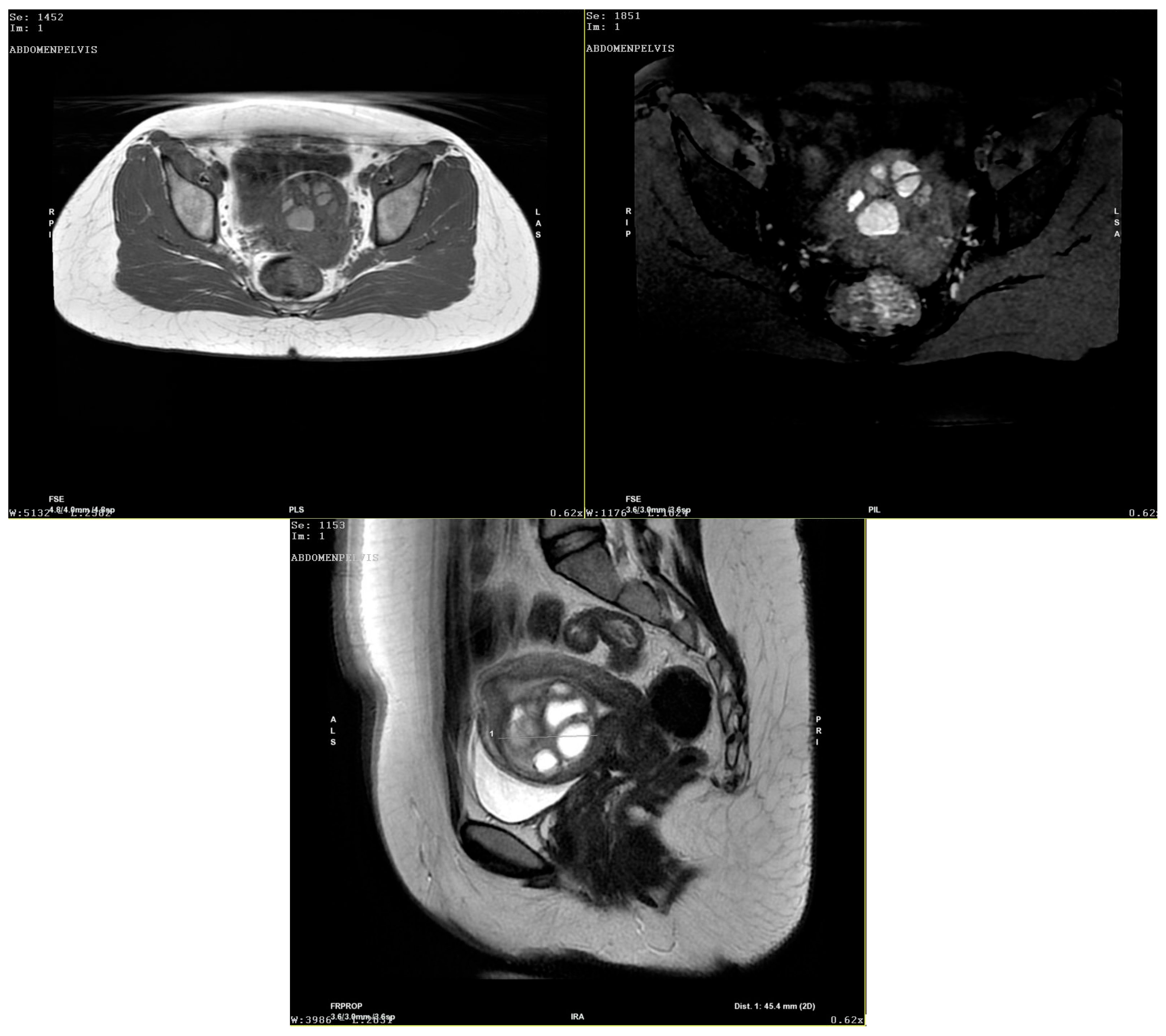
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FIGO | International Federation of Gynecology and Obstetrics |
| STUMP | Smooth muscle tumor of uncertain malignant potential |
| ER | Estrogen receptor |
| MRI | Magnetic resonance imaging |
| AUB | Abnormal uterine bleeding |
| LDH | Lactate dehydrogenase |
References
- Munro, M.G.; Critchley, H.O.; Fraser, I.S. Corrigendum to “The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions”. Int. J. Gynaecol. Obstet. 2019, 144, 237. [Google Scholar] [CrossRef] [PubMed]
- Byar, K.L.; Fredericks, T. Uterine Leiomyosarcoma. J. Adv. Pract. Oncol. 2022, 13, 70–76. [Google Scholar] [PubMed]
- Ferron, G.; Bataillon, G.; Martinez, A.; Chibon, F.; Valentin, T. Gynecological sarcomas, surgical management: Primary, metastatic, and recurrent disease. Int. J. Gynecol. Cancer 2024, 34, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Wang, D.; Wang, W.; Cao, D.; Yang, J.; Wu, M.; Yang, J.; Xiang, Y. Oncologic and Reproductive Outcomes of Uterine Smooth Muscle Tumor of Uncertain Malignant Potential: A Single Center Retrospective Study of 67 Cases. Front. Oncol. 2020, 10, 647. [Google Scholar] [CrossRef]
- Liu, H.-T.; Wong, C.-N.; Wong, C.-N.; Liu, F.-S. Uterine smooth muscle tumor of uncertain malignant potential: A review of current knowledge. Taiwan J. Obstet. Gynecol. 2022, 61, 935–940. [Google Scholar] [CrossRef]
- Al Khuri, M.; Al Salmi, I.; Al Ajmi, H.; Al Hadidi, A.; Alabousi, A.; Haider, E.; Vasudev, P.; Al Salmi, A.; Jose, S.; Alrahbi, N. Validating the diagnostic accuracy of an MRI-based scoring system for differentiating benign uterine leiomyomas from leiomyosarcomas. Int. J. Gynecol. Cancer 2024, 34, 1027–1033. [Google Scholar] [CrossRef]
- Russo, C.; Camilli, S.; Martire, F.G.; Di Giovanni, A.; Lazzeri, L.; Malzoni, M.; Zupi, E.; Exacoustos, C. Ultrasound features of highly vascularized uterine myomas (uterine smooth muscle tumors) and correlation with histopathology. Ultrasound Obs. Gynecol. 2022, 60, 269–276. [Google Scholar] [CrossRef]
- Cotrino, I.; Carosso, A.; Macchi, C.; Poma, C.B.; Cosma, S.; Ribotta, M.; Viora, E.; Sciarrone, A.; Borella, F.; Zola, P. Ultrasound and clinical characteristics of uterine smooth muscle tumors of uncertain malignant potential (STUMPs). Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 251, 167–172. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Liu, X.-B.; Mao, Y.-Y.; Lin, M.-H. Smooth muscle tumor of uncertain malignant potential (STUMP): A clinicopathologic analysis of 26 cases. Int. J. Clin. Exp. Pathol. 2020, 13, 818–826. [Google Scholar]
- Lin, G.; Yang, L.; Huang, Y.; Ng, K.; Ng, S.; Ueng, S.; Chao, A.; Yen, T.; Chang, T.; Lai, C. Comparison of the diagnostic accuracy of contrast-enhanced MRI and diffusion-weighted MRI in the differentiation between uterine leiomyosarcoma / smooth muscle tumor with uncertain malignant potential and benign leiomyoma. J. Magn. Reson. Imaging 2016, 43, 333–342. [Google Scholar] [CrossRef]
- Goidescu, I.; Nemeti, G.; Caracostea, G.; Eniu, D.T.; Chiorean, A.; Pintican, R.; Cruciat, G.; Muresan, D. The role of imaging techniques in the diagnosis, staging and choice of therapeutic conduct in pregnancy associated breast cancer. Med. Ultrason. 2019, 21, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.-C.; Fang, Y.-H.D.; Lin, G.; Ueng, S.-H.; Wu, T.-I.; Lai, C.-H.; Chueh, H.-Y.; Chao, A.; Chang, T.-C.; Yen, T.-C. Presurgical Identification of Uterine Smooth Muscle Malignancies through the Characteristic FDG Uptake Pattern on PET Scans. Contrast Media Mol. Imaging 2018, 2018, 7890241. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, A.D.; Saponara, M.; DE Leo, A.; Perrone, A.M.; DE Iaco, P.; Pantaleo, M.A.; Nannini, M. Recurrent Uterine Smooth-Muscle Tumors of Uncertain Malignant Potential (STUMP): State of The Art. Anticancer Res. 2020, 40, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Laury, A.L.; Nucci, M.R.; Quade, B.J. Predictors of adverse outcome in uterine smooth muscle tumours of uncertain malignant potential (STUMP): A clinicopathological analysis of 22 cases with a proposal for the inclusion of additional histological parameters. Histopathology 2018, 73, 284–298. [Google Scholar] [CrossRef]
- Lu, B.; Chen, Q.; Zhang, X.; Cheng, L. Serous carcinoma arising from uterine adenomyosis/adenomyotic cyst of the cervical stump: A report of 3 cases. Diagn. Pathol. 2016, 11, 46. [Google Scholar] [CrossRef]
- Izadi-Mood, N.; Samadi, N.; Sarmadi, S.; Eftekhar, Z. Papillary serous carcinoma arising from adenomyosis presenting as intramural leiomyoma. Arch Iran. Med. 2007, 10, 258–260. [Google Scholar]
- Abushahin, N.; Zhang, T.; Chiang, S.; Zhang, X.; Hatch, K.; Zheng, W. Serous endometrial intraepithelial carcinoma arising in adenomyosis: A report of 5 cases. Int. J. Gynecol. Pathol. 2011, 30, 271–281. [Google Scholar] [CrossRef]
- Koshiyama, M.; Suzuki, A.; Ozawa, M.; Fujita, K.; Sakakibara, A.; Kawamura, M.; Takahashi, S.; Fujii, H.; Hirano, T.; Okagaki, A.; et al. Adenocarcinomas arising from uterine adenomyosis: A report of four cases. Int. J. Gynecol. Pathol. 2002, 21, 239–245. [Google Scholar] [CrossRef]
- Talwar, A.; Behera, P.; Ahuja, A.; Sarkar, B.; Phulware, R.H. Endometrial Serous Carcinoma Arising From Adenomyosis: A Clinico-Pathological Insight. J. Fam. Reprod. Health 2021, 15, 125–129. [Google Scholar] [CrossRef]
- Jing, Q.; He, Y. Synchronous serous carcinoma arising in adenomyosis and small cell carcinoma of ovary-pulmonary type (SCCOPT): A case report and literature review. Eur. J. Gynaecol. Oncol. 2022, 43, 113–121. [Google Scholar] [CrossRef]
- Taran, F.A.; Weaver, A.L.; Coddington, C.C.; Stewart, E.A. Characteristics indicating adenomyosis coexisting with leiomyomas: A case-control study. Hum. Reprod. 2010, 25, 1177–1182. [Google Scholar] [CrossRef]
- Pandey, H.; Rizvi, G.; Pant, H.; Chufal, S.; Pant, P.; H, P.; Ss, C. Histopathological correlation of adenomyosis and leiomyoma in hysterectomy specimens as the cause of abnormal uterine bleeding in women in different age groups in the Kumaon region: A retroprospective study. J. Midlife Health 2013, 4, 27–30. [Google Scholar] [CrossRef]
- Ranaei, M.; Kohsari, E.; Galeshi, M.; Yazdani, S. The Correlation of Adenomyosis with Benign Endometrial Lesions in Hysterectomy Samples. J. Obstet. Gynecol. Cancer Res. 2022, 4, 99–104. [Google Scholar] [CrossRef]
- Nava, H.J. Highly Cellular Leiomyoma Mixed with a Focus of Adenomyosis. Cureus 2022, 14, e28129. [Google Scholar] [CrossRef]
- Wang, J.-H.; Wu, R.-J.; Xu, K.-H.; Lin, J. Single large cystic adenomyoma of the uterus after cornual pregnancy and curettage. Fertil. Steril. 2007, 88, 965–967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, Z.; Pang, Y.; Zhao, J.; Liang, J.; Ma, X. Cystic adenomyoma of the uterus: Case report and literature review. Open Life Sci. 2024, 19, 20220846. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Liu, C.; Lin, Y.; Zhu, J.; Wang, Z.; Wang, Z. Submucosal cystic adenomyosis: A report of five cases and review of the literature. BMC Women’s Health 2024, 24, 493. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Shen, J.; Tang, R.; Sun, F.; Chen, W.; Yang, J. Uterine cystic adenomyosis: A case report. AME Case Rep. 2024, 9, 23. [Google Scholar] [CrossRef]
- Kaur, B.D.; Kaur, P.; Kaur, M.; Kaur, M. Unraveling Cystic Adenomyosis: Diagnostic Odyssey and Surgical Resolution in a Multiparous Woman. J. Midlife Health 2024, 15, 302–305. [Google Scholar] [CrossRef]
- Brosens, I.; Gordts, S.; Habiba, M.; Benagiano, G. Uterine Cystic Adenomyosis: A Disease of Younger Women. J. Pediatr. Adolesc. Gynecol. 2015, 28, 420–426. [Google Scholar] [CrossRef]
- Xu, T.; Li, Y.; Jiang, L.; Liu, Q.; Liu, K. Subserous Cystic Adenomyosis: A Case Report and Review of the Literature. Front. Surg. 2022, 9, 807676. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemeti, G.; Cruciat, G.; Goidescu, I.G.; Roberta, C.; Ticala, V.M.; Surcel, M.; Goidescu, C.M.; Staicu, A.; Boitor-Borza, D.; Fetica, B.; et al. STUMP Swiftly Followed by Large Adenomyoma in a Young Nulliparous Patient. Diagnostics 2025, 15, 3018. https://doi.org/10.3390/diagnostics15233018
Nemeti G, Cruciat G, Goidescu IG, Roberta C, Ticala VM, Surcel M, Goidescu CM, Staicu A, Boitor-Borza D, Fetica B, et al. STUMP Swiftly Followed by Large Adenomyoma in a Young Nulliparous Patient. Diagnostics. 2025; 15(23):3018. https://doi.org/10.3390/diagnostics15233018
Chicago/Turabian StyleNemeti, Georgiana, Gheorghe Cruciat, Iulian Gabriel Goidescu, Chereches Roberta, Vasile Marian Ticala, Mihai Surcel, Cerasela Mihaela Goidescu, Adelina Staicu, Dan Boitor-Borza, Bogdan Fetica, and et al. 2025. "STUMP Swiftly Followed by Large Adenomyoma in a Young Nulliparous Patient" Diagnostics 15, no. 23: 3018. https://doi.org/10.3390/diagnostics15233018
APA StyleNemeti, G., Cruciat, G., Goidescu, I. G., Roberta, C., Ticala, V. M., Surcel, M., Goidescu, C. M., Staicu, A., Boitor-Borza, D., Fetica, B., Rotar, I. C., & Muresan, D. (2025). STUMP Swiftly Followed by Large Adenomyoma in a Young Nulliparous Patient. Diagnostics, 15(23), 3018. https://doi.org/10.3390/diagnostics15233018





