Recent Trend of Laboratory Tests in Common Gastrointestinal Tract Disorders
Abstract
1. Introduction
2. Common GI Disorders and Related Laboratory Tests
2.1. Helicobacter pylori Infection
2.1.1. Urea Breath Test
2.1.2. H. pylori Stool Antigen Test (HpSAT)
2.1.3. H. pylori Serology
2.1.4. H. pylori Molecular Testing
2.2. Celiac Disease
2.3. Inflammatory Bowel Disease
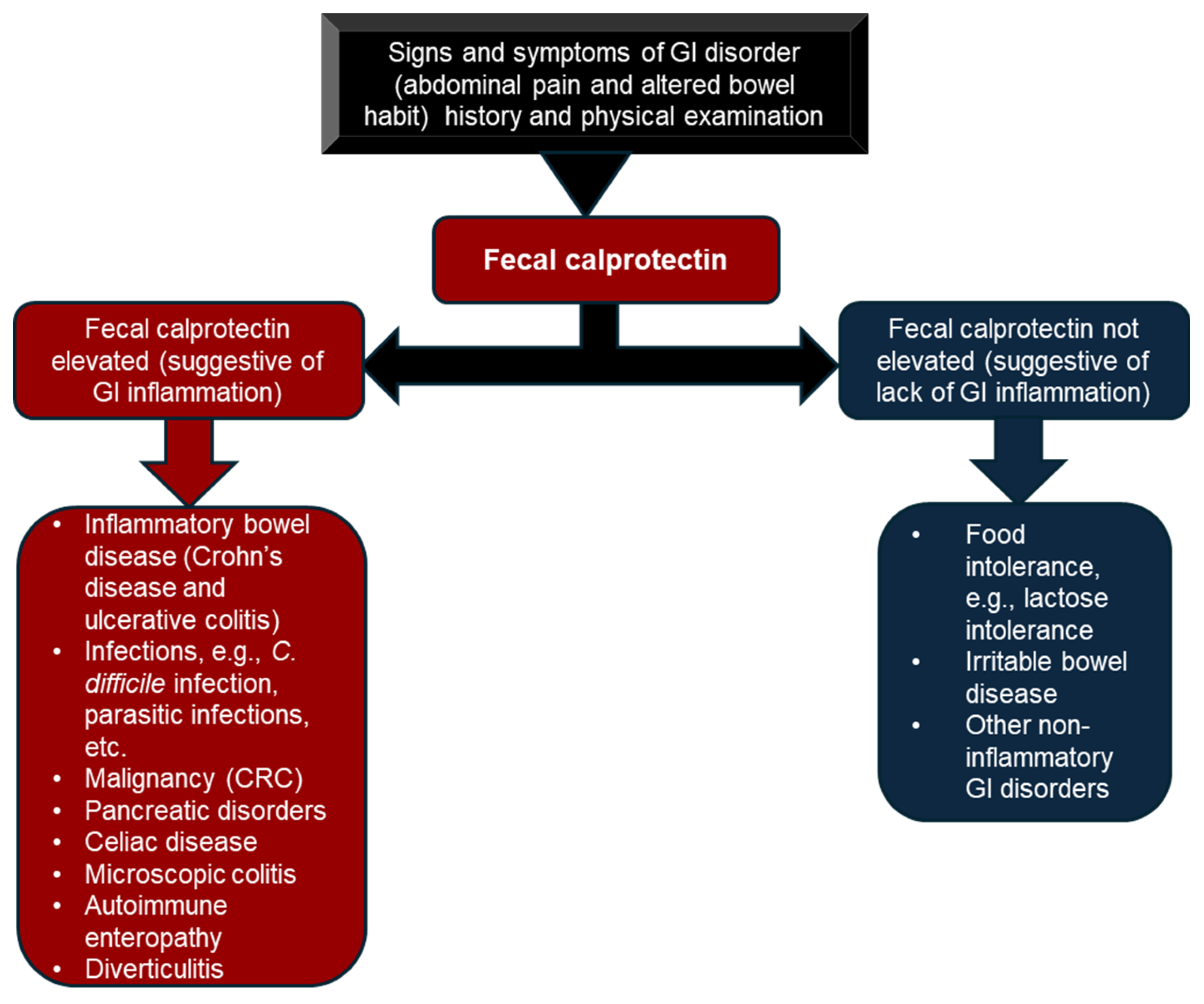
2.3.1. Fecal Calprotectin
2.3.2. Fecal Lactoferrin
2.3.3. C-Reactive Protein (CRP)
2.3.4. Perinuclear Anti-Neutrophil Cytoplasmic Antibody (pANCA) and Anti-Saccharomyces cerevisiae Antibody (ASCA)
2.4. Irritable Bowel Syndrome
2.5. Colorectal Cancer
3. Emerging Laboratory Tests and Biomarkers in GI Diseases
3.1. Gut Microbiota as a Diagnostic Tool in IBD
3.2. Gut Microbiome-Associated Serum Metabolites (GMSMs) in IBD and CRC
3.3. Other Potential Biomarkers in IBD
3.3.1. Anti-Integrin αvβ6 Autoantibodies
3.3.2. Oncostatin
3.3.3. MicroRNA (miRNA) as a Potential Biomarker of IBD
3.4. Interleukin 2 (IL-2) for the Diagnosis of Celiac Disease
3.5. Gluten Immunogenic Peptide (GIP) for Diet Compliance and Unintentional Gluten Exposure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skok, K.; Vihar, B.; Maver, U.; Gradišnik, L.; Bräutigam, K.; Trapecar, M.; Skok, P. Gastrointestinal tract, its pathophysiology and in-vitro models: A “quick” reference guide to translational studies. World J. Gastroenterol. 2025, 31, 108297. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hu, D.; Sun, H.; Yan, Z.; Wang, Y.; Wang, L.; Zhang, T.; Meng, N.; Zhai, C.; Zong, Q.; et al. Global, regional, and national burden of digestive diseases: Findings from the global burden of disease study 2019. Front. Public Health 2023, 11, 1202980. [Google Scholar] [CrossRef]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef]
- Peery, A.F.; Dellon, E.S.; Lund, J.; Crockett, S.D.; McGowan, C.E.; Bulsiewicz, W.J.; Gangarosa, L.M.; Thiny, M.T.; Stizenberg, K.; Morgan, D.R.; et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012, 143, 1179–1187.e3. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Kuo, F.-C.; Liu, C.-J.; Wu, M.-C.; Shih, H.-Y.; Wang, S.S.W.; Wu, J.-Y.; Kuo, C.-H.; Huang, Y.-K.; Wu, D.-C. Diagnosis of Helicobacter pylori infection: Current options and developments. World J. Gastroenterol. 2015, 21, 11221–11235. [Google Scholar] [CrossRef] [PubMed]
- Kalali, B.; Formichella, L.; Gerhard, M. Diagnosis of Helicobacter pylori: Changes towards the Future. Diseases 2015, 3, 122–135. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Peleteiro, B.; Bastos, A.; Ferro, A.; Lunet, N. Prevalence of Helicobacter pylori infection worldwide: A systematic review of studies with national coverage. Dig. Dis. Sci. 2014, 59, 1698–1709. [Google Scholar] [CrossRef]
- Li, Y.; Choi, H.; Leung, K.; Jiang, F.; Graham, D.Y.; Leung, W.K. Global prevalence of Helicobacter pylori infection between 1980 and 2022: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 553–564. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.-M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Primers 2023, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Miftahussurur, M. Helicobacter pylori urease for diagnosis of Helicobacter pylori infection: A mini review. J. Adv. Res. 2018, 13, 51–57. [Google Scholar] [CrossRef]
- Guimarães, N.; Azevedo, N.F.; Figueiredo, C.; Keevil, C.W.; Vieira, M.J. Development and Application of a Novel Peptide Nucleic Acid Probe for the Specific Detection of Helicobacter pylori in Gastric Biopsy Specimens. J. Clin. Microbiol. 2007, 45, 3089–3094. [Google Scholar] [CrossRef]
- Cerqueira, L.; Fernandes, R.M.; Ferreira, R.M.; Carneiro, F.; Dinis-Ribeiro, M.; Figueiredo, C.; Keevil, C.W.; Azevedo, N.F.; Vieira, M.J. PNA-FISH as a new diagnostic method for the determination of clarithromycin resistance of Helicobacter pylori. BMC Microbiol. 2011, 11, 101. [Google Scholar] [CrossRef]
- Thaker, Y.; Moon, A.; Afzali, A. Helicobacter pylori: A Review of Epidemiology, Treatment, and Management. J. Clin. Gastroenterol. Treat. 2016, 2, 1–5. [Google Scholar] [CrossRef]
- Sabbagh, P.; Mohammadnia-Afrouzi, M.; Javanian, M.; Babazadeh, A.; Koppolu, V.; Vasigala, V.R.; Nouri, H.R.; Ebrahimpour, S. Diagnostic methods for Helicobacter pylori infection: Ideals, options, and limitations. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 55–66. [Google Scholar] [CrossRef]
- Taj, Y.; Essa, F.; Kazmi, S.U.; Abdullah, E. Sensitivity and specificity of various diagnostic tests in the detection of Helicobacter pylori. J. Coll. Physicians Surg. Pak. 2003, 13, 90–93. [Google Scholar]
- Ogata, S.K.; Godoy, A.P.O.; da Silva Patricio, F.R.; Kawakami, E. High Helicobacter pylori resistance to metronidazole and clarithromycin in Brazilian children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 645–648. [Google Scholar] [CrossRef]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori Infection, Its Laboratory Diagnosis, and Antimicrobial Resistance: A Perspective of Clinical Relevance. Clin. Microbiol. Rev. 2022, 35, e0025821. [Google Scholar] [CrossRef] [PubMed]
- Velayos, B.; Fernández-Salazar, L.; Pons-Renedo, F.; Muñoz, M.F.; Almaraz, A.; Aller, R.; Ruíz, L.; Del Olmo, L.; Gisbert, J.P.; González-Hernández, J.M. Accuracy of urea breath test performed immediately after emergency endoscopy in peptic ulcer bleeding. Dig. Dis. Sci. 2012, 57, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; de la Morena, F.; Abraira, V. Accuracy of monoclonal stool antigen test for the diagnosis of H. pylori infection: A systematic review and meta-analysis. Am. J. Gastroenterol. 2006, 101, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Iannone, A.; Giorgio, F.; Russo, F.; Riezzo, G.; Girardi, B.; Pricci, M.; Palmer, S.C.; Barone, M.; Principi, M.; Strippoli, G.F.; et al. New fecal test for non-invasive Helicobacter pylori detection: A diagnostic accuracy study. World J. Gastroenterol. 2018, 24, 3021–3029. [Google Scholar] [CrossRef]
- de Carvalho Costa Cardinali, L.; Rocha, G.A.; Rocha, A.M.C.; de Moura, S.B.; de Figueiredo Soares, T.; Esteves, A.M.B.; Nogueira, A.M.M.F.; Cabral, M.M.D.A.; de Carvalho, A.S.T.; Bitencourt, P.; et al. Evaluation of [13C]urea breath test and Helicobacter pylori stool antigen test for diagnosis of H. pylori infection in children from a developing country. J. Clin. Microbiol. 2003, 41, 3334–3335. [Google Scholar] [CrossRef]
- Gatta, L.; Vakil, N.; Ricci, C.; Osborn, J.F.; Tampieri, A.; Perna, F.; Miglioli, M.; Vaira, D. Effect of proton pump inhibitors and antacid therapy on 13C urea breath tests and stool test for Helicobacter pylori infection. Am. J. Gastroenterol. 2004, 99, 823–829. [Google Scholar] [CrossRef]
- Costa, L.C.M.C.; Carvalho, M.d.G.; Vale, F.F.; Marques, A.T.; Rasmussen, L.T.; Chen, T.; Barros-Pinheiro, M. Helicobacter pylori in oral cavity: Current knowledge. Clin. Exp. Med. 2024, 24, 209. [Google Scholar] [CrossRef] [PubMed]
- Day, A.S.; Sherman, P.M. Accuracy of office-based immunoassays for the diagnosis of Helicobacter pylori infection in children. Helicobacter 2002, 7, 205–209. [Google Scholar] [CrossRef]
- Kawai, S.; Arai, K.; Lin, Y.; Nishiyama, T.; Sasakabe, T.; Wang, C.; Miwa, H.; Kikuchi, S. Comparison of the detection of Helicobacter pylori infection by commercially available serological testing kits and the 13C-urea breath test. J. Infect. Chemother. 2019, 25, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Formichella, L.; Romberg, L.; Meyer, H.; Bolz, C.; Vieth, M.; Geppert, M.; Göttner, G.; Nölting, C.; Schepp, W.; Schneider, A.; et al. Validation of a Novel Immunoline Assay for Patient Stratification according to Virulence of the Infecting Helicobacter pylori Strain and Eradication Status. J. Immunol. Res. 2017, 2017, 8394593. [Google Scholar] [CrossRef]
- Coelho, L.G.V.; Marinho, J.R.; Genta, R.; Ribeiro, L.T.; Passos, M.d.C.F.; Zaterka, S.; Assumpção, P.P.; Barbosa, A.J.A.; Barbuti, R.; Braga, L.L.; et al. IVTH Brazilian Consensus Conference on Helicobacter pylori Infection. Arq. Gastroenterol. 2018, 55, 97–121. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef]
- Jones, N.L.; Koletzko, S.; Goodman, K.; Bontems, P.; Cadranel, S.; Casswall, T.; Czinn, S.; Gold, B.D.; Guarner, J.; Elitsur, Y.; et al. Joint ESPGHAN/NASPGHAN Guidelines for the Management of Helicobacter pylori in Children and Adolescents (Update 2016). J. Pediatr. Gastroenterol. Nutr. 2017, 64, 991–1003. [Google Scholar] [CrossRef]
- Marrero Rolon, R.; Cunningham, S.A.; Mandrekar, J.N.; Polo, E.T.; Patel, R. Clinical Evaluation of a Real-Time PCR Assay for Simultaneous Detection of Helicobacter pylori and Genotypic Markers of Clarithromycin Resistance Directly from Stool. J. Clin. Microbiol. 2021, 59, e03040-20. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.R.; Ciacci, C.; Ciclitira, P.J.; Green, P.H.R.; Hadjivassiliou, M.; Holdoway, A.; van Heel, D.A.; et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [CrossRef]
- Akobeng, A.K.; Thomas, A.G. Systematic review: Tolerable amount of gluten for people with coeliac disease. Aliment. Pharmacol. Ther. 2008, 27, 1044–1052. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Silvester, J.A.; Lebwohl, B.; Leffler, D.A.; Anderson, R.P.; Therrien, A.; Kelly, C.P.; Verdu, E.F. Society for the Study of Celiac Disease position statement on gaps and opportunities in coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 875–884. [Google Scholar] [CrossRef]
- Bai, J.C.; Ciacci, C. World Gastroenterology Organisation Global Guidelines: Celiac Disease February 2017. J. Clin. Gastroenterol. 2017, 51, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Al-Toma, A.; Goerres, M.S.; Meijer, J.W.R.; Peña, A.S.; Crusius, J.B.A.; Mulder, C.J.J. Human leukocyte antigen-DQ2 homozygosity and the development of refractory celiac disease and enteropathy-associated T-cell lymphoma. Clin. Gastroenterol. Hepatol. 2006, 4, 315–319. [Google Scholar] [CrossRef]
- Pietzak, M.M.; Schofield, T.C.; McGinniss, M.J.; Nakamura, R.M. Stratifying risk for celiac disease in a large at-risk United States population by using HLA alleles. Clin. Gastroenterol. Hepatol. 2009, 7, 966–971. [Google Scholar] [CrossRef]
- Kim, C.-Y.; Quarsten, H.; Bergseng, E.; Khosla, C.; Sollid, L.M. Structural basis for HLA-DQ2-mediated presentation of gluten epitopes in celiac disease. Proc. Natl. Acad. Sci. USA 2004, 101, 4175–4179. [Google Scholar] [CrossRef]
- Henderson, K.N.; Tye-Din, J.A.; Reid, H.H.; Chen, Z.; Borg, N.A.; Beissbarth, T.; Tatham, A.; Mannering, S.I.; Purcell, A.W.; Dudek, N.L.; et al. A structural and immunological basis for the role of human leukocyte antigen DQ8 in celiac disease. Immunity 2007, 27, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Hill, I.D.; Semrad, C.; Kelly, C.P.; Greer, K.B.; Limketkai, B.N.; Lebwohl, B. American College of Gastroenterology Guidelines Update: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2023, 118, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Werkstetter, K.J.; Korponay-Szabó, I.R.; Popp, A.; Villanacci, V.; Salemme, M.; Heilig, G.; Lillevang, S.T.; Mearin, M.L.; Ribes-Koninckx, C.; Thomas, A.; et al. Accuracy in Diagnosis of Celiac Disease Without Biopsies in Clinical Practice. Gastroenterology 2017, 153, 924–935. [Google Scholar] [CrossRef]
- Husby, S.; Choung, R.S.; Crawley, C.; Lillevang, S.T.; Murray, J.A. Laboratory Testing for Celiac Disease: Clinical and Methodological Considerations. Clin. Chem. 2024, 70, 1208–1219. [Google Scholar] [CrossRef]
- Toftedal, P.; Nielsen, C.; Madsen, J.T.; Titlestad, K.; Husby, S.; Lillevang, S.T. Positive predictive value of serological diagnostic measures in celiac disease. Clin. Chem. Lab. Med. 2010, 48, 685–691. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef]
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The histopathology of coeliac disease: Time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194. [Google Scholar] [CrossRef]
- Marsh, M.N.; Hinde, J. Morphometric analysis of small intestinal mucosa. III. The quantitation of crypt epithelial volumes and lymphoid cell infiltrates, with reference to celiac sprue mucosae. Virchows Arch. A Pathol. Anat. Histopathol. 1986, 409, 11–22. [Google Scholar] [CrossRef]
- Marsh, M.N.; Johnson, M.W.; Rostami, K. Mucosal histopathology in celiac disease: A rebuttal of Oberhuber’s sub-division of Marsh III. Gastroenterol. Hepatol. Bed Bench 2015, 8, 99–109. [Google Scholar]
- Marsh, M.N.; Rostami, K. What Is A Normal Intestinal Mucosa? Gastroenterology 2016, 151, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Taavela, J.; Koskinen, O.; Huhtala, H.; Lähdeaho, M.-L.; Popp, A.; Laurila, K.; Collin, P.; Kaukinen, K.; Kurppa, K.; Mäki, M. Validation of morphometric analyses of small-intestinal biopsy readouts in celiac disease. PLoS ONE 2013, 8, e76163. [Google Scholar] [CrossRef] [PubMed]
- Hujoel, I.A.; Jansson-Knodell, C.L.; Hujoel, P.P.; Hujoel, M.L.A.; Choung, R.S.; Murray, J.A.; Rubio-Tapia, A. Estimating the Impact of Verification Bias on Celiac Disease Testing. J. Clin. Gastroenterol. 2021, 55, 327–334. [Google Scholar] [CrossRef]
- Loftus, E.V. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef]
- Podolsky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 1991, 325, 1008–1016. [Google Scholar] [CrossRef]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef]
- Knights, D.; Lassen, K.G.; Xavier, R.J. Advances in inflammatory bowel disease pathogenesis: Linking host genetics and the microbiome. Gut 2013, 62, 1505–1510. [Google Scholar] [CrossRef]
- Rosen, M.J.; Dhawan, A.; Saeed, S.A. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015, 169, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; Colitis Foundation of America; Bousvaros, A.; Antonioli, D.A.; Colletti, R.B.; Dubinsky, M.C.; Glickman, J.N.; Gold, B.D.; Griffiths, A.M.; Jevon, G.P.; et al. Differentiating ulcerative colitis from Crohn disease in children and young adults: Report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 653–674. [Google Scholar] [CrossRef] [PubMed]
- Kugathasan, S.; Judd, R.H.; Hoffmann, R.G.; Heikenen, J.; Telega, G.; Khan, F.; Weisdorf-Schindele, S.; San Pablo, W.; Perrault, J.; Park, R.; et al. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: A statewide population-based study. J. Pediatr. 2003, 143, 525–531. [Google Scholar] [CrossRef] [PubMed]
- De Matos, V.; Russo, P.A.; Cohen, A.B.; Mamula, P.; Baldassano, R.N.; Piccoli, D.A. Frequency and clinical correlations of granulomas in children with Crohn disease. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 392–398. [Google Scholar] [CrossRef]
- Sostegni, R.; Daperno, M.; Scaglione, N.; Lavagna, A.; Rocca, R.; Pera, A. Review article: Crohn’s disease: Monitoring disease activity. Aliment. Pharmacol. Ther. 2003, 17 (Suppl. S2), 11–17. [Google Scholar] [CrossRef]
- Bager, P.; Chauhan, U.; Greveson, K.; Jäghult, S.; Moortgat, L.; Kemp, K. Systematic review: Advice lines for patients with inflammatory bowel disease. Scand. J. Gastroenterol. 2018, 53, 506–512. [Google Scholar] [CrossRef]
- Levine, A.; Koletzko, S.; Turner, D.; Escher, J.C.; Cucchiara, S.; de Ridder, L.; Kolho, K.-L.; Veres, G.; Russell, R.K.; Paerregaard, A.; et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 795–806. [Google Scholar] [CrossRef]
- Knowles, S.R.; Keefer, L.; Wilding, H.; Hewitt, C.; Graff, L.A.; Mikocka-Walus, A. Quality of Life in Inflammatory Bowel Disease: A Systematic Review and Meta-analyses-Part II. Inflamm. Bowel Dis. 2018, 24, 966–976. [Google Scholar] [CrossRef]
- Kyle, B.D.; Agbor, T.A.; Sharif, S.; Chauhan, U.; Marshall, J.; Halder, S.L.S.; Ip, S.; Khan, W.I. Fecal Calprotectin, CRP and Leucocytes in IBD Patients: Comparison of Biomarkers With Biopsy Results. J. Can. Assoc. Gastroenterol. 2021, 4, 84–90. [Google Scholar] [CrossRef]
- Nakashige, T.G.; Zhang, B.; Krebs, C.; Nolan, E.M. Human calprotectin is an iron-sequestering host-defense protein. Nat. Chem. Biol. 2015, 11, 765–771. [Google Scholar] [CrossRef] [PubMed]
- van Rheenen, P.F.; Van de Vijver, E.; Fidler, V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: Diagnostic meta-analysis. BMJ 2010, 341, c3369. [Google Scholar] [CrossRef]
- Desai, D.; Faubion, W.A.; Sandborn, W.J. Review article: Biological activity markers in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2007, 25, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Kostakis, I.D.; Cholidou, K.G.; Vaiopoulos, A.G.; Vlachos, I.S.; Perrea, D.; Vaos, G. Fecal calprotectin in pediatric inflammatory bowel disease: A systematic review. Dig. Dis. Sci. 2013, 58, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Kapel, N.; Ouni, H.; Benahmed, N.A.; Barbot-Trystram, L. Fecal Calprotectin for the Diagnosis and Management of Inflammatory Bowel Diseases. Clin. Transl. Gastroenterol. 2023, 14, e00617. [Google Scholar] [CrossRef]
- Menees, S.B.; Powell, C.; Kurlander, J.; Goel, A.; Chey, W.D. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am. J. Gastroenterol. 2015, 110, 444–454. [Google Scholar] [CrossRef]
- Orlando, A.; Modesto, I.; Castiglione, F.; Scala, L.; Scimeca, D.; Rispo, A.; Teresi, S.; Mocciaro, F.; Criscuoli, V.; Marrone, C.; et al. The role of calprotectin in predicting endoscopic post-surgical recurrence in asymptomatic Crohn’s disease: A comparison with ultrasound. Eur. Rev. Med. Pharmacol. Sci. 2006, 10, 17–22. [Google Scholar]
- Boschetti, G.; Laidet, M.; Moussata, D.; Stefanescu, C.; Roblin, X.; Phelip, G.; Cotte, E.; Passot, G.; Francois, Y.; Drai, J.; et al. Levels of Fecal Calprotectin Are Associated With the Severity of Postoperative Endoscopic Recurrence in Asymptomatic Patients With Crohn’s Disease. Am. J. Gastroenterol. 2015, 110, 865–872. [Google Scholar] [CrossRef]
- Sakurai, T.; Saruta, M. Positioning and Usefulness of Biomarkers in Inflammatory Bowel Disease. Digestion 2023, 104, 30–41. [Google Scholar] [CrossRef]
- Reenaers, C.; Bossuyt, P.; Hindryckx, P.; Vanpoucke, H.; Cremer, A.; Baert, F. Expert opinion for use of faecal calprotectin in diagnosis and monitoring of inflammatory bowel disease in daily clinical practice. United Eur. Gastroenterol. J. 2018, 6, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, F.S.; Burri, E.; Beglinger, C. The role and utility of faecal markers in inflammatory bowel disease. Ther. Adv. Gastroenterol. 2015, 8, 23–36. [Google Scholar] [CrossRef]
- Mumolo, M.G.; Bertani, L.; Ceccarelli, L.; Laino, G.; Di Fluri, G.; Albano, E.; Tapete, G.; Costa, F. From bench to bedside: Fecal calprotectin in inflammatory bowel diseases clinical setting. World J. Gastroenterol. 2018, 24, 3681–3694. [Google Scholar] [CrossRef] [PubMed]
- Husebye, E.; Tøn, H.; Johne, B. Biological variability of fecal calprotectin in patients referred for colonoscopy without colonic inflammation or neoplasm. Am. J. Gastroenterol. 2001, 96, 2683–2687. [Google Scholar] [CrossRef]
- Lasson, A.; Stotzer, P.-O.; Öhman, L.; Isaksson, S.; Sapnara, M.; Strid, H. The intra-individual variability of faecal calprotectin: A prospective study in patients with active ulcerative colitis. J. Crohn’s Colitis 2015, 9, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pei, F.; Wang, X.; Sun, Z.; Hu, C.; Dou, H. Diagnostic accuracy of fecal lactoferrin for inflammatory bowel disease: A meta-analysis. Int. J. Clin. Exp. Pathol. 2015, 8, 12319–12332. [Google Scholar]
- Gisbert, J.P.; Bermejo, F.; Pérez-Calle, J.-L.; Taxonera, C.; Vera, I.; McNicholl, A.G.; Algaba, A.; López, P.; López-Palacios, N.; Calvo, M.; et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm. Bowel Dis. 2009, 15, 1190–1198. [Google Scholar] [CrossRef]
- Heida, A.; Van de Vijver, E.; van Ravenzwaaij, D.; Van Biervliet, S.; Hummel, T.Z.; Yuksel, Z.; Gonera-de Jong, G.; Schulenberg, R.; Muller Kobold, A.; van Rheenen, P.F.; et al. Predicting inflammatory bowel disease in children with abdominal pain and diarrhoea: Calgranulin-C versus calprotectin stool tests. Arch. Dis. Child. 2018, 103, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Gewurz, H.; Mold, C.; Siegel, J.; Fiedel, B. C-reactive protein and the acute phase response. Adv. Intern. Med. 1982, 27, 345–372. [Google Scholar] [CrossRef]
- Boras, E.; Slevin, M.; Alexander, M.Y.; Aljohi, A.; Gilmore, W.; Ashworth, J.; Krupinski, J.; Potempa, L.A.; Al Abdulkareem, I.; Elobeid, A.; et al. Monomeric C-reactive protein and Notch-3 co-operatively increase angiogenesis through PI3K signalling pathway. Cytokine 2014, 69, 165–179. [Google Scholar] [CrossRef]
- Solem, C.A.; Loftus, E.V.; Tremaine, W.J.; Harmsen, W.S.; Zinsmeister, A.R.; Sandborn, W.J. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm. Bowel Dis. 2005, 11, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Mosli, M.H.; Zou, G.; Garg, S.K.; Feagan, S.G.; MacDonald, J.K.; Chande, N.; Sandborn, W.J.; Feagan, B.G. C-Reactive Protein, Fecal Calprotectin, and Stool Lactoferrin for Detection of Endoscopic Activity in Symptomatic Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2015, 110, 802–819; quiz 820. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Marín, A.C.; Chaparro, M. Systematic review: Factors associated with relapse of inflammatory bowel disease after discontinuation of anti-TNF therapy. Aliment. Pharmacol. Ther. 2015, 42, 391–405. [Google Scholar] [CrossRef]
- Saxon, A.; Shanahan, F.; Landers, C.; Ganz, T.; Targan, S. A distinct subset of antineutrophil cytoplasmic antibodies is associated with inflammatory bowel disease. J. Allergy Clin. Immunol. 1990, 86, 202–210. [Google Scholar] [CrossRef]
- Sendid, B.; Colombel, J.F.; Jacquinot, P.M.; Faille, C.; Fruit, J.; Cortot, A.; Lucidarme, D.; Camus, D.; Poulain, D. Specific antibody response to oligomannosidic epitopes in Crohn’s disease. Clin. Diagn. Lab. Immunol. 1996, 3, 219–226. [Google Scholar] [CrossRef]
- Quinton, J.F.; Sendid, B.; Reumaux, D.; Duthilleul, P.; Cortot, A.; Grandbastien, B.; Charrier, G.; Targan, S.R.; Colombel, J.F.; Poulain, D. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: Prevalence and diagnostic role. Gut 1998, 42, 788–791. [Google Scholar] [CrossRef]
- Pang, Y.; Ruan, H.; Wu, D.; Lang, Y.; Sun, K.; Xu, C. Assessment of clinical activity and severity using serum ANCA and ASCA antibodies in patients with ulcerative colitis. Allergy Asthma Clin. Immunol. 2020, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Israeli, E.; Grotto, I.; Gilburd, B.; Balicer, R.D.; Goldin, E.; Wiik, A.; Shoenfeld, Y. Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease. Gut 2005, 54, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- D’Incà, R.; Sturniolo, G. Biomarkers in IBD: What to Utilize for the Diagnosis? Diagnostics 2023, 13, 2931. [Google Scholar] [CrossRef] [PubMed]
- Kaul, A.; Hutfless, S.; Liu, L.; Bayless, T.M.; Marohn, M.R.; Li, X. Serum anti-glycan antibody biomarkers for inflammatory bowel disease diagnosis and progression: A systematic review and meta-analysis. Inflamm. Bowel Dis. 2012, 18, 1872–1884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, C.; Zhao, X.; Lv, C.; He, Q.; Lei, S.; Guo, Y.; Zhi, F. Anti-Saccharomyces cerevisiae antibodies associate with phenotypes and higher risk for surgery in Crohn’s disease: A meta-analysis. Dig. Dis. Sci. 2012, 57, 2944–2954. [Google Scholar] [CrossRef]
- Kim, J.M.; Choi, Y.M.; Jung, S.A.; Yang, H.R. Diagnostic utility, disease activity, and disease phenotype correlation of serum ASCA, pANCA, and PR3-ANCA in pediatric inflammatory bowel disease. J. Pediatr. 2024, 100, 204–211. [Google Scholar] [CrossRef]
- Bossuyt, X. Serologic markers in inflammatory bowel disease. Clin. Chem. 2006, 52, 171–181. [Google Scholar] [CrossRef]
- Sultan, S.; Malhotra, A. Irritable Bowel Syndrome. Ann. Intern. Med. 2017, 166, ITC81–ITC96. [Google Scholar] [CrossRef]
- Drossman, D.A.; Hasler, W.L. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 17–44. [Google Scholar] [CrossRef]
- Smalley, W.; Falck-Ytter, C.; Carrasco-Labra, A.; Wani, S.; Lytvyn, L.; Falck-Ytter, Y. AGA Clinical Practice Guidelines on the Laboratory Evaluation of Functional Diarrhea and Diarrhea-Predominant Irritable Bowel Syndrome in Adults (IBS-D). Gastroenterology 2019, 157, 851–854. [Google Scholar] [CrossRef]
- Pimentel, M.; Morales, W.; Rezaie, A.; Marsh, E.; Lembo, A.; Mirocha, J.; Leffler, D.A.; Marsh, Z.; Weitsman, S.; Chua, K.S.; et al. Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects. PLoS ONE 2015, 10, e0126438. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.; Park, S.C.; Morales, W.; Marsh, E.; Lembo, A.; Kim, J.H.; Weitsman, S.; Chua, K.S.; Barlow, G.M.; Pimentel, M. Assessment of Anti-vinculin and Anti-cytolethal Distending Toxin B Antibodies in Subtypes of Irritable Bowel Syndrome. Dig. Dis. Sci. 2017, 62, 1480–1485. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Ansa, B.E.; Coughlin, S.S.; Alema-Mensah, E.; Smith, S.A. Evaluation of Colorectal Cancer Incidence Trends in the United States (2000–2014). J. Clin. Med. 2018, 7, 22. [Google Scholar] [CrossRef]
- Austin, H.; Henley, S.J.; King, J.; Richardson, L.C.; Eheman, C. Changes in colorectal cancer incidence rates in young and older adults in the United States: What does it tell us about screening. Cancer Causes Control 2014, 25, 191–201. [Google Scholar] [CrossRef]
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J. Natl. Cancer Inst. 2017, 109, djw322. [Google Scholar] [CrossRef]
- Brenner, D.R.; Heer, E.; Sutherland, R.L.; Ruan, Y.; Tinmouth, J.; Heitman, S.J.; Hilsden, R.J. National Trends in Colorectal Cancer Incidence Among Older and Younger Adults in Canada. JAMA Netw. Open 2019, 2, e198090. [Google Scholar] [CrossRef]
- Kalyta, A.; De Vera, M.A.; Peacock, S.; Telford, J.J.; Brown, C.J.; Donnellan, F.; Gill, S.; Loree, J.M. Canadian Colorectal Cancer Screening Guidelines: Do They Need an Update Given Changing Incidence and Global Practice Patterns? Curr. Oncol. 2021, 28, 1558–1570. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Gupta, S. Screening for Colorectal Cancer. Hematol. Oncol. Clin. N. Am. 2022, 36, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, M.; Prathiraja, O.; Caldera, D.; Jena, R.; Coffie-Pierre, J.A.; Silva, M.S.; Siddiqui, O.S. Colon Cancer Screening Methods: 2023 Update. Cureus 2023, 15, e37509. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force; Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965–1977. [Google Scholar] [CrossRef]
- Allison, J.E.; Tekawa, I.S.; Ransom, L.J.; Adrain, A.L. A comparison of fecal occult-blood tests for colorectal-cancer screening. N. Engl. J. Med. 1996, 334, 155–159. [Google Scholar] [CrossRef]
- van Rossum, L.G.; van Rijn, A.F.; Laheij, R.J.; van Oijen, M.G.; Fockens, P.; van Krieken, H.H.; Verbeek, A.L.; Jansen, J.B.; Dekker, E. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008, 135, 82–90. [Google Scholar] [CrossRef]
- Selby, K.; Levin, T.R.; Corley, D.A. Influence of Varying Quantitative Fecal Immunochemical Test Positivity Thresholds on Colorectal Cancer Detection. Ann. Intern. Med. 2019, 170, 736–737. [Google Scholar] [CrossRef]
- Shaukat, A.; Levin, T.R. Current and future colorectal cancer screening strategies. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 521–531. [Google Scholar] [CrossRef]
- Lidgard, G.P.; Domanico, M.J.; Bruinsma, J.J.; Light, J.; Gagrat, Z.D.; Oldham-Haltom, R.L.; Fourrier, K.D.; Allawi, H.; Yab, T.C.; Taylor, W.R.; et al. Clinical performance of an automated stool DNA assay for detection of colorectal neoplasia. Clin. Gastroenterol. Hepatol. 2013, 11, 1313–1318. [Google Scholar] [CrossRef]
- Shaukat, A.; Kahi, C.J.; Burke, C.A.; Rabeneck, L.; Sauer, B.G.; Rex, D.K. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am. J. Gastroenterol. 2021, 116, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Loktionov, A. Biomarkers for detecting colorectal cancer non-invasively: DNA, RNA or proteins? World J. Gastrointest. Oncol. 2020, 12, 124–148. [Google Scholar] [CrossRef] [PubMed]
- Potter, N.T.; Hurban, P.; White, M.N.; Whitlock, K.D.; Lofton-Day, C.E.; Tetzner, R.; Koenig, T.; Quigley, N.B.; Weiss, G. Validation of a real-time PCR-based qualitative assay for the detection of methylated SEPT9 DNA in human plasma. Clin. Chem. 2014, 60, 1183–1191. [Google Scholar] [CrossRef]
- Johnson, D.A.; Barclay, R.L.; Mergener, K.; Weiss, G.; König, T.; Beck, J.; Potter, N.T. Plasma Septin9 versus fecal immunochemical testing for colorectal cancer screening: A prospective multicenter study. PLoS ONE 2014, 9, e98238. [Google Scholar] [CrossRef]
- Pittayanon, R.; Lau, J.T.; Leontiadis, G.I.; Tse, F.; Yuan, Y.; Surette, M.; Moayyedi, P. Differences in Gut Microbiota in Patients With vs Without Inflammatory Bowel Diseases: A Systematic Review. Gastroenterology 2020, 158, 930–946.e1. [Google Scholar] [CrossRef]
- Swidsinski, A.; Ladhoff, A.; Pernthaler, A.; Swidsinski, S.; Loening-Baucke, V.; Ortner, M.; Weber, J.; Hoffmann, U.; Schreiber, S.; Dietel, M.; et al. Mucosal flora in inflammatory bowel disease. Gastroenterology 2002, 122, 44–54. [Google Scholar] [CrossRef]
- Lepage, P.; Häsler, R.; Spehlmann, M.E.; Rehman, A.; Zvirbliene, A.; Begun, A.; Ott, S.; Kupcinskas, L.; Doré, J.; Raedler, A.; et al. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology 2011, 141, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Y.; Xiang, J.; Xiang, S.; Zhao, Y.; Xiao, M.; Du, F.; Ji, H.; Kaboli, P.J.; Wu, X.; et al. Metagenome Analysis of Intestinal Bacteria in Healthy People, Patients With Inflammatory Bowel Disease and Colorectal Cancer. Front. Cell Infect. Microbiol. 2021, 11, 599734. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, Q.; Zhang, M.; Liu, C.; Su, Q.; Zhang, L.; Xu, Z.; Lu, W.; Ching, J.; Tang, W.; et al. Noninvasive, microbiome-based diagnosis of inflammatory bowel disease. Nat. Med. 2024, 30, 3555–3567. [Google Scholar] [CrossRef]
- Kang, D.-Y.; Park, J.-L.; Yeo, M.-K.; Kang, S.-B.; Kim, J.-M.; Kim, J.S.; Kim, S.-Y. Diagnosis of Crohn’s disease and ulcerative colitis using the microbiome. BMC Microbiol. 2023, 23, 336. [Google Scholar] [CrossRef]
- Pratt, M.; Forbes, J.D.; Knox, N.C.; Bernstein, C.N.; Van Domselaar, G. Microbiome-Mediated Immune Signaling in Inflammatory Bowel Disease and Colorectal Cancer: Support From Meta-omics Data. Front. Cell Dev. Biol. 2021, 9, 716604. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, S.; Schulte, B.; Al-Massad, N.; Thieme, F.; Schulte, D.M.; Bethge, J.; Rehman, A.; Tran, F.; Aden, K.; Häsler, R.; et al. Increased Tryptophan Metabolism Is Associated with Activity of Inflammatory Bowel Diseases. Gastroenterology 2017, 153, 1504–1516.e2. [Google Scholar] [CrossRef]
- Garrett, W.S. The gut microbiota and colon cancer. Science 2019, 364, 1133–1135. [Google Scholar] [CrossRef]
- Yachida, S.; Mizutani, S.; Shiroma, H.; Shiba, S.; Nakajima, T.; Sakamoto, T.; Watanabe, H.; Masuda, K.; Nishimoto, Y.; Kubo, M.; et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 2019, 25, 968–976. [Google Scholar] [CrossRef]
- Dejea, C.M.; Fathi, P.; Craig, J.M.; Boleij, A.; Taddese, R.; Geis, A.L.; Wu, X.; DeStefano Shields, C.E.; Hechenbleikner, E.M.; Huso, D.L.; et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 2018, 359, 592–597. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.-F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.; Rosella, O.; Nov, R.; Young, G. Colonic epithelium is diffusely abnormal in ulcerative colitis and colorectal cancer. Gut 1995, 36, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, L.; Bi, J.; Häkkinen, L.; Larjava, H. Integrin αvβ6: Structure, function and role in health and disease. Int. J. Biochem. Cell Biol. 2018, 99, 186–196. [Google Scholar] [CrossRef]
- Livanos, A.E.; Dunn, A.; Fischer, J.; Ungaro, R.C.; Turpin, W.; Lee, S.-H.; Rui, S.; Del Valle, D.M.; Jougon, J.J.; Martinez-Delgado, G.; et al. Anti-Integrin αvβ6 Autoantibodies Are a Novel Biomarker That Antedate Ulcerative Colitis. Gastroenterology 2023, 164, 619–629. [Google Scholar] [CrossRef]
- Rydell, N.; Ekoff, H.; Hellström, P.M.; Movérare, R. Measurement of Serum IgG Anti-Integrin αvβ6 Autoantibodies Is a Promising Tool in the Diagnosis of Ulcerative Colitis. J. Clin. Med. 2022, 11, 1881. [Google Scholar] [CrossRef]
- Kuwada, T.; Shiokawa, M.; Kodama, Y.; Ota, S.; Kakiuchi, N.; Nannya, Y.; Yamazaki, H.; Yoshida, H.; Nakamura, T.; Matsumoto, S.; et al. Identification of an Anti-Integrin αvβ6 Autoantibody in Patients With Ulcerative Colitis. Gastroenterology 2021, 160, 2383–2394.e21. [Google Scholar] [CrossRef]
- West, N.R.; Hegazy, A.N.; Owens, B.M.J.; Bullers, S.J.; Linggi, B.; Buonocore, S.; Coccia, M.; Görtz, D.; This, S.; Stockenhuber, K.; et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat. Med. 2017, 23, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Ross, C.; Chande, N.; Gregor, J.; Ponich, T.; Khanna, R.; Sey, M.; Beaton, M.; Yan, B.; Kim, R.B.; et al. High oncostatin M predicts lack of clinical remission for patients with inflammatory bowel disease on tumor necrosis factor α antagonists. Sci. Rep. 2022, 12, 1185. [Google Scholar] [CrossRef]
- Suri, K.; Bubier, J.A.; Wiles, M.V.; Shultz, L.D.; Amiji, M.M.; Hosur, V. Role of MicroRNA in Inflammatory Bowel Disease: Clinical Evidence and the Development of Preclinical Animal Models. Cells 2021, 10, 2204. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, O.G.; Russell, A.K.; Henneken, L.M.; Fothergill, L.; Motyer, A.; Reid, H.; Rossjohn, J.; Bryant, V.; Anderson, R.P.; Hardy, M.Y.; et al. Blood-Based T-Cell Diagnosis of Celiac Disease. Gastroenterology 2025, 169, 1253–1267.e5. [Google Scholar] [CrossRef] [PubMed]
- Coto, L.; Mendia, I.; Sousa, C.; Bai, J.C.; Cebolla, A. Determination of gluten immunogenic peptides for the management of the treatment adherence of celiac disease: A systematic review. World J. Gastroenterol. 2021, 27, 6306–6321. [Google Scholar] [CrossRef]
- Gkikas, K.; Gianolio, L.; Kavanagh, M.; White, B.; Kerbiriou, C.; Lima, M.; Svolos, V.; Hansen, R.; Russell, R.K.; Gerasimidis, K. Comparing urine and stool gluten immunogenic peptides for detecting compliance to gluten-free diets. Pediatr. Res. 2025. [Google Scholar] [CrossRef]
- Guz-Mark, A.; Perets, T.T.; Biran, N.; Jack, Y.; Zevit, N.; Silbermintz, A.; Matar, M.; Nachmias-Friedler, V.; Waisbourd-Zinman, O.; Bar-Lev, M.R.; et al. Gluten Immunogenic Peptides Are Not Correlated With Reported Adherence to Gluten-Free Diet in Children With Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2023, 77, 244–248. [Google Scholar] [CrossRef]

| Laboratory Test | Clinical Utility | Advantages | Limitations |
|---|---|---|---|
| Urea breath test (UBT) | Detection of active H. pylori infection, disease monitoring, and effectiveness of treatment. |
|
|
| H. pylori stool antigen test (HpSAT) | Detection of active H. pylori infection, disease monitoring, and effectiveness of treatment. |
|
|
| Serology | Detection of H. pylori infection. |
|
|
| H. pylori molecular tests | Detection of H. pylori infection and antibiotic resistance. |
|
|
| Laboratory Test | Clinical Utility | Advantages | Limitations |
|---|---|---|---|
| Anti-tTG IgA serology | First-line screening test for celiac disease. |
|
|
| EMA-IgA/IgG Serology | Second-line screening test and confirmatory test. |
|
|
| DGP-IgG | Useful in children <2 years old and in IgA deficiency. |
|
|
| HLA-DQ2/DQ8 | Used as a rule-out test for celiac (negative results, rules out celiac disease). |
|
|
| Laboratory Test | Clinical Utility | Advantages | Limitations |
|---|---|---|---|
| Fecal calprotectin | Helps to differentiate IBD from IBS, assess mucosal healing, where concentrations reflect active inflammation, monitor therapy, and predict relapse. |
|
|
| CRP | Levels correlate with disease activity and active inflammation; levels correlate with clinical relapse and can be used to predict treatment discontinuation. |
|
|
| pANCA | Used in combination with ASCA to discriminate between UC and CD. Positive pANCA and negative ASCA specific for UC; helps to distinguish between UC and CD. Antibody levels correlate with disease severity. |
|
|
| ASCA | When used in together with pANCA, positive ASCA is specific to CD and can be used to distinguish between CD and UC and predict disease. Antibody levels correlate with disease localization in pediatrics. |
|
|
| Laboratory Test | Clinical Utility | Advantages | Limitations |
|---|---|---|---|
| FOBT | Screening test for CRC. |
|
|
| FIT | Screening test for CRC. |
|
|
| mts-DNA (Cologuard) | Screening for CRC. |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agbor, T.A.; Khan, W.I. Recent Trend of Laboratory Tests in Common Gastrointestinal Tract Disorders. Diagnostics 2025, 15, 2998. https://doi.org/10.3390/diagnostics15232998
Agbor TA, Khan WI. Recent Trend of Laboratory Tests in Common Gastrointestinal Tract Disorders. Diagnostics. 2025; 15(23):2998. https://doi.org/10.3390/diagnostics15232998
Chicago/Turabian StyleAgbor, Terence A., and Waliul I. Khan. 2025. "Recent Trend of Laboratory Tests in Common Gastrointestinal Tract Disorders" Diagnostics 15, no. 23: 2998. https://doi.org/10.3390/diagnostics15232998
APA StyleAgbor, T. A., & Khan, W. I. (2025). Recent Trend of Laboratory Tests in Common Gastrointestinal Tract Disorders. Diagnostics, 15(23), 2998. https://doi.org/10.3390/diagnostics15232998





