Complementary Role of Ultrasound and Clinical Features in Assessing Carpal Tunnel Syndrome Severity: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Clinical Assessment
2.3. Ultrasound Assessment
2.4. Electrophysiological Assessment
2.5. Statistical Analysis
3. Results
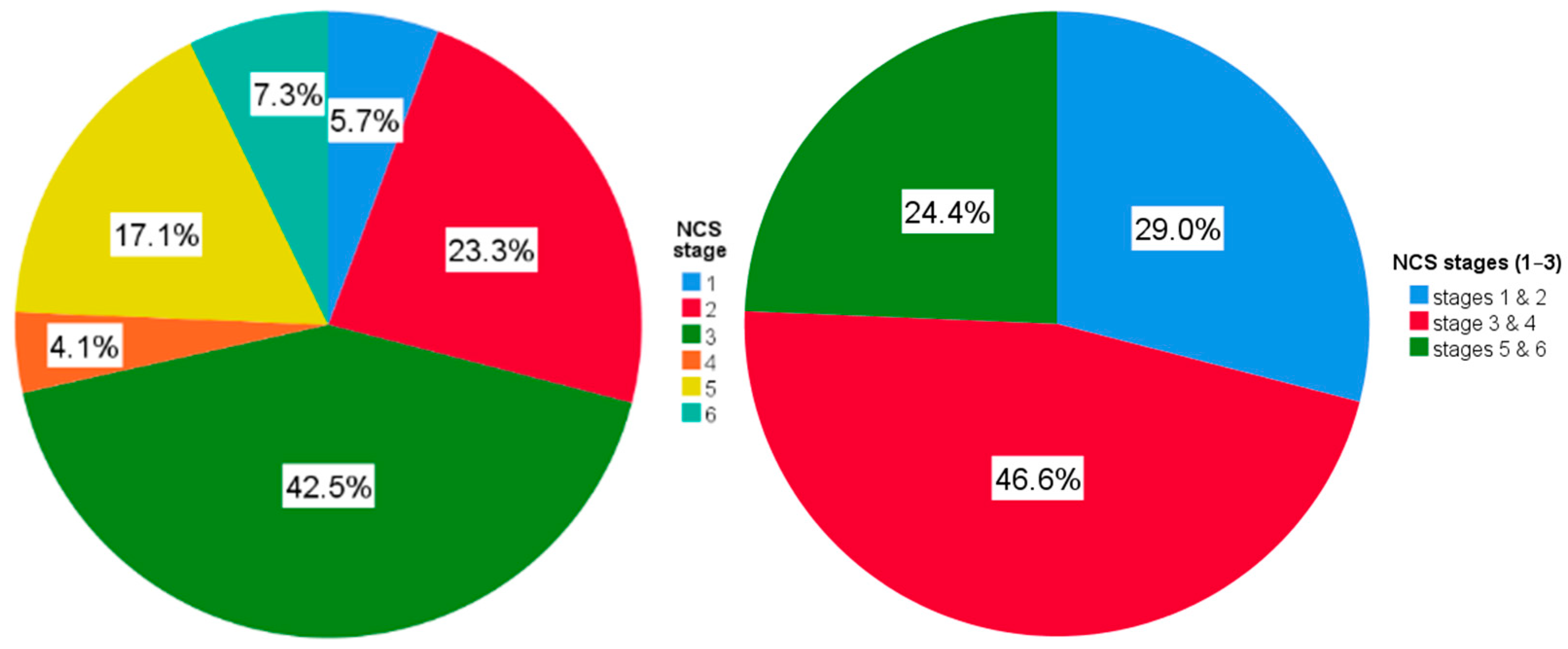
3.1. Demographic, Clinical, Ultrasound and NCS Characteristics
3.2. Univariate Analysis
3.3. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| BCTQ | Boston Carpal Tunnel Questionnaire |
| CI | Confidence interval |
| CMAP | Compound Muscle Action Potential |
| CSA | Cross Sectional Area |
| CTS | Carpal Tunnel Syndrome |
| EMG | Electromyography |
| FRB | flexor retinaculum bowing |
| GEE | Generalized Estimating Equations |
| i/oFR | Flattening ratio at the carpal tunnel inlet/outlet |
| MN | Median nerve |
| NCS | Nerve Conduction Studies |
| OR | Odds ratio |
| PD | Power Doppler |
| QIC | Quasi-likelihood under the independence model criterion |
| SD | Standard deviation |
| SNAP | Sensory Nerve Action Potential |
References
- Hulkkonen, S.; Lampainen, K.; Auvinen, J.; Miettunen, J.; Karppinen, J.; Ryhänen, J. Incidence and operations of median, ulnar and radial entrapment neuropathies in Finland: A nationwide register study. J. Hand Surg. Eur. Vol. 2020, 45, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.B.; Fundaun, J.; Tampin, B. Entrapment neuropathies: A contemporary approach to pathophysiology, clinical assessment, and management. Pain. Rep. 2020, 5, e829. [Google Scholar] [CrossRef] [PubMed]
- Padua, L.; Coraci, D.; Erra, C.; Pazzaglia, C.; Paolasso, I.; Loreti, C.; Caliandro, P.; Hobson-Webb, L.D. Carpal tunnel syndrome: Clinical features, diagnosis, and management. Lancet Neurol. 2016, 15, 1273–1284. [Google Scholar] [CrossRef]
- Aroori, S.; Spence, R.A. Carpal tunnel syndrome. Ulst. Med. J. 2008, 77, 6–17. [Google Scholar]
- Atroshi, I.; Gummesson, C.; Johnsson, R.; Ornstein, E.; Ranstam, J.; Rosén, I. Prevalence of carpal tunnel syndrome in a general population. JAMA 1999, 282, 153–158. [Google Scholar] [CrossRef]
- Padua, L.; Mondelli, M.; Italian CTS Study Group. Evolution of hand dysfunction and symptoms in untreated carpal tunnel syndrome. Muscle Nerve 2005, 32, 545–547. [Google Scholar] [CrossRef]
- Mondelli, M.; Rossi, S.; Monti, E.; Aprile, I.; Caliandro, P.; Pazzaglia, C.; Romano, C.; Padua, L. Long term follow-up of carpal tunnel syndrome during pregnancy: A cohort study and review of the literature. Electromyogr. Clin. Neurophysiol. 2007, 47, 259–271. [Google Scholar]
- Foley, M.; Silverstein, B. The long-term burden of work-related carpal tunnel syndrome relative to upper-extremity fractures and dermatitis in Washington State. Am. J. Ind. Med. 2015, 58, 1255–1269. [Google Scholar] [CrossRef]
- Foley, M.; Silverstein, B.; Polissar, N. The economic burden of carpal tunnel syndrome: Long-term earnings of CTS claimants in Washington State. Am. J. Ind. Med. 2007, 50, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, M.S.; Hobson-Webb, L.D.; Boon, A.J.; Alter, K.E.; Hunt, C.H.; Flores, V.H.; Werner, R.A.; Shook, S.J.; Thomas, T.D.; Primack, S.J.; et al. Evidence-based guideline: Neuromuscular ultrasound for the diagnosis of carpal tunnel syndrome. Muscle Nerve 2012, 46, 287–293. [Google Scholar] [CrossRef]
- Salah, A.; AbdelAzim, G.S.; Ahmed, S.F.; Elmesiry, A.M. Ultrasonography as A diagnostic Tool for Clinically Manifested Carpal Tunnel Syndrome with Normal Nerve Conduction Study. Int. J. Med. Arts 2025, 7, 5682–5688. [Google Scholar] [CrossRef]
- Chen, J.; Fowler, J.R. Ultrasound Findings in Patients with Normal Nerve Conduction despite Clinical Signs and Symptoms Consistent with Carpal Tunnel Syndrome. Plast. Reconstr. Surg. 2022, 150, 1025e–1032e. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.D.P. Use of nerve conduction studies in carpal tunnel syndrome. J. Hand Surg. Eur. Vol. 2023, 48, 976–985. [Google Scholar] [CrossRef]
- Song, C.; Lee, S.J.; Kim, S.W.; Kim, S.H.; Jeon, H.R. Electrophysiological Changes in Patients with Carpal Tunnel Syndrome after Open Carpal Tunnel Release. J. Electrodiagn. Neuromuscul. Dis. 2022, 24, 79–83. [Google Scholar] [CrossRef]
- Tan, P.S.; Tan, S.Y.; Lee, L.Y.; Gunasagaran, J.; Khoo, S.S.; Tan, C.Y. Influence of Preoperative Nerve Conduction Studies on the Outcome of Carpal Tunnel Release Surgery. Acta Orthop. Belg. 2025, 91, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Birsanu, L.; Covali, R.; Roman, E.M.; Chirap-Mitulschi, I.A.; Ciubotaru, A.; Turliuc, M.-D. Romanian validation study of the Boston questionare in patients with carpal tunnel syndrome. Balneo PRM Res. J. 2024, 15, 751. [Google Scholar] [CrossRef]
- Möller, I.; Janta, I.; Backhaus, M.; Ohrndorf, S.; Bong, D.A.; Martinoli, C.; Filippucci, E.; Sconfienza, L.M.; Terslev, L.; Damjanov, N.; et al. The 2017 EULAR standardised procedures for ultrasound imaging in rheumatology. Ann. Rheum. Dis. 2017, 76, 1974–1979. [Google Scholar] [CrossRef]
- Battaglia, F.; Troisi, L.; Cigna, E.; D’alcontres, F.S.; Rizzo, V.; Delia, G. Standardized High-Resolution Ultrasound Protocol for the Diagnosis and Monitoring of Carpal Tunnel Syndrome: A Mixed-Design Observational Study. Diagnostics 2025, 15, 1593. [Google Scholar] [CrossRef]
- Kanagasabai, K. Ultrasound of Median Nerve in the Diagnosis of Carpal Tunnel Syndrome-Correlation with Electrophysiological Studies. Indian J. Radiol. Imaging 2022, 32, 16–29. [Google Scholar] [CrossRef]
- Osiak, K.; Elnazir, P.; Walocha, J.A.; Pasternak, A. Carpal tunnel syndrome: State-of-the-art review. Folia Morphol. 2022, 81, 851–862. [Google Scholar] [CrossRef]
- Luo, Y.T.; Huang, Y.T.; Chiu, V.; Chang, Y.-W.; Horng, Y.-S. Diagnostic meta-analysis of the efficacy of ultrasonography for diagnosing carpal tunnel syndrome: A comparison between Asian and non-Asian populations. J. Formos. Med. Assoc. 2025, 124, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Takata, S.C.; Kysh, L.; Mack, W.J.; Roll, S.C. Sonographic reference values of median nerve cross-sectional area: A protocol for a systematic review and meta-analysis. Syst. Rev. 2019, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, C.; Alexander, M.; Kane, D. The role of ultrasound in the diagnosis and management of carpal tunnel syndrome: A new paradigm. Rheumatology 2015, 54, 9–19. [Google Scholar] [CrossRef]
- Sarría, L.; Cabada, T.; Cozcolluela, R.; Martínez-Berganza, T.; García, S. Carpal tunnel syndrome: Usefulness of sonography. Eur. Radiol. 2000, 10, 1920–1925. [Google Scholar] [CrossRef]
- Ziswiler, H.R.; Reichenbach, S.; Vögelin, E.; Bachmann, L.M.; Villiger, P.M.; Jüni, P. Diagnostic value of sonography in patients with suspected carpal tunnel syndrome: A prospective study. Arthritis Rheum. 2005, 52, 304–311. [Google Scholar] [CrossRef]
- Ng, A.W.H.; Griffith, J.F.; Lee, R.K.L.; Tse, W.; Wong, C.; Ho, P. Ultrasound carpal tunnel syndrome: Additional criteria for diagnosis. Clin. Radiol. 2018, 73, 214.e11–214.e18. [Google Scholar] [CrossRef]
- Akcar, N.; Ozkan, S.; Mehmetoglu, O.; Calisir, C.; Adapinar, B. Value of power Doppler and gray-scale US in the diagnosis of carpal tunnel syndrome: Contribution of cross-sectional area just before the tunnel inlet as compared with the cross-sectional area at the tunnel. Korean J. Radiol. 2010, 11, 632–639. [Google Scholar] [CrossRef]
- Hobson-Webb, L.D.; Massey, J.M.; Juel, V.C.; Sanders, D.B. The ultrasonographic wrist-to-forearm median nerve area ratio in carpal tunnel syndrome. Clin. Neurophysiol. 2008, 119, 1353–1357. [Google Scholar] [CrossRef]
- Mezian, K.; Ricci, V.; Guvener, O.; Jačisko, J.; Novotny, T.; Kara, M.; Ata, A.M.; Wu, W.-T.; Chang, K.-V.; Stecco, C.; et al. EURO-MUSCULUS/USPRM Dynamic Ultrasound Protocols for Wrist and Hand. Am. J. Phys. Med. Rehabil. 2022, 101, e132–e138. [Google Scholar] [CrossRef]
- Bland, J.D. A neurophysiological grading scale for carpal tunnel syndrome. Muscle Nerve 2000, 23, 1280–1283. [Google Scholar] [CrossRef] [PubMed]
- Paniker, P.; Iype, T. Nerve Ultrasound Findings in Carpal Tunnel Syndrome and its Correlation with Clinical and Electrophysiological Data. J. Med. Sci. Health 2022, 8, 59–64. [Google Scholar] [CrossRef]
- Potuznik, P.; Hosek, P.; Kotas, R. Median nerve ultrasonography examination correlates with electrodiagnostic studies for the diagnosis of moderate to severe carpal tunnel syndrome. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2023, 167, 192–198. [Google Scholar] [CrossRef] [PubMed]
- El-Shewi, I.E.-H.; Tawfeek, A.A.; Mohamed, A.A.; Mostafa, M.A. Role of conventional ultrasound and shear wave elastography of median nerve in diagnosis and differentiation of carpal tunnel syndrome severity in correlation with electrodiagnostic studies. Egypt. J. Radiol. Nucl. Med. 2024, 55, 53. [Google Scholar] [CrossRef]
- Rayegani, S.M.; Malekmahmoodi, R.; Aalipour, K.; Nouri, F. The relationship between ultrasound and electrodiagnostic findings in relation of the severity of carpal tunnel syndrome. BMC Musculoskelet. Disord. 2024, 25, 864. [Google Scholar] [CrossRef]
- Rossmann, T.; Pruidze, P.; Veldeman, M.; Weninger, W.J.; Grisold, W.; Chang, K.-V.; Meng, S. Successful evaluation of a new image-based parameter for the diagnosis of carpal tunnel syndrome: Ultrasound assessment of longitudinal median nerve gliding in patients, healthy volunteers, and cadavers. Eur. J. Phys. Rehabil. Med. 2024, 60, 671–679. [Google Scholar] [CrossRef]
- Thomas, V.; Sudhakaran, S.; George, M.; Sebastian; Ponnezhathu, J. Sonographic analysis of transverse mobility of median nerve in carpal tunnel syndrome: A novel technique. Kerala J. Orthop. 2022, 1, 26–31. [Google Scholar] [CrossRef]
- Lo, I.N.; Hsu, P.C.; Huang, Y.C.; Yeh, C.-K.; Yang, Y.-C.; Wang, J.-C. Dynamic Ultrasound Assessment of Median Nerve Mobility Changes Following Corticosteroid Injection and Carpal Tunnel Release in Patients with Carpal Tunnel Syndrome. Front. Neurol. 2021, 12, 710511. [Google Scholar] [CrossRef] [PubMed]
- Padua, L.; Padua, R.; Aprile, I.; Tonali, P. Italian multicentre study of carpal tunnel syndrome. Differences in the clinical and neurophysiological features between male and female patients. J. Hand Surg. Br. 1999, 24, 579–582. [Google Scholar] [CrossRef]
- Mitake, T.; Iwatsuki, K.; Hirata, H. Differences in characteristics of carpal tunnel syndrome between male and female patients. J. Orthop. Sci. 2020, 25, 843–846. [Google Scholar] [CrossRef]
- Mondelli, M.; Aprile, I.; Ballerini, M.; Ginanneschi, F.; Reale, F.; Romano, C.; Rossi, S.; Padua, L. Sex differences in carpal tunnel syndrome: Comparison of surgical and non-surgical populations. Eur. J. Neurol. 2005, 12, 976–983. [Google Scholar] [CrossRef]
- Mathew, A.E.; John, T. A Clinical and Neurophysiological Analysis of Idiopathic Carpal Tunnel Syndrome with Respect to Gender and Occupation. Ann. Indian Acad. Neurol. 2021, 24, 865–872. [Google Scholar] [CrossRef]
- McDiarmid, M.; Oliver, M.; Ruser, J.; Gucer, P. Male and female rate differences in carpal tunnel syndrome injuries: Personal attributes or job tasks? Environ. Res. 2000, 83, 23–32. [Google Scholar] [CrossRef]
- Sassi, S.A.; Giddins, G. Gender differences in carpal tunnel relative cross-sectional area: A possible causative factor in idiopathic carpal tunnel syndrome. J. Hand Surg. Eur. Vol. 2016, 41, 638–642. [Google Scholar] [CrossRef]
- Warren, J.R.; Link, R.C.; Cheng, A.L.; Sinclair, M.K.; Sorensen, A.A. Carpal tunnel syndrome and sleep, a systematic review and meta-analysis. Hand Surg. Rehabil. 2024, 43, 101698. [Google Scholar] [CrossRef]
- Rubin, G.; Orbach, H.; Rinott, M.; Rozen, N. Relationship between electrodiagnostic findings and sleep disturbance in carpal tunnel syndrome: A controlled objective and subjective study. J. Int. Med. Res. 2020, 48, 300060519862673. [Google Scholar] [CrossRef]
- Goorman, A.M.; Dawson, S.; Schneck, C.; Pierce, D. Association of Sleep and Hand Function in People with Carpal Tunnel Syndrome. Am. J. Occup. Ther. 2019, 73, 7306205050p1–7306205050p7. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Pulido, C.; Ortiz-Corredor, F. Measurement properties of the Boston Carpal Tunnel Questionnaire in subjects with neurophysiological confirmation of carpal tunnel syndrome: A Rasch analysis perspective. Qual. Life Res. 2021, 30, 2697–2710. [Google Scholar] [CrossRef]
- Forcelini, C.M.; Chichelero, E.R.; de Oliveira, A.T.; da Silva, F.T.; Durigan, P.H.B.; Ramos, N.O.; Bianchini, L.; Battistel, B.L.I.; Borghetti, V. Boston Carpal Tunnel Questionnaire and Severity of Carpal Tunnel Syndrome. J. Clin. Neuromuscul. Dis. 2022, 23, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.; Prakash, H.R.; Challawala, S. Association between Bilateral Grip and Pinch Strength in Sub-clinical Carpal Tunnel Syndrome in Patients with Unilateral Carpal Tunnel Syndrome. Int. Res. J. Multidiscip. Scope 2024, 5, 9–17. [Google Scholar] [CrossRef]
- Gonzalez-Suarez, C.B.; Buenavente, L.D.; Cua, R.C.A.; Fidel, M.B.C.; Cabrera, J.-T.C.; Regala, C.F.G. Inter-Rater and Intra-Rater Reliability of Sonographic Median Nerve and Wrist Measurements. J. Med. Ultrasound 2018, 26, 14–23. [Google Scholar] [CrossRef]
- Yao, B.; Roll, S.C. An ultrasound study of the mobility of the median nerve during composite finger movement in the healthy young wrist. Muscle Nerve 2022, 65, 82–88. [Google Scholar] [CrossRef] [PubMed]
| Parameter Name | Definition of Parameter | Cut-Off Value | In Sample (n = 193) |
|---|---|---|---|
| iCSA | Measured at the pisiform * | 10 mm2 [23] | 13.8 ± 3.8 |
| oCSA | Measured at the hamate’s hook * | 10 mm2 [24] | 9.8 ± 2.7 |
| mCSA | Largest CSA measured, irrespective of bony landmarks * | 10 mm2 [25] | 15.3 ± 4.2 |
| pCSA | CSA at the proximal third of the pronator quadratus * | - | 6.8 ± 1.4 |
| iFR | Ratio of long to short axis of the median nerve at the tunnel inlet | >2.5 [26] | 3.1 ± 0.8 |
| oFR | Ratio of long to short axis of the median nerve at the tunnel outlet | >2.6 [27] | 2.9 ± 0.7 |
| CSA ratio | Ratio of CSA at the carpal tunnel inlet to CSA at the proximal third of the pronator quadratus | 1.4 [28] | 2.1 ± 0.6 |
| PD signal | Detection of intraneural vascular signal within the MN | absent/ present | 68.4% |
| MN echogenicity | Evaluation of internal pattern of the median nerve (e.g., normal, hypoechoic, hyperechoic) | normal **/ abnormal | 96.9% |
| MN mobility | Assessment of nerve displacement during wrist and finger movement | normal ***/ abnormal [29] | 60.1% |
| FRB | Perpendicular distance from the flexor retinaculum to the trapezium–pisiform line | 2.5 [24] | 3.8 ± 1.2 |
| inlet median nerve tapering | Visual/qualitative alteration in median nerve diameter at the inlet in longitudinal view | absent/ present | 90.7% |
| outlet median nerve tapering | Visual/qualitative alteration of the MN’s caliber at the carpal tunnel outlet in longitudinal view | absent/ present | 20.2% |
| anatomical variants | Detection of bifid MN, persistent median artery, or accessory tendons/muscles | absent/ present | 11.9% |
| Patients (n = 100) | Hands (n = 193) | ||
|---|---|---|---|
| Age (y) | 58.5 ± 9.8 | Age at CTS onset (years) | 54 ± 11 |
| Female | 93.0% | Age at CTS diagnosis (years) | 57 ± 10 |
| Menopause age (y) | 47.8 ± 5.6 | Vitamin B supplements | 48.2% |
| Urban dwelling | 62.0% | CTS symptoms during sleep | 75.1% |
| Secondary school | 10.0% | Worsening symptoms in elevated position of hand | 77.2% |
| High school | 64.0% | Worsening symptoms with repetitive hand movement | 66.3% |
| College | 26.0% | Worsening symptoms upon changing hand position | 71.5% |
| Professional active | 57.0% | Improving symptoms with hand shaking/flicking | 67.9% |
| Smoking, current | 18.0% | Tinel’s sign | 39.9% |
| Smoking, ever | 37.0% | Phalen’s sign | 64.8% |
| Alcohol, frequent | 48.0% | Morning hand stiffness | 74.1% |
| BMI (kg/m2) | 29.2 ± 5.0 | Decrease/loss of sensitivity in median nerve territory | 36.8% |
| Arterial hypertension | 57.0% | Muscle weakness/atrophy in median nerve territory | 20.7% |
| Raynaud phenomena | 3.0% | Dry skin in median nerve territory | 11.4% |
| BCTQ (sensory) | 32 ± 10 | ||
| BCTQ (motor) | 21 ± 8 | ||
| Grip strength (kgf) | 19.4 ± 5.8 | ||
| Clinical and Ultrasound Variables | ρ | p | 95% CI |
|---|---|---|---|
| Age at study entry | 0.229 | 0.003 | 0.076–0.383 |
| Body mass index | 0.169 | 0.027 | 0.028–0.308 |
| Age at CTS onset | 0.197 | 0.010 | 0.049–0.356 |
| Age at CTS diagnosis | 0.188 | 0.014 | 0.040–0.351 |
| BCTQ (sensitive) | 0.252 | 0.001 | 0.111–0.389 |
| BCTQ (motor) | 0.365 | 0.000 | 0.235–0.487 |
| mCSA | 0.298 | 0.000 | 0.154–0.426 |
| iCSA | 0.240 | 0.002 | 0.086–0.372 |
| FRB | 0.344 | 0.000 | 0.184–0.478 |
| CSA ratio | 0.196 | 0.010 | 0.043–0.330 |
| χ2 | p | Cramer’s V | |
| Sex | 11.9 | 0.037 | 0.249 |
| Arterial hypertension | 12.5 | 0.029 | 0.256 |
| Vitamin supplements | 19.2 | 0.002 | 0.317 |
| CTS symptoms during sleep | 12.8 | 0.025 | 0.259 |
| Tinel’s sign | 12.4 | 0.030 | 0.254 |
| Phalen’s sign | 10.9 | 0.048 | 0.239 |
| Decrease/loss of sensitivity in median nerve territory | 26.1 | 0.000 | 0.370 |
| Muscle weakness/atrophy in median nerve territory | 54.5 | 0.000 | 0.534 |
| MN Doppler signal | 25.1 | 0.000 | 0.362 |
| Partial/no median nerve mobility | 23.9 | 0.000 | 0.353 |
| Predictor (OR, 95% CI)/Model | 1 | 2 | 3.1 | 3.2 | 3.3 | 3.4 | 3.5 | 3.6 | 3.7 |
|---|---|---|---|---|---|---|---|---|---|
| Age at study entry (y) | 1.03 | 0.99 | 1.01 | 1 | 1.03 | 0.99 | 1 | 1.02 | 1.03 |
| 0.99–1.08 | 0.93–1.04 | 0.95–1.06 | 0.94–1.06 | 0.97–1.09 | 0.93–1.05 | 0.93–1.06 | 0.96–1.09 | 0.97–1.10 | |
| Sex (male) | 2.26 | 6.16 * | 9.99 * | 5.2 | 9.12 * | 6.20 * | 5.16 | 8.59 * | 3.88 |
| 0.58–8.85 | 1.34–28.2 | 1.13–88.5 | 0.96–28.0 | 1.42–58.6 | 1.37–28.1 | 0.92–29.1 | 1.46–50.4 | 0.59–25.3 | |
| Body mass index (kg/m2) | 1.04 | 1 | 1 | 1.01 | 0.97 | 1.01 | 1 | 0.98 | 1.01 |
| 0.97–1.12 | 0.88–1.14 | 0.89–1.13 | 0.88–1.14 | 0.85–1.11 | 0.89–1.13 | 0.89–1.13 | 0.87–1.12 | 0.90–1.13 | |
| Arterial hypertension (no) | 0.78 | 0.6 | 0.52 | 0.7 | 0.62 | 0.59 | 0.64 | 0.67 | 1.1 |
| 0.34–1.80 | 0.20–1.79 | 0.18–1.52 | 0.23–2.15 | 0.19–2.01 | 0.20–1.77 | 0.22–1.87 | 0.20–2.22 | 0.40–3.02 | |
| Supplements (no) | - | 1.46 | 1.47 | 1.64 | 1.68 | 1.48 | 1.8 | 1.5 | 1.22 |
| 0.56–3.77 | 0.54–3.95 | 0.64–4.19 | 0.66–4.31 | 0.57–3.87 | 0.70–4.61 | 0.58–3.86 | 0.53–2.83 | ||
| Symptoms in sleep (no) | - | 0.38 * | 0.42 * | 0.36 * | 0.37 | 0.38 * | 0.36 * | 0.37 * | 0.33 * |
| 0.19–0.77 | 0.18–0.96 | 0.15–0.85 | 0.12–1.14 | 0.18–0.81 | 0.15–0.85 | 0.14–0.99 | 0.11–0.98 | ||
| Tinel’s sign (no) | - | 0.8 | 1.07 | 0.77 | 1.12 | 0.83 | 0.81 | 1.14 | 0.7 |
| 0.46–1.42 | 0.49–2.30 | 0.37–1.60 | 0.44–2.88 | 0.44–1.58 | 0.38–1.72 | 0.47–2.73 | 0.25–1.98 | ||
| Phalen’s sign (no) | - | 1.34 | 1.48 | 1.45 | 1.62 | 1.39 | 1.47 | 1.56 | 1.7 |
| 0.55–2.29 | 0.27–1.46 | 0.44–2.30 | 0.28–1.44 | 0.52–2.25 | 0.40–2.11 | 0.32–1.26 | 0.55–1.80 | ||
| Decrease/loss of sensitivity (no) | - | 0.43 * | 0.67 | 0.44 * | 0.49 | 0.42 * | 0.44 * | 0.44 * | 0.61 |
| 0.23–0.82 | 0.23–1.89 | 0.22–0.86 | 0.21–1.11 | 0.23–0.79 | 0.23–0.82 | 0.21–0.93 | 0.30–1.24 | ||
| Muscle weakness/atrophy (no) | - | 0.26 * | 0.28 * | 0.32 * | 0.37 | 0.24 * | 0.30 * | 0.39 | 0.35 |
| 0.10–0.64 | 0.10–0.83 | 0.12–0.89 | 0.12–1.15 | 0.10–0.60 | 0.10–0.90 | 0.14–1.10 | 0.12–1.01 | ||
| Motor BCTQ | - | 1.06 | 1.02 | 1.05 | 1.03 | 1.06 | 1.05 | 1.03 | 1.01 |
| 0.98–1.15 | 0.96–1.09 | 0.96–1.16 | 0.96–1.11 | 0.98–1.14 | 0.95–1.15 | 0.96–1.11 | 0.90–1.14 | ||
| MN maximum area (mm2) | - | - | 1.12 | - | 1.15 * | - | - | 1.16 * | 1.09 |
| 0.98–1.28 | 1.02–1.29 | 1.03–1.30 | 0.96–1.24 | ||||||
| Retinaculum-SP line distance (mm) | - | - | - | 1.15 | 1.23 | - | 1.14 | 1.2 | 1.22 |
| 0.83–1.60 | 0.93–1.64 | 0.82–1.59 | 0.85–1.68 | 0.73–2.05 | |||||
| MN Doppler signal (no) | - | - | - | - | - | 1.13 | 1.01 | 0.91 | 1.06 |
| 0.55–2.30 | 0.34–2.99 | 0.42–2.00 | 0.19–6.02 | ||||||
| Partial/no MN mobility (no) | - | - | - | - | - | - | - | - | 0.36 * |
| 0.17–0.74 | |||||||||
| Log pseudolikelihood | −195 | −169 | −162 | −158 | −154 | −164 | −152 | −150 | −146 |
| pseudo-R2 | 0.04 | 0.17 | 0.19 | 0.18 | 0.2 | 0.19 | 0.21 | 0.22 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, D.N.; Popescu, C.C.; Morari, O.; Blidaru, N.; Dima, A.; Catanoiu, I.A.; Rakoczy, A.; Otobic, I.; Parvu, M.I.; Codreanu, C.; et al. Complementary Role of Ultrasound and Clinical Features in Assessing Carpal Tunnel Syndrome Severity: A Cross-Sectional Study. Diagnostics 2025, 15, 2985. https://doi.org/10.3390/diagnostics15232985
Popescu DN, Popescu CC, Morari O, Blidaru N, Dima A, Catanoiu IA, Rakoczy A, Otobic I, Parvu MI, Codreanu C, et al. Complementary Role of Ultrasound and Clinical Features in Assessing Carpal Tunnel Syndrome Severity: A Cross-Sectional Study. Diagnostics. 2025; 15(23):2985. https://doi.org/10.3390/diagnostics15232985
Chicago/Turabian StylePopescu, Daniela Nicoleta, Claudiu Costinel Popescu, Oana Morari, Natalia Blidaru, Alina Dima, Ioana Adriana Catanoiu, Alice Rakoczy, Ioana Otobic, Magda Ileana Parvu, Catalin Codreanu, and et al. 2025. "Complementary Role of Ultrasound and Clinical Features in Assessing Carpal Tunnel Syndrome Severity: A Cross-Sectional Study" Diagnostics 15, no. 23: 2985. https://doi.org/10.3390/diagnostics15232985
APA StylePopescu, D. N., Popescu, C. C., Morari, O., Blidaru, N., Dima, A., Catanoiu, I. A., Rakoczy, A., Otobic, I., Parvu, M. I., Codreanu, C., & Enache, L. (2025). Complementary Role of Ultrasound and Clinical Features in Assessing Carpal Tunnel Syndrome Severity: A Cross-Sectional Study. Diagnostics, 15(23), 2985. https://doi.org/10.3390/diagnostics15232985






