Pulmonary Embolism and ABO Blood Type: A Systematic Review
Abstract
1. Introduction
- Assess whether non-O blood groups confer a higher risk of incident PE.
- Compare risk profiles among specific ABO phenotypes (A, B, AB).
- Explore associations with PE prognosis, including recurrence, mortality, and CTEPH.
2. Materials and Methods
2.1. Study Design and Reporting Framework
2.2. Information Sources and Search Strategy
2.3. Eligibility Criteria (PICO Framework)
- •
- Population: Adults (≥18 years), any ancestry.
- •
- Exposure: ABO blood group phenotype or ABO locus genotype.
- •
- Comparator: O vs. non-O, or individual subgroups (A, B, AB vs. O).
- •
- Outcomes: Incident PE and recurrent PE (primary); secondary: short-term mortality (in-hospital/30 days), long-term mortality (≥90 days/1 year), severity markers (hemodynamic instability, right ventricular (RV) dysfunction, Pulmonary Embolism Severity Index (PESI/sPESI), and PE complications (CTEPH).
- •
- Study types: Cohort, case–control, registry-based analyses.
- •
- Excluded: Pediatric-only studies, case reports, small case series, conference abstracts without full text, non-human studies, meta-analyses, systematic reviews, and narrative reviews.
- •
- Timeframe: All years up to 1 August 2025.
- •
- Language: English.
2.4. Variables and Definitions
2.5. Data Extraction
2.6. Data Synthesis
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Association Between ABO Blood Group and Pulmonary Embolism
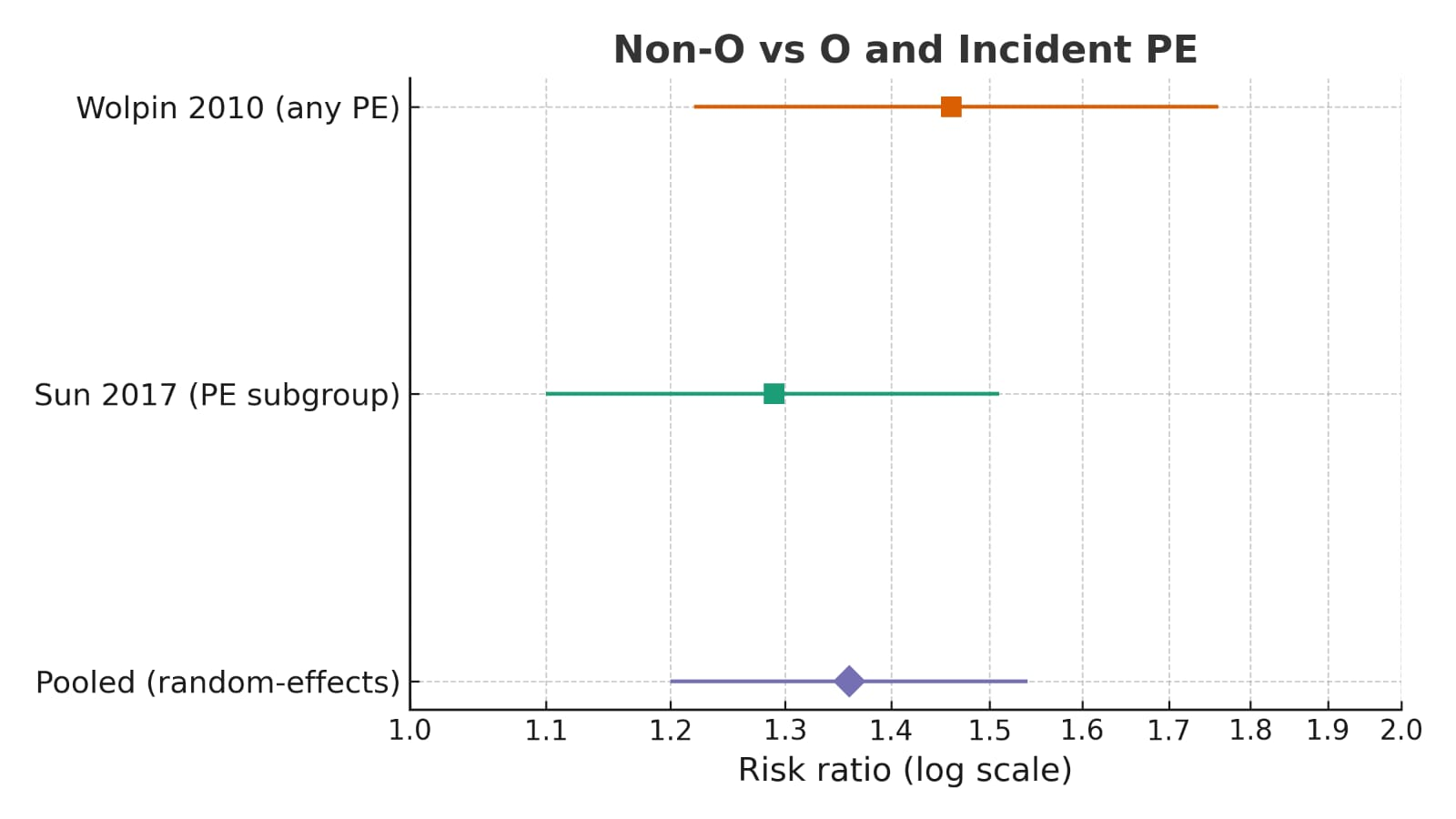
3.4. Pooled Evidence
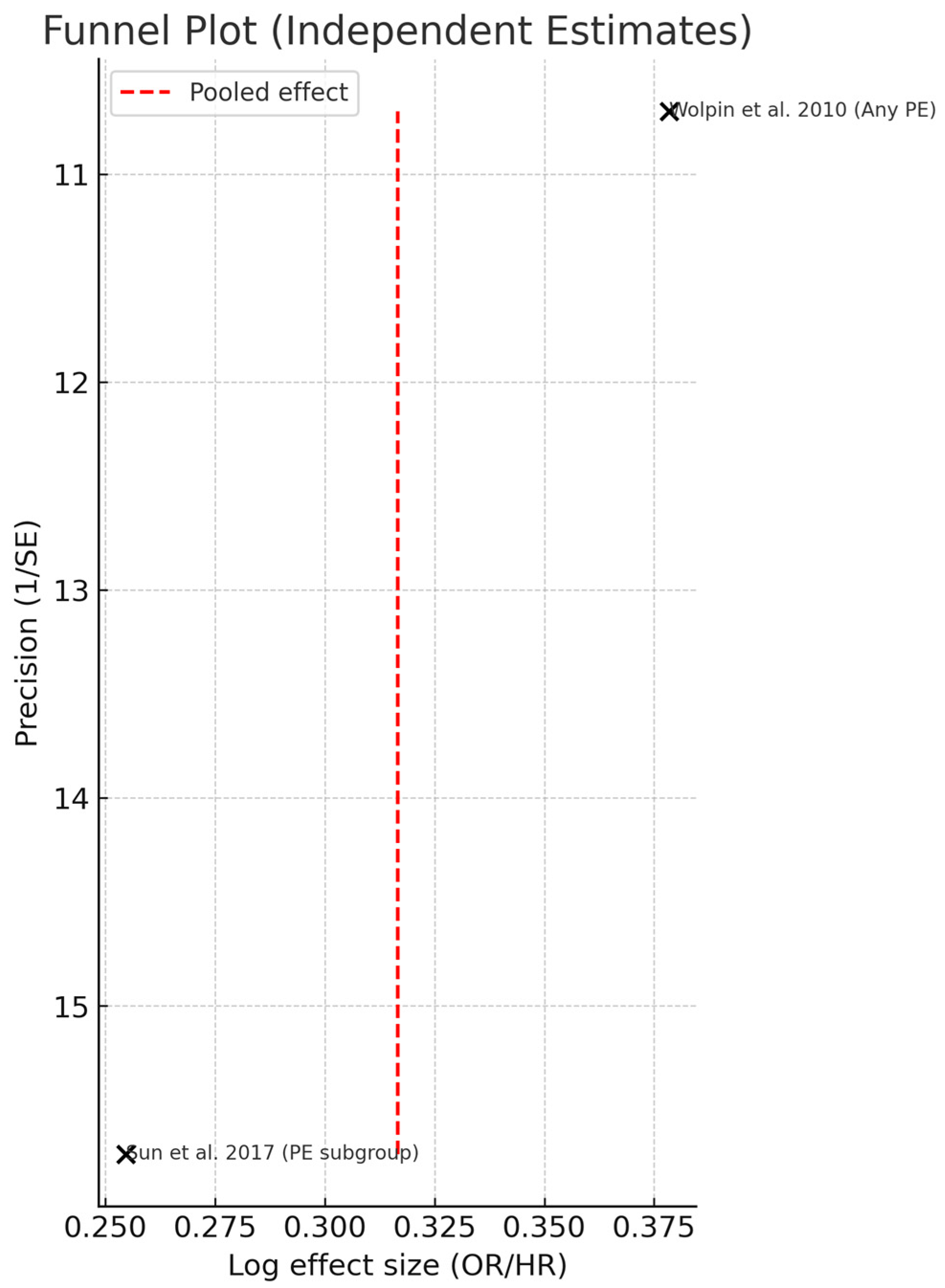
3.5. Publication Bias Assessment
4. Discussion
5. Conclusions
5.1. Summary of Findings
5.2. Clinical and Research Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTEPH | Chronic Thromboembolic Pulmonary Hypertension |
| CTPA | Computed Tomography Pulmonary Angiography |
| DVTDeep | Vein Thrombosis |
| PE | Pulmonary Embolism |
| PESI | Pulmonary Embolism Severity Index |
| sPESI | Simplified Pulmonary Embolism Severity Index |
| VTE | Venous Thromboembolism |
| vWF | von Willebrand Factor |
References
- Wendelboe, A.M.; Raskob, G.E. Global burden of thrombosis: Epidemiologic aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef]
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, A.; Konstantinides, S.V.; McCumber, M.; et al. ISTH Steering Committee for World Thrombosis. Thrombosis: A major contributor to global disease burden. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2363–2371. [Google Scholar] [CrossRef]
- Park, B.; Messina, L.; Dargon, P.; Huang, W.; Ciocca, R.; Anderson, F.A. Recent trends in clinical outcomes and resource utilization for pulmonary embolism in the United States. Chest 2009, 136, 983–990. [Google Scholar] [CrossRef]
- Heit, J.A. Epidemiology of venous thromboembolism. Nat. Rev. Cardiol. 2015, 12, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Horlander, K.T.; Mannino, D.M.; Leeper, K.V. Pulmonary embolism mortality in the United States, 1979–1998. Arch. Intern. Med. 2003, 163, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Filopei, J.; Frank, M.; Ramesh, N.; Verzosa, S.; Ehrlich, M.; Bondarsky, E.; Miller, A.; Steiger, D. Pulmonary infarction secondary to pulmonary embolism: An evolving paradigm. Respirology 2018, 23, 866–872. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Welsby, I. Is ABO blood group truly a risk factor for thrombosis and adverse outcomes? World J. Cardiol. 2014, 6, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Kyrle, P.A.; Rosendaal, F.R.; Eichinger, S. Risk assessment for recurrent venous thrombosis. Lancet 2010, 376, 203–212. [Google Scholar] [CrossRef]
- Martinelli, I. von Willebrand factor and factor VIII as risk factors for arterial and venous thrombosis. Semin. Hematol. 2005, 42, 49–55. [Google Scholar] [CrossRef]
- Geerts, W.H.; Heit, J.A.; Clagett, G.P.; Pineo, G.F.; Colwell, C.W.; Anderson, F.A.; Wheeler, H.B. Prevention of venous thromboembolism. Chest 2008, 133, 381S–453S. [Google Scholar] [CrossRef] [PubMed]
- Folsom, A.R.; Cushman, M.; Tsai, M.Y.; Aleksic, N.; Heckbert, S.R.; Boland, L.L.; Tsai, A.W.; Yanez, N.D.; Rosamond, W.D. A prospective study of venous thromboembolism in relation to factor V Leiden and related factors. Blood 2002, 99, 2720–2725. [Google Scholar] [CrossRef]
- Wu, O.; Bayoumi, N.; Vickers, M.A.; Clark, P. ABO(H) blood groups and vascular disease: A systematic review and meta-analysis. J. Thromb. Haemost. 2008, 6, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Wautrecht, J.C.; Galle, C.; Motte, S.; Dereume, J.P.; Dramaix, M. The role of ABO blood groups in the incidence of deep vein thrombosis. Thromb. Haemost. 1998, 79, 688–689. [Google Scholar] [CrossRef]
- Yamamoto, F.; Cid, E.; Yamamoto, M.; Blancher, A. ABO research in the modern era of genomics. Transfus. Med. Rev. 2012, 26, 103–118. [Google Scholar] [CrossRef]
- Robert, A.; Aillaud, M.F.; Eschwège, V.; Randrianjohany, A.; Scarabin, Y.; Juhan-Vague, I. ABO blood group and risk of venous thrombosis in heterozygous carriers of factor V Leiden. Thromb. Haemost. 2000, 83, 630–631. [Google Scholar] [CrossRef]
- Gill, J.C.; Endres-Brooks, J.; Bauer, P.J.; Marks, W.J., Jr.; Montgomery, R.R. Effect of ABO blood group on diagnosis of von Willebrand disease. Blood 1987, 69, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.V.; O’Donnell, J.S. ABO blood group determines plasma von Willebrand factor levels. Transfusion 2006, 46, 1836–1844. [Google Scholar] [CrossRef]
- Dentali, F.; Sironi, A.P.; Ageno, W.; Turato, S.; Bonfanti, C.; Frattini, F.; Crestani, S.; Franchini, M. Non-O blood type is the commonest genetic risk factor for VTE: Results from a meta-analysis of the literature. Semin. Thromb. Hemost. 2012, 38, 535–548. [Google Scholar] [CrossRef]
- Vasan, S.K.; Rostgaard, K.; Majeed, A.; Ullum, H.; Titlestad, K.E.; Pedersen, O.B.; Erikstrup, C.; Nielsen, K.R.; Melbye, M.; Nyrén, O.; et al. ABO blood group and risk of venous thromboembolism in 1.5 million blood donors. Circulation 2016, 133, 1449–1457. [Google Scholar] [CrossRef]
- Clark, P.; Wu, O. ABO blood groups and thrombosis: A causal association? Future Cardiol. 2011, 7, 191–201. [Google Scholar] [CrossRef]
- Wolpin, B.M.; Kabrhel, C.; Varraso, R.; Kraft, P.; Rimm, E.B.; Goldhaber, S.Z.; Camargo, C.A., Jr.; Fuchs, C.S. Prospective study of ABO blood type and the risk of pulmonary embolism in two large cohort studies. Thromb. Haemost. 2010, 104, 962–971. [Google Scholar] [CrossRef]
- Ohira, T.; Cushman, M.; Tsai, M.Y.; Zhang, Y.; Heckbert, S.R.; Zakai, N.A.; Rosamond, W.D.; Folsom, A.R. ABO blood group, other risk factors and venous thromboembolism: The LITE study. J. Thromb. Haemost. 2007, 5, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.B.; Johnsen, S.P.; Gislum, M.; Møller, C.A.I.; Larsen, H.; Sørensen, H.T. ABO blood group and risk of venous thromboembolism during pregnancy and puerperium. J. Thromb. Haemost. 2005, 3, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Dentali, F.; Sironi, A.P.; Ageno, W.; Crestani, S.; Franchini, M. ABO blood group and vascular disease: An update. Semin. Thromb. Hemost. 2014, 40, 49–59. [Google Scholar] [CrossRef]
- Barbalic, M.; Dupuis, J.; Dehghan, A.; Bis, J.C.; Hoogeveen, R.C.; Schnabel, R.B.; Nambi, V.; Bretler, M.; Smith, N.L.; Peters, A.; et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum. Mol. Genet. 2010, 19, 1863–1872. [Google Scholar] [CrossRef]
- Mercier, B.; Oger, E.; Le Gal, G.; Mottier, D.; Ferec, C. Phenotypic but not allelic ABO blood group association with risk of venous thrombosis. Thromb. Haemost. 2005, 93, 388–389. [Google Scholar] [CrossRef]
- Muellner, S.K.; Haut, E.R.; Streiff, M.B.; Holcomb, J.B.; Cotton, B.A. ABO as a risk factor for venous thromboembolism in trauma. Thromb. Haemost. 2011, 105, 5–13. [Google Scholar] [CrossRef]
- Franchini, M.; Capra, F.; Targher, G.; Montagnana, M.; Lippi, G. Relationship between ABO blood group and von Willebrand factor levels. Thromb. J. 2007, 5, 14. [Google Scholar] [CrossRef]
- Gallinaro, L.; Cattini, M.G.; Sztukowska, M.; Padrini, R.; Sartorello, F.; Pontara, E.; Bertomoro, A.; Daidone, V.; Pagnan, A.; Casonato, A. ABO determinants and von Willebrand factor survival. Blood 2008, 111, 3540–3545. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.E.; O’Sullivan, J.M.; O’Donnell, J.S. The relationship between ABO blood group, von Willebrand factor, and primary hemostasis. Blood 2020, 136, 2864–2874. [Google Scholar] [CrossRef] [PubMed]
- Tirado, I.; Mateo, J.; Soria, J.M.; Oliver, A.; Martínez-Sánchez, E.; Vallvé, C.; Borrell, M.; Urrutia, T.; Fontcuberta, J. The ABO blood group genotype and factor VIII levels as independent risk factors for venous thromboembolism. Thromb. Haemost. 2005, 93, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Nossent, A.Y.; van Marion, V.; van Tilburg, N.H.; Rosendaal, F.R.; Bertina, R.M.; van Mourik, J.A.; Eikenboom, H.C. von Willebrand factor and its propeptide: Secretion, clearance, and risk of venous thrombosis. J. Thromb. Haemost. 2006, 4, 2556–2562. [Google Scholar] [CrossRef]
- Souto, J.C.; Almasy, L.; Muñiz-Diaz, E.; Soria, J.M.; Borrell, M.; Bayén, L.; Mateo, J.; Madoz, P.; Stone, W.; Blangero, J.; et al. Functional effects of the ABO locus polymorphism on plasma levels of von Willebrand factor, factor VIII, and activated partial thromboplastin time. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2024–2028. [Google Scholar] [CrossRef]
- Morelli, V.M.; de Visser, M.C.; van Tilburg, N.H.; Vos, H.L.; Eikenboom, J.C.; Rosendaal, F.R.; Bertina, R.M. ABO blood group genotypes, plasma von Willebrand factor levels and loading of von Willebrand factor with A and B antigens. Thromb. Haemost. 2007, 97, 534–541. [Google Scholar] [CrossRef]
- Paterson, A.D.; Lopes-Virella, M.F.; Waggott, D.; Boright, A.P.; Hosseini, S.M.; Carter, R.E.; Shen, E.; Mirea, L.; Bharaj, B.; Sun, L.; et al. Genome-wide association identifies the ABO blood group as a major locus associated with serum levels of soluble E-selectin. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1958–1967. [Google Scholar] [CrossRef]
- Chan, N.; Hirsh, J. Exploring the ABO–VTE connection. Blood 2025, 145, 2544–2545. [Google Scholar] [CrossRef]
- Sun, X.; Feng, J.; Wu, W.; Peng, M.; Shi, J. ABO blood types associated with the risk of venous thromboembolism in Han Chinese people: A hospital-based study of 200,000 patients. Sci. Rep. 2017, 7, 42925. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, R.; Kavandi, H.; Nadiri, M.; Ghaffari, S. Association of ABO blood group with incidence and outcome of acute pulmonary embolism. Turk Kardiyol Dern Ars. 2016, 44, 397–403. [Google Scholar] [CrossRef]
- Wang, X.J.; Wang, X.D.; Wang, Z.H. Association between ABO blood type and risk of pulmonary embolism: A systematic review and meta-analysis. Clin. Appl. Thromb. Hemost. 2025, 31, 10760296251364267. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffman, T.C.; Mulrow, C.D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Rees, D.C.; Cox, M.; Clegg, J.B. World distribution of factor V Leiden. Lancet 1995, 346, 1133–1134. [Google Scholar] [CrossRef]
- Kereš, T.; Jukić, I.; Svaguša, T.; Prkačin, I.; Bingulac-Popović, J.; Vinković, M.; Hećimović, A.; Živković, M.; Parašilovac, N. A1B and BB blood group genotypes as risk factors for pulmonary embolism. Wien. Klin. Wochenschr. 2021, 133, 1179–1185. [Google Scholar] [CrossRef]
- Cushman, M.; Tsai, A.W.; White, R.H.; Heckbert, S.R.; Rosamond, W.D.; Enright, P.; Folsom, A.R. Deep vein thrombosis and pulmonary embolism in two cohorts. Am. J. Med. 2004, 117, 19–25. [Google Scholar] [CrossRef]
- Jukic, I.; Bingulac-Popovic, J.; Dogic, V.; Babić, I.; Culej, J.; Tomičić, M.; Vuk, T.; Šarlija, D.; Balija, M. ABO blood groups and genetic risk factors for thrombosis in the Croatian population. Croat. Med. J. 2009, 50, 550–558. [Google Scholar] [CrossRef]
- Dentali, F.; Riva, N.; Turato, S.; Grazioli, S.; Squizzato, A.; Steidl, L.; Guasti, L.; Grandi, A.M.; Ageno, W. Pulmonary embolism severity index predicts long-term mortality. J. Thromb. Haemost. 2013, 11, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Streiff, M.B.; Segal, J.; Grossman, S.A.; Kickler, T.S.; Weir, E.G. ABO blood group is a risk factor for venous thromboembolism in malignant gliomas. Cancer 2004, 100, 1717–1723. [Google Scholar] [CrossRef]
- Aujesky, D.; Obrosky, D.S.; Stone, R.A.; Auble, T.E.; Perrier, A.; Cornuz, J.; Roy, P.M.; Fine, M.J. Derivation and validation of a prognostic model for pulmonary embolism. Am. J. Respir. Crit. Care Med. 2005, 172, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, D.; Aujesky, D.; Moores, L.; Gómez, V.; Lobo, J.L.; Uresandi, F.; Otero, R.; Monreal, M.; Muriel, A.; Yusen, R.D.; et al. Simplification of the pulmonary embolism severity index. Arch. Intern. Med. 2010, 170, 1383–1389. [Google Scholar] [CrossRef]
- Onsaker, A.L.; Arntzen, A.Y.; Trégouët, D.A.; Nøst, T.H.; Tang, W.; Guan, W.; Jonasson, C.; Morange, P.-E.; Hindberg, H.D.; Folsom, A.R.; et al. Histo–blood group ABO system transferase plasma levels and risk of future venous thromboembolism. Blood 2025, 145, 2656–2665. [Google Scholar] [CrossRef] [PubMed]
- Munsch, G.; Goumidi, L.; van Hylckama Vlieg, A.; Ibrahim-Kosta, M.; Bruzelius, M.; Deleuze, J.F.; Rosendaal, F.L.; Jacqmin-Gadda, H.; Morange, P.-E.; Trégouët, D.-A. Association of ABO blood groups with venous thrombosis recurrence in middle-aged patients: Weighted Cox analysis for ambispective design. BMC Med. Res. Methodol. 2023, 23, 1471–2288. [Google Scholar] [CrossRef]
- Hernaningsih, Y. ABO Blood Group and Thromboembolic Diseases. In Blood Groups—More than Inheritance of Antigenic Substances; IntechOpen: London, UK, 2022. [Google Scholar]
- White, R.H.; Zhou, H.; Romano, P.S. Incidence of idiopathic deep venous thrombosis and secondary thromboembolism among ethnic groups in California. Ann. Intern Med. 1998, 128, 737–740. [Google Scholar] [CrossRef]
- Zakai, N.A.; McClure, L.A. Racial differences in venous thromboembolism. J. Thromb. Haemost. 2011, 9, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.H.; Guo, J.K.; Xing, W.Y.; Bai, X.L.; Chang, Y.J.; Lu, Z.; Yang, M.; Yang, Y.; Li, W.; Jia, X.-X.; et al. ABO and Rhesus blood groups and multiple health outcomes: An umbrella review of systematic reviews with meta-analyses of observational studies. BMC Med. 2024, 22, 206. [Google Scholar]
- Perera, M.A.; Cavallari, L.H.; Limdi, N.A.; Gamazon, E.R.; Konkashbaev, A.; Daneshjou, R.; Pluzhnikov, A.; Crawford, D.C.; Wang, J.; Liu, N.; et al. Genetic variants associated with warfarin dose in African-American individuals: Genome-wide association study. Lancet 2013, 382, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Harst, H.; Villegas Sierra, L.E.; Said, M.A.; Lipsic, E.; Karper, J.C.; van der Harst, P. Genetically determined ABO blood group and its associations with health and disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 830–838. [Google Scholar] [CrossRef]
- Urabe, F.; Kimura, S.; Iwatani, K.; Yasue, K.; Koike, Y.; Tashiro, K.; Tsuzuki, S.; Sasaki, H.; Kimura, T.; Egawa, S. The impact of ABO blood type on developing venous thromboembolism in cancer patients: Meta-analysis. J. Clin. Med. 2021, 10, 3692. [Google Scholar] [CrossRef]


| Author (Year, Country) | Design/Population | Sample Size (PE Cases/Controls or Cohort) | Exposure | Comparators | Outcomes Assessed |
|---|---|---|---|---|---|
| Wolpin (2010, USA) [22] | Prospective cohort; Nurses’ Health Study and Health Professionals Follow-up Study, general U.S. population | >100,000 participants followed over~1 million person-years; 499 incident PE | Phenotype | O vs. non-O as primary; subgroup analyses of A, B, and AB | Incident PE identified prospectively during follow-up |
| Hajizadeh (2016, Iran) [39] | Case–control; hospitalized PE patients vs. blood donors and staff controls | 230 PE/230 controls | Phenotype | O vs. non-O; subgroup comparisons A, B, AB | Incident acute PE; short-term (in-hospital) mortality; follow-up mortality |
| Sun (2017, China) [38] | Retrospective hospital-based cohort; large inpatient population | >200,000 inpatients screened; 1412 VTE cases (441 isolated PE; 371 PE + DVT) | Phenotype | O vs. non-O; subgroup analyses separating PE-only from combined PE + DVT | Incident PE identified within hospitalization; subgroup analysis by isolated PE vs. combined PE + DVT |
| Kereš (2021, Croatia) [43] | Case–control; confirmed PE patients vs. healthy controls | 74 PE/303 controls | Genotype | O1O1 vs. non-O1O1 genotypes; subgroup analyses of A1B, BB, and other allele combinations | Incident PE; severity assessed by PESI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamal Eddin, A.; Tunea, O.E.; Mozos, I.M.; Damian, A.D.; Stanciugelu, S.-I. Pulmonary Embolism and ABO Blood Type: A Systematic Review. Diagnostics 2025, 15, 2973. https://doi.org/10.3390/diagnostics15232973
Jamal Eddin A, Tunea OE, Mozos IM, Damian AD, Stanciugelu S-I. Pulmonary Embolism and ABO Blood Type: A Systematic Review. Diagnostics. 2025; 15(23):2973. https://doi.org/10.3390/diagnostics15232973
Chicago/Turabian StyleJamal Eddin, Abdulkader, Oana Elena Tunea, Ioana Monica Mozos, Arnaldo Dario Damian, and Stefan-Iulian Stanciugelu. 2025. "Pulmonary Embolism and ABO Blood Type: A Systematic Review" Diagnostics 15, no. 23: 2973. https://doi.org/10.3390/diagnostics15232973
APA StyleJamal Eddin, A., Tunea, O. E., Mozos, I. M., Damian, A. D., & Stanciugelu, S.-I. (2025). Pulmonary Embolism and ABO Blood Type: A Systematic Review. Diagnostics, 15(23), 2973. https://doi.org/10.3390/diagnostics15232973