Exploring the Role of CT-Based Delta-Radiomics in Unresectable Vulvar Cancer
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Treatment, CT Acquisition Parameters, and Segmentation
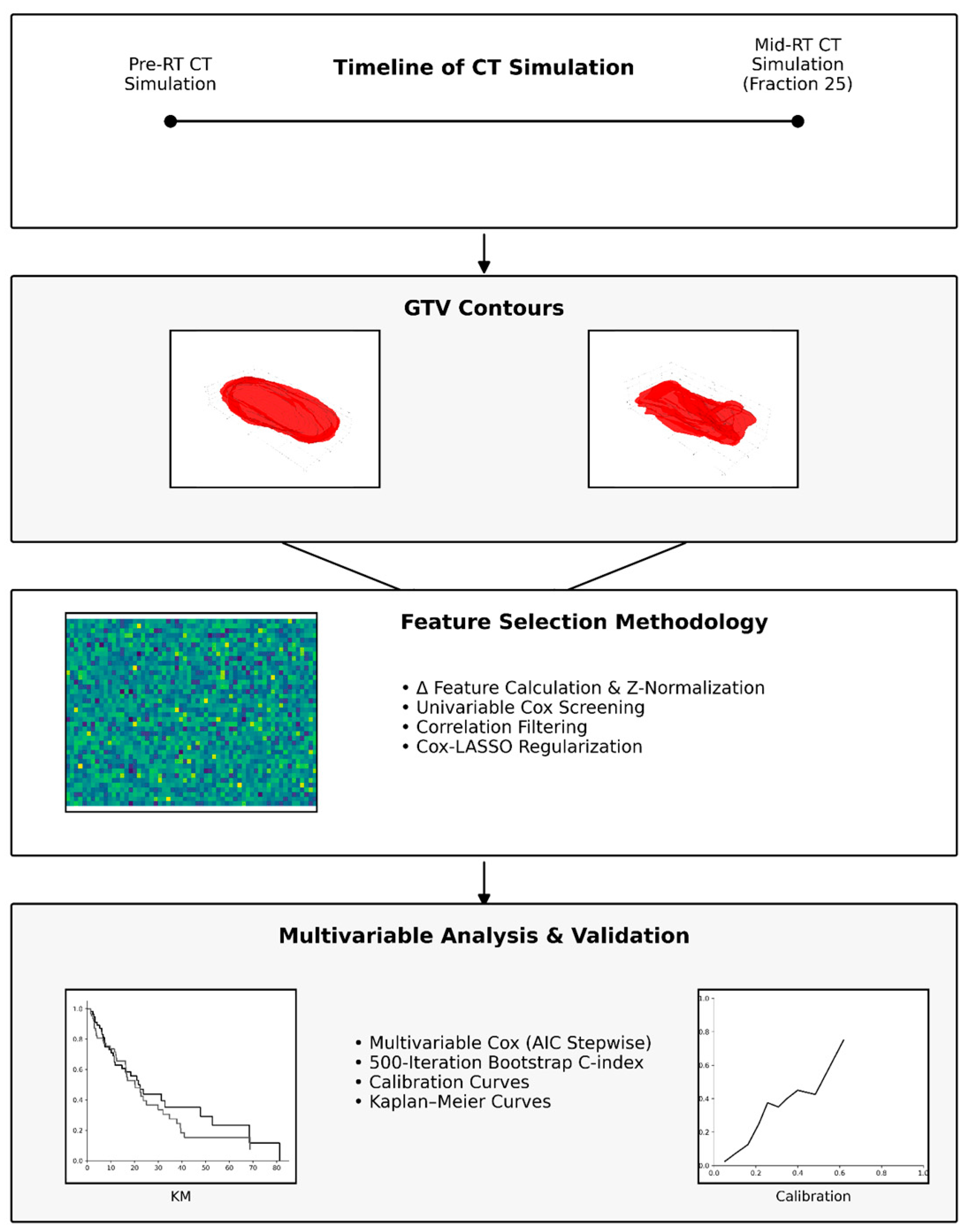
2.3. Image Preprocessing and Radiomic Workflow Overview
2.4. DICOM Image and RT Structure Acquisition
2.5. ROI Mask Generation
2.6. Radiomic Feature Extraction
2.7. Calculation of Delta-Radiomics
2.8. Survival Endpoints
2.9. Feature Selection for Survival Outcomes
2.10. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Treatment Outcomes
3.3. Univariable Analyses and Feature Selection
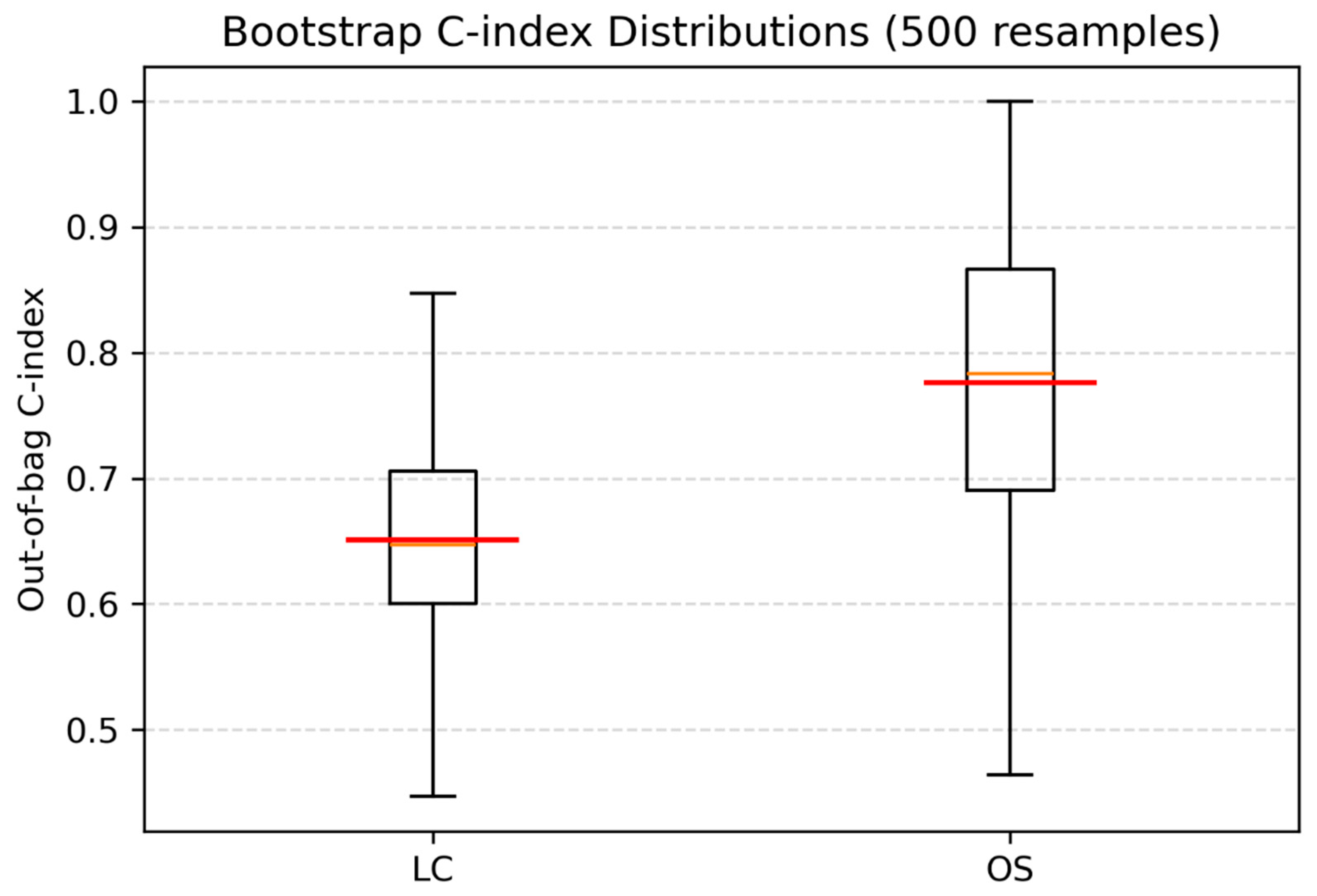
3.4. Multivariable Analyses and Internal Validation
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Buttmann-Schweiger, N.; Klug, S.J.; Luyten, A.; Holleczek, B.; Heitz, F.; Du Bois, A.; Kraywinkel, K. Incidence Patterns and Temporal Trends of Invasive Nonmelanotic Vulvar Tumors in Germany 1999–2011. A Population-Based Cancer Registry Analysis. PLoS ONE 2015, 10, e0128073. [Google Scholar] [CrossRef]
- Alkatout, I.; Schubert, M.; Garbrecht, N.; Weigel, M.T.; Jonat, W.; Mundhenke, C.; Günther, V. Vulvar cancer: Epidemiology, clinical presentation, and management options. Int. J. Womens Health 2015, 7, 305–313. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Yashar, C.M.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Crispens, M.A.; et al. Vulvar Cancer, Version 3.2024. JNCCN J. Natl. Compr. Cancer Netw. 2024, 22, 117–135. [Google Scholar] [CrossRef]
- Rao, Y.J.; Chin, R.I.; Hui, C.; Mutch, D.G.; Powell, M.A.; Schwarz, J.K.; Grigsby, P.W.; Markovina, S. Improved survival with definitive chemoradiation compared to definitive radiation alone in squamous cell carcinoma of the vulva: A review of the National Cancer Database. Gynecol. Oncol. 2017, 146, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Macchia, G.; Lancellotta, V.; Ferioli, M.; Casà, C.; Pezzulla, D.; Pappalardi, B.; Laliscia, C.; Ippolito, E.; Di Muzio, J.; Huscher, A.; et al. Definitive chemoradiation in vulvar squamous cell carcinoma: Outcome and toxicity from an observational multicenter Italian study on vulvar cancer (OLDLADY 1.1). Radiol. Med. 2023, 129, 152. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, J.Y.; Kim, J.Y.; Lee, N.K.; Kim, J.H.; Kim, Y.B.; Kim, Y.S.; Kim, J.; Kim, Y.S.; Yang, D.S.; et al. Treatment outcomes of curative radiotherapy in patients with vulvar cancer: Results of the retrospective KROG 1203 study. Radiat. Oncol. J. 2015, 33, 198. [Google Scholar] [CrossRef]
- Prieske, K.; Haeringer, N.; Grimm, D.; Trillsch, F.; Eulenburg, C.; Burandt, E.; Schmalfeldt, B.; Mahner, S.; Mueller, V.; Woelber, L. Patterns of distant metastases in vulvar cancer. Gynecol. Oncol. 2016, 142, 427–434. [Google Scholar] [CrossRef]
- Salom, E.M.; Penalver, M. Recurrent vulvar cancer. Curr. Treat. Options Oncol. 2002, 3, 143–153. [Google Scholar] [CrossRef]
- Zach, D.; Åvall-Lundqvist, E.; Falconer, H.; Hellman, K.; Johansson, H.; Flöter Rådestad, A. Patterns of recurrence and survival in vulvar cancer: A nationwide population-based study. Gynecol. Oncol. 2021, 161, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.L.; Huynh, Q.H.; Nguyen, P.N. Assessing the Clinical Characteristics and the Role of Imaging Modalities in Uterine Sarcoma: A Single-Center Retrospective Study From Vietnam. J. Clin. Ultrasound 2025, 53, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Sakala, M.D.; Shampain, K.L.; Wasnik, A.P. Advances in MR Imaging of the Female Pelvis. Magn. Reson. Imaging Clin. N. Am. 2020, 28, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Virarkar, M.; Vulasala, S.S.; Daoud, T.; Javadi, S.; Lall, C.; Bhosale, P. Vulvar Cancer: 2021 Revised FIGO Staging System and the Role of Imaging. Cancers 2022, 14, 2264. [Google Scholar] [CrossRef]
- Serrado, M.A.; Horta, M.; Cunha, T.M. State of the art in vulvar cancer imaging. Radiol. Bras. 2019, 52, 316. [Google Scholar] [CrossRef]
- Gaffney, D.K.; King, B.; Viswanathan, A.N.; Barkati, M.; Beriwal, S.; Eifel, P.; Erickson, B.; Fyles, A.; Goulart, J.; Harkenrider, M.; et al. Consensus recommendations for radiation therapy contouring and treatment of vulvar carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1191–1200. [Google Scholar] [CrossRef]
- Aerts, H.J.W.L.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Cavalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef]
- Manganaro, L.; Nicolino, G.M.; Dolciami, M.; Martorana, F.; Stathis, A.; Colombo, I.; Rizzo, S. Radiomics in cervical and endometrial cancer. Br. J. Radiol. 2021, 94, 20201314. [Google Scholar] [CrossRef]
- Zhou, C.; Hou, L.; Tang, X.; Liu, C.; Meng, Y.; Jia, H.; Yang, H.; Zhou, S. CT-based radiomics nomogram may predict who can benefit from adaptive radiotherapy in patients with local advanced-NSCLC patients. Radiother. Oncol. 2023, 183, 109637. [Google Scholar] [CrossRef]
- Kasai, A.; Miyoshi, J.; Sato, Y.; Okamoto, K.; Miyamoto, H.; Kawanaka, T.; Tonoiso, C.; Harada, M.; Goto, M.; Yoshida, T.; et al. A novel CT-based radiomics model for predicting response and prognosis of chemoradiotherapy in esophageal squamous cell carcinoma. Sci. Rep. 2024, 14, 2039. [Google Scholar] [CrossRef]
- Nafchi, E.R.; Fadavi, P.; Amiri, S.; Cheraghi, S.; Garousi, M.; Nabavi, M.; Daneshi, I.; Gomar, M.; Molaie, M.; Nouraeinejad, A. Radiomics model based on computed tomography images for prediction of radiation-induced optic neuropathy following radiotherapy of brain and head and neck tumors. Heliyon 2025, 11, e41409. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wang, Y.; Shi, X.; Pang, H.; Li, Y. Computed tomography–based radiomics modeling to predict patient overall survival in cervical cancer with intensity-modulated radiotherapy combined with concurrent chemotherapy. J. Int. Med. Res. 2025, 53, 03000605251325996. [Google Scholar] [CrossRef]
- Shur, J.D.; Doran, S.J.; Kumar, S.; Ap Dafydd, D.; Downey, K.; O’connor, J.P.B.; Papanikolaou, N.; Messiou, C.; Koh, D.M.; Orton, M.R. Radiomics in oncology: A practical guide. Radiographics 2021, 41, 1717–1732. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.R.; Zhou, Y.M.; Xie, X.Y.; Chen, J.Y.; Quan, K.R.; Wei, Y.T.; Xia, X.Y.; Chen, W.J. Delta radiomics analysis for prediction of intermediary- and high-risk factors for patients with locally advanced cervical cancer receiving neoadjuvant therapy. Sci. Rep. 2023, 13, 19409. [Google Scholar] [CrossRef]
- Wagner-Larsen, K.S.; Lura, N.; Gulati, A.; Ryste, S.; Hodneland, E.; Fasmer, K.E.; Woie, K.; Bertelsen, B.I.; Salvesen, Ø.; Halle, M.K.; et al. MRI delta radiomics during chemoradiotherapy for prognostication in locally advanced cervical cancer. BMC Cancer 2025, 25, 122. [Google Scholar] [CrossRef] [PubMed]
- Collarino, A.; Garganese, G.; Fragomeni, S.M.; Pereira Arias-Bouda, L.M.; Ieria, F.P.; Boellaard, R.; Rufini, V.; De Geus-Oei, L.F.; Scambia, G.; Valdés Olmos, R.A.; et al. Radiomics in Vulvar Cancer: First Clinical Experience Using 18F-FDG PET/CT Images. J. Nucl. Med. 2019, 60, 199. [Google Scholar] [CrossRef]
- Reade, C.J.; Eiriksson, L.R.; Mackay, H. Systemic therapy in squamous cell carcinoma of the vulva: Current status and future directions. Gynecol. Oncol. 2014, 132, 780–789. [Google Scholar] [CrossRef]
- Xia, P.; Murray, E. 3D treatment planning system—Pinnacle system. Med. Dosim. 2018, 43, 118–128. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J.W.L. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Parmar, C.; Grossmann, P.; Rietveld, D.; Rietbergen, M.M.; Lambin, P.; Aerts, H.J.W.L. Radiomic Machine-Learning Classifiers for Prognostic Biomarkers of Head and Neck Cancer. Front. Oncol. 2015, 5, 272. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Limkin, E.J.; Sun, R.; Dercle, L.; Zacharaki, E.I.; Robert, C.; Reuzé, S.; Schernberg, A.; Paragios, N.; Deutsch, E.; Ferté, C. Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann. Oncol. 2017, 28, 1191–1206. [Google Scholar] [CrossRef]
- Vallières, M.; Kay-Rivest, E.; Perrin, L.J.; Liem, X.; Furstoss, C.; Aerts, H.J.W.L.; Khaouam, N.; Nguyen-Tan, P.F.; Wang, C.S.; Sultanem, K.; et al. Radiomics strategies for risk assessment of tumour failure in head-and-neck cancer. Sci. Rep. 2017, 7, 10117. [Google Scholar] [CrossRef]
- Atiya, H.I.; Gorecki, G.; Garcia, G.L.; Frisbie, L.G.; Baruwal, R.; Coffman, L. Stromal-Modulated Epithelial-to-Mesenchymal Transition in Cancer Cells. Biomolecules 2023, 13, 1604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Peng, Z.; Weng, Y.; Fang, Z.; Xiao, F.; Zhang, C.; Fan, Z.; Huang, K.; Zhu, Y.; et al. The progress of multimodal imaging combination and subregion based radiomics research of cancers. Int. J. Biol. Sci. 2022, 18, 3458–3469. [Google Scholar] [CrossRef] [PubMed]
- Nooij, L.S.; Brand, F.A.M.; Gaarenstroom, K.N.; Creutzberg, C.L.; de Hullu, J.A.; van Poelgeest, M.I.E. Risk factors and treatment for recurrent vulvar squamous cell carcinoma. Crit. Rev. Oncol. Hematol. 2016, 106, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Woelber, L.; Eulenburg, C.; Kosse, J.; Neuser, P.; Heiss, C.; Hantschmann, P.; Mallmann, P.; Tanner, B.; Pfisterer, J.; Jückstock, J.; et al. Predicting the course of disease in recurrent vulvar cancer—A subset analysis of the AGO-CaRE-1 study. Gynecol. Oncol. 2019, 154, 571–576. [Google Scholar] [CrossRef]
- Ger, R.B.; Wei, L.; El Naqa, I.; Wang, J. The Promise and Future of Radiomics for Personalized Radiotherapy Dosing and Adaptation. Semin. Radiat. Oncol. 2023, 33, 252. [Google Scholar] [CrossRef]
- Tanaka, S.; Kadoya, N.; Sugai, Y.; Umeda, M.; Ishizawa, M.; Katsuta, Y.; Ito, K.; Takeda, K.; Jingu, K. A deep learning-based radiomics approach to predict head and neck tumor regression for adaptive radiotherapy. Sci. Rep. 2022, 12, 8899. [Google Scholar] [CrossRef]
- Abuhijla, F.; Salah, S.; Al-Hussaini, M.; Mohamed, I.; Jaradat, I.; Dayyat, A.; Almasri, H.; Allozi, A.; Arjan, A.; Almousa, A.; et al. Factors influencing the use of adaptive radiation therapy in vulvar carcinoma. Rep. Pract. Oncol. Radiother. 2020, 25, 709. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Harrell, F.E.; Lee, K.L.; Califf, R.M.; Pryor, D.B.; Rosati, R.A. Regression modelling strategies for improved prognostic prediction. Stat. Med. 1984, 3, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Oonk, M.H.M.; Planchamp, F.; Baldwin, P.; Mahner, S.; Mirza, M.R.; Fischerová, D.; Creutzberg, C.L.; Guillot, E.; Garganese, G.; Lax, S.; et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 1023–1043. [Google Scholar] [CrossRef] [PubMed]

| Variable | Median | IQR | |
|---|---|---|---|
| Age at diagnosis (years) | 57 | 50.0–67.0 | |
| Total vulvar dose (Gy) | 65 | 64.0–66.6 | |
| First-phase external-beam radiotherapy dose in Gy | 45 | 45.0–50.4 | |
| External-beam radiotherapy number of fractions | 25 | 25.0–28.0 | |
| Nodal dose (Gy) | 63 | 54.0–66.0 | |
| EBRT time (days) | 54 | 51.0–61.0 | |
| Chemotherapy cycles | 5 | 4.0–5.0 | |
| Variable | Category | Count | Percentage (%) |
| Chronic comorbidities | Diabetes mellitus | 5 | 23.8 |
| Hypertension | 7 | 33.3 | |
| Ischemic heart disease | 2 | 9.5 | |
| Chronic obstructive pulmonary disease | 1 | 4.8 | |
| Systemic lupus erythematosus | 1 | 4.8 | |
| Medically free otherwise | 10 | 47.6 | |
| Grade | 1 | 1 | 4.8 |
| 2 | 15 | 71.4 | |
| 3 | 4 | 19 | |
| Missing | 1 | 4.8 | |
| HPV categories | Not associated | 12 | 57.1 |
| HPV-associated | 7 | 33.3 | |
| Missing | 2 | 9.5 | |
| FIGO stage | II | 1 | 4.8 |
| IIIA | 1 | 4.8 | |
| IIIB | 7 | 33.3 | |
| IIIC | 1 | 4.8 | |
| IVA | 2 | 9.5 | |
| IVB | 6 | 28.6 | |
| Missing | 3 | 14.3 | |
| Concurrent chemotherapy | Yes | 20 | 95.2 |
| No | 1 | 4.8 | |
| Nodal debulking surgery prior to radiotherapy | Yes | 3 | 14.3 |
| No | 18 | 85.7 | |
| Endpoint | Events (n) | Total (n) | Event Rate (%) | Events ≤ 24 Months (n) | ≤24 Months (%) |
|---|---|---|---|---|---|
| Local Control | 8 | 21 | 38.1 | 7 | 87.5 |
| Regional Control | 5 | 21 | 23.8 | 5 | 100.0 |
| Distant Metastasis-Free | 9 | 21 | 42.9 | 9 | 100.0 |
| Progression-Free | 12 | 21 | 57.1 | 11 | 91.7 |
| Overall Survival | 9 | 21 | 42.9 | 7 | 77.8 |
| Endpoint | Selected Δ Features |
|---|---|
| LC | GLCM Inverse Difference Moment (IDM) |
| GLRLM Run Length Non-Uniformity Normalized (RLNU_norm) | |
| GLRLM Run Percentage (RP) | |
| First-Order Entropy | |
| GLCM Difference Entropy (DiffEnt) | |
| GLCM Cluster Prominence (ClusProm) | |
| RC | none retained |
| DMFS | none retained |
| PFS | none retained |
| OS | GLCM Difference Average (DiffAvg) |
| Shape Surface–Volume Ratio (SVR) | |
| GLCM Difference Variance (DiffVar) | |
| GLDM Large Dependence Low Gray-Level Emphasis (LDLGLE) | |
| GLSZM Size-Zone Non-Uniformity (SZNU) | |
| GLSZM Gray-Level Non-Uniformity Normalized (GLNU_norm) | |
| GLSZM Zone Entropy (ZoneEnt) | |
| GLSZM Gray-Level Variance (GLVar) | |
| First-Order Energy |
| Multivariable Cox Model for LC (Retaining One Variable) | ||||
|---|---|---|---|---|
| Δ Feature | Coef | HR | 95% CI | p-Value * |
| GLRLM Run-Length Non-Uniformity Normalized (RLNU_norm) | 0.9625 | 2.618 | [1.05, 6.52] | 0.0388 |
| Multivariable Cox Model for OS (time-varying) | ||||
| Feature | Coef | HR | 95% CI | p-value * |
| GLCM Difference Average (DiffAvg) | −9.0097 | 0.00012 | [3 × 10−8, 0.48] | 0.0327 |
| Shape Surface–Volume Ratio (SVR) | 5.7666 | 319.45 | [1.74, 5.9 × 104] | 0.0302 |
| GLCM Difference Variance (DiffVar) | 5.7931 | 328.02 | [1.33, 8.1 × 104] | 0.0393 |
| GLDM Large Dependence Low Gray-Level Emphasis (LDLGLE) | −8.3732 | 0.00023 | [1 × 10−8, 5.28] | 0.1021 |
| GLSZM Gray-Level Non-Uniformity Normalized (GLNU_norm) | −9.2533 | 0.00010 | [3 × 10−8, 0.33] | 0.0259 |
| First-Order Energy | −1.6158 | 0.1987 | [0.03, 1.22] | 0.0807 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzibdeh, A.; Hammadeh, B.M.; Alnajjar, R.; Abd Al-Raheem, M.; Mheidat, R.; Al Matairi, A.; Qamber, M.; Almasri, H.; Altalla’, B.; Al-Omari, A.; et al. Exploring the Role of CT-Based Delta-Radiomics in Unresectable Vulvar Cancer. Diagnostics 2025, 15, 2972. https://doi.org/10.3390/diagnostics15232972
Alzibdeh A, Hammadeh BM, Alnajjar R, Abd Al-Raheem M, Mheidat R, Al Matairi A, Qamber M, Almasri H, Altalla’ B, Al-Omari A, et al. Exploring the Role of CT-Based Delta-Radiomics in Unresectable Vulvar Cancer. Diagnostics. 2025; 15(23):2972. https://doi.org/10.3390/diagnostics15232972
Chicago/Turabian StyleAlzibdeh, Abdulla, Bara M. Hammadeh, Rahaf Alnajjar, Mohammad Abd Al-Raheem, Rima Mheidat, Alzahra’a Al Matairi, Mohamed Qamber, Hanan Almasri, Bayan Altalla’, Amal Al-Omari, and et al. 2025. "Exploring the Role of CT-Based Delta-Radiomics in Unresectable Vulvar Cancer" Diagnostics 15, no. 23: 2972. https://doi.org/10.3390/diagnostics15232972
APA StyleAlzibdeh, A., Hammadeh, B. M., Alnajjar, R., Abd Al-Raheem, M., Mheidat, R., Al Matairi, A., Qamber, M., Almasri, H., Altalla’, B., Al-Omari, A., & Abuhijla, F. (2025). Exploring the Role of CT-Based Delta-Radiomics in Unresectable Vulvar Cancer. Diagnostics, 15(23), 2972. https://doi.org/10.3390/diagnostics15232972







