Osteoporosis and Fracture Risk in Ovarian Cancer: Beyond the Oncologic Burden
Abstract
1. Introduction
1.1. Osteoporosis: Epidemiology and Diagnostic Tools
1.2. Ovarian Cancer: Epidemiology and Clinical Context
1.3. Mechanisms of Bone Loss in Ovarian Cancer
1.4. Hormonal Mechanisms
1.5. Tumor-Driven Pathways
1.6. Treatment-Related Mechanisms
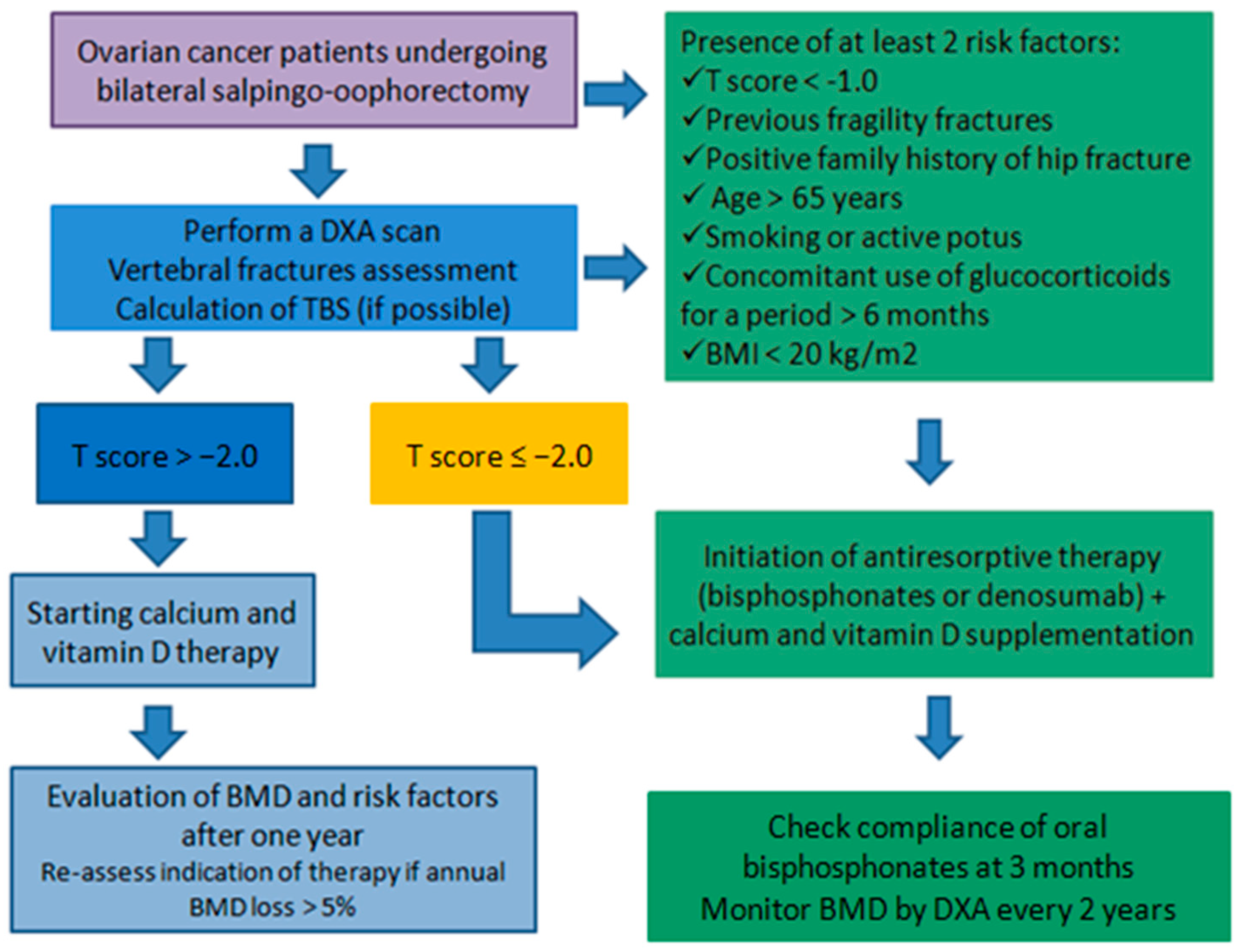
1.7. Assessment of Bone Health and Management of Osteoporosis in Ovarian Cancer
2. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker-Bone, K.; Walter, G.; Cooper, C. Recent Developments in the Epidemiology of Osteoporosis. Curr. Opin. Rheumatol. 2002, 14, 411–415. [Google Scholar] [CrossRef]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The Global Prevalence of Osteoporosis in the World: A Comprehensive Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef]
- Armas, L.A.G.; Recker, R.R. Pathophysiology of Osteoporosis. Endocrinol. Metab. Clin. N. Am. 2012, 41, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Leslie, W.D. Fracture Risk and Assessment in Adults with Cancer. Osteoporos. Int. 2023, 34, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Morin, S.N.; Lix, L.M.; McCloskey, E.V.; Johansson, H.; Harvey, N.C.; Kanis, J.A.; Leslie, W.D. Age at First Fracture and Later Fracture Risk in Older Adults Undergoing Osteoporosis Assessment. JAMA Netw. Open 2024, 7, e2448208. [Google Scholar] [CrossRef]
- Ye, C.; Leslie, W.D.; Al-Azazi, S.; Yan, L.; Lix, L.M.; Czaykowski, P.; Singh, H. Fractures and Long-Term Mortality in Cancer Patients: A Population-Based Cohort Study. Osteoporos. Int. 2022, 33, 2629–2635. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.; Wolfe, R.A.; Klem, M.L. Risk Factors for Falls in Adult Cancer Survivors: An Integrative Review. Rehabil. Nurs. 2018, 43, 201–213. [Google Scholar] [CrossRef]
- Xiao, P.-L.; Cui, A.-Y.; Hsu, C.-J.; Peng, R.; Jiang, N.; Xu, X.-H.; Ma, Y.-G.; Liu, D.; Lu, H.-D. Global, Regional Prevalence, and Risk Factors of Osteoporosis According to the World Health Organization Diagnostic Criteria: A Systematic Review and Meta-Analysis. Osteoporos. Int. 2022, 33, 2137–2153. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Xie, N.; Sun, X.-D.; Nice, E.C.; Liou, Y.-C.; Huang, C.; Zhu, H.; Shen, Z. Insights and Implications of Sexual Dimorphism in Osteoporosis. Bone Res. 2024, 12, 8. [Google Scholar] [CrossRef]
- Van Der Klift, M.; Pols, H.A.P.; Geleijnse, J.M.; Van Der Kuip, D.A.M.; Hofman, A.; De Laet, C.E.D.H. Bone Mineral Density and Mortality in Elderly Men and Women: The Rotterdam Study. Bone 2002, 30, 643–648. [Google Scholar] [CrossRef]
- Vilaca, T.; Eastell, R.; Schini, M. Osteoporosis in Men. Lancet Diabetes Endocrinol. 2022, 10, 273–283. [Google Scholar] [CrossRef]
- International Osteoporosis Foundation; Borgström, F.; Karlsson, L.; Ortsäter, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; et al. Fragility Fractures in Europe: Burden, Management and Opportunities. Arch. Osteoporos. 2020, 15, 59. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Hamann, C.; Ebeling, P.R. Approach to the Patient with Secondary Osteoporosis. Eur. J. Endocrinol. 2010, 162, 1009–1020. [Google Scholar] [CrossRef]
- Fuggle, N.R.; Reginster, J.-Y.; Al-Daghri, N.; Bruyere, O.; Burlet, N.; Campusano, C.; Cooper, C.; Perez, A.D.; Halbout, P.; Ghi, T.; et al. Radiofrequency Echographic Multi Spectrometry (REMS) in the Diagnosis and Management of Osteoporosis: State of the Art. Aging Clin. Exp. Res. 2024, 36, 135. [Google Scholar] [CrossRef]
- Lalli, P.; Mautino, C.; Busso, C.; Bardesono, F.; Di Monaco, M.; Lippi, L.; Invernizzi, M.; Minetto, M.A. Reproducibility and Accuracy of the Radiofrequency Echographic Multi-Spectrometry for Femoral Mineral Density Estimation and Discriminative Power of the Femoral Fragility Score in Patients with Primary and Disuse-Related Osteoporosis. J. Clin. Med. 2022, 11, 3761. [Google Scholar] [CrossRef]
- Pisani, P.; Conversano, F.; Muratore, M.; Adami, G.; Brandi, M.L.; Caffarelli, C.; Casciaro, E.; Di Paola, M.; Franchini, R.; Gatti, D.; et al. Fragility Score: A REMS-Based Indicator for the Prediction of Incident Fragility Fractures at 5 Years. Aging Clin. Exp. Res. 2023, 35, 763–773. [Google Scholar] [CrossRef]
- Ehresman, J.; Pennington, Z.; Schilling, A.; Lubelski, D.; Ahmed, A.K.; Cottrill, E.; Khan, M.; Sciubba, D.M. Novel MRI-Based Score for Assessment of Bone Density in Operative Spine Patients. Spine J. 2020, 20, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Cianferotti, L.; Cipriani, C.; Corbetta, S.; Corona, G.; Defeudis, G.; Lania, A.G.; Messina, C.; Napoli, N.; Mazziotti, G. Bone Quality in Endocrine Diseases: Determinants and Clinical Relevance. J. Endocrinol. Investig. 2023, 46, 1283–1304. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, M.; Guermazi, A.; Roemer, F.W.; Delmas, P.D.; Genant, H.K. Recognizing and Reporting Osteoporotic Vertebral Fractures. Eur. Spine J. 2003, 12, S104–S112. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Ebeling, P.; Kline, G. Osteoporosis. Lancet 2025, 406, 2003–2016. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Sambasivan, S. Epithelial Ovarian Cancer: Review Article. Cancer Treat. Res. Commun. 2022, 33, 100629. [Google Scholar] [CrossRef]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian Cancer in the World: Epidemiology and Risk Factors. Int. J. Womens Health 2019, 11, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Kauff, N.D.; Satagopan, J.M.; Robson, M.E.; Scheuer, L.; Hensley, M.; Hudis, C.A.; Ellis, N.A.; Boyd, J.; Borgen, P.I.; Barakat, R.R.; et al. Risk-Reducing Salpingo-Oophorectomy in Women with a BRCA1 or BRCA2 Mutation. N. Engl. J. Med. 2002, 346, 1609–1615. [Google Scholar] [CrossRef]
- Höhn, A.K.; Brambs, C.E.; Hiller, G.G.R.; May, D.; Schmoeckel, E.; Horn, L.-C. 2020 WHO Classification of Female Genital Tumors. Geburtshilfe Frauenheilkd. 2021, 81, 1145–1153. [Google Scholar] [CrossRef]
- Lele, S. (Ed.) Ovarian Cancer; Exon Publications: Brisbane, Australia, 2022; ISBN 978-0-645-33208-7. [Google Scholar]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.; Cristea, M.; DeRosa, M.; Eisenhauer, E.L.; et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 191–226. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Alvarez, R.D.; Backes, F.J.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.; Chitiyo, V.C.; Cristea, M.; et al. NCCN Guidelines® Insights: Ovarian Cancer, Version 3.2022: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2022, 20, 972–980. [Google Scholar] [CrossRef]
- Angioli, R.; Luvero, D.; Armento, G.; Capriglione, S.; Plotti, F.; Scaletta, G.; Lopez, S.; Montera, R.; Gatti, A.; Serra, G.B.; et al. Hormone Replacement Therapy in Cancer Survivors: Utopia? Crit. Rev. Oncol./Hematol. 2018, 124, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Rees, M.; Angioli, R.; Coleman, R.L.; Glasspool, R.; Plotti, F.; Simoncini, T.; Terranova, C. European Menopause and Andropause Society (EMAS) and International Gynecologic Cancer Society (IGCS) Position Statement on Managing the Menopause after Gynecological Cancer: Focus on Menopausal Symptoms and Osteoporosis. Maturitas 2020, 134, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N. Estrogen Deficiency and Bone Loss: An Inflammatory Tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef]
- Khosla, S. Minireview: The OPG/RANKL/RANK System. Endocrinology 2001, 142, 5050–5055. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.J.; Tom, C.; Guemes, M.; Polanco, G.; Mayorga, M.E.; Wend, K.; Miranda-Carboni, G.A.; Krum, S.A. ERα Signaling Regulates MMP3 Expression to Induce FasL Cleavage and Osteoclast Apoptosis. J. Bone Miner. Res. 2013, 28, 283–290. [Google Scholar] [CrossRef]
- Krum, S.A.; Chang, J.; Miranda-Carboni, G.; Wang, C.-Y. Novel Functions for NFκB: Inhibition of Bone Formation. Nat. Rev. Rheumatol. 2010, 6, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Cenci, S.; Weitzmann, M.N.; Roggia, C.; Namba, N.; Novack, D.; Woodring, J.; Pacifici, R. Estrogen Deficiency Induces Bone Loss by Enhancing T-Cell Production of TNF-Alpha. J. Clin. Investig. 2000, 106, 1229–1237. [Google Scholar] [CrossRef]
- Pantschenko, A.G.; Zhang, W.; Nahounou, M.; Mccarthy, M.B.; Stover, M.L.; Lichtler, A.C.; Clark, S.H.; Gronowicz, G.A. Effect of Osteoblast-Targeted Expression of Bcl-2 in Bone: Differential Response in Male and Female Mice. J. Bone Miner. Res. 2005, 20, 1414–1429. [Google Scholar] [CrossRef] [PubMed]
- Shi, V.; Morgan, E.F. Estrogen and Estrogen Receptors Mediate the Mechanobiology of Bone Disease and Repair. Bone 2024, 188, 117220. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, C.Y.; Lee, E.; Ji, Y.I. Effect of Gynecological Cancer and Its Treatment on Bone Mineral Density and the Risk of Osteoporosis and Osteoporotic Fracture. Obstet. Gynecol. Sci. 2020, 63, 470–479. [Google Scholar] [CrossRef]
- Sobecki, J.; Weigman, B.; Anderson-Carter, I.; Barroilhet, L.; Chandereng, T.; Kliewer, M.; Hartenbach, E. Opportunistic Osteoporosis Screening Using Routine Computed Tomography Images to Identify Bone Loss in Gynecologic Cancer Survivors. Int. J. Gynecol. Cancer 2022, 32, 1050–1055. [Google Scholar] [CrossRef]
- Garcia, C.; Lyon, L.; Conell, C.; Littell, R.D.; Powell, C.B. Osteoporosis Risk and Management in BRCA1 and BRCA2 Carriers Who Undergo Risk-Reducing Salpingo-Oophorectomy. Gynecol. Oncol. 2015, 138, 723–726. [Google Scholar] [CrossRef]
- Vesco, K.K.; Marshall, L.M.; Nelson, H.D.; Humphrey, L.; Rizzo, J.; Pedula, K.L.; Cauley, J.A.; Ensrud, K.E.; Hochberg, M.C.; Antoniucci, D.; et al. Surgical Menopause and Nonvertebral Fracture Risk among Older US Women. Menopause 2012, 19, 510–516. [Google Scholar] [CrossRef]
- Beekman, M.J.; Terra, L.; Stuursma, A.; Heemskerk-Gerritsen, B.A.M.; van Lennep, J.E.R.; van Beurden, M.; van Doorn, L.C.; de Hullu, J.A.; van Dorst, E.B.L.; Mom, C.H.; et al. Long-Term Effects of Premenopausal Risk-Reducing Salpingo-Oophorectomy on Bone Mineral Density. In Osteoporosis International; Springer: Berlin/Heidelberg, Germany, 2025. [Google Scholar] [CrossRef]
- Fakkert, I.E.; Abma, E.M.; Westrik, I.G.; Lefrandt, J.D.; Wolffenbuttel, B.H.R.; Oosterwijk, J.C.; Slart, R.H.J.A.; Van Der Veer, E.; De Bock, G.H.; Mourits, M.J.E. Bone Mineral Density and Fractures after Risk-Reducing Salpingo-Oophorectomy in Women at Increased Risk for Breast and Ovarian Cancer. Eur. J. Cancer 2015, 51, 400–408. [Google Scholar] [CrossRef]
- Sherwin, S.A.; Twardzik, D.R.; Bohn, W.H.; Cockley, K.D.; Todaro, G.J. High-Molecular-Weight Transforming Growth Factor Activity in the Urine of Patients with Disseminated Cancer. Cancer Res. 1983, 43, 403–407. [Google Scholar]
- Zhao, B. Does TNF Promote or Restrain Osteoclastogenesis and Inflammatory Bone Resorption? Crit. Rev. Immunol. 2018, 38, 253–261. [Google Scholar] [CrossRef]
- Pin, F.; Jones, A.J.; Huot, J.R.; Narasimhan, A.; Zimmers, T.A.; Bonewald, L.F.; Bonetto, A. RANKL Blockade Reduces Cachexia and Bone Loss Induced by Non-Metastatic Ovarian Cancer in Mice. J. Bone Miner. Res. 2020, 37, 381–396. [Google Scholar] [CrossRef]
- Inoue, D.; Matsumoto, T. Parathyroid Hormone-Related Peptide and Bone: Pathological and Physiological Aspects. Biomed. Pharmacother. 2000, 54 (Suppl. 1), 32s–41s. [Google Scholar] [CrossRef]
- Liu, X.-H.; Kirschenbaum, A.; Weinstein, B.M.; Zaidi, M.; Yao, S.; Levine, A.C. Prostaglandin E2 Modulates Components of the Wnt Signaling System in Bone and Prostate Cancer Cells. Biochem. Biophys. Res. Commun. 2010, 394, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Essex, A.L.; Pin, F.; Huot, J.R.; Bonewald, L.F.; Plotkin, L.I.; Bonetto, A. Bisphosphonate Treatment Ameliorates Chemotherapy-Induced Bone and Muscle Abnormalities in Young Mice. Front. Endocrinol. 2019, 10, 809. [Google Scholar] [CrossRef]
- Douchi, T.; Kosha, S.; Kan, R.; Nakamura, S.; Oki, T.; Nagata, Y. Predictors of Bone Mineral Loss in Patients with Ovarian Cancer Treated with Anticancer Agents. Obstet. Gynecol. 1997, 90, 12–15. [Google Scholar] [CrossRef]
- Hui, S.K.; Khalil, A.; Zhang, Y.; Coghill, K.; Le, C.; Dusenbery, K.; Froelich, J.; Yee, D.; Downs, L. Longitudinal Assessment of Bone Loss from Diagnostic Computed Tomography Scans in Gynecologic Cancer Patients Treated with Chemotherapy and Radiation. Am. J. Obstet. Gynecol. 2010, 203, 353.e1–353.e7. [Google Scholar] [CrossRef] [PubMed]
- Nishio, K.; Tanabe, A.; Maruoka, R.; Nakamura, K.; Takai, M.; Sekijima, T.; Tunetoh, S.; Terai, Y.; Ohmichi, M. Bone Mineral Loss Induced by Anticancer Treatment for Gynecological Malignancies in Premenopausal Women. Endocr. Connect. 2013, 2, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, C.L.; Van Poznak, C.; Lacchetti, C.; Kirshner, J.; Eastell, R.; Gagel, R.; Smith, S.; Edwards, B.J.; Frank, E.; Lyman, G.H.; et al. Management of Osteoporosis in Survivors of Adult Cancers With Nonmetastatic Disease: ASCO Clinical Practice Guideline. JCO 2019, 37, 2916–2946. [Google Scholar] [CrossRef]
- Hadji, P.; Aapro, M.S.; Body, J.-J.; Gnant, M.; Brandi, M.L.; Reginster, J.Y.; Zillikens, M.C.; Glüer, C.-C.; De Villiers, T.; Baber, R.; et al. Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in Postmenopausal Women with Hormone Sensitive Breast Cancer: Joint Position Statement of the IOF, CABS, ECTS, IEG, ESCEO, IMS, and SIOG. J. Bone Oncol. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Waqas, K.; Lima Ferreira, J.; Tsourdi, E.; Body, J.-J.; Hadji, P.; Zillikens, M.C. Updated Guidance on the Management of Cancer Treatment-Induced Bone Loss (CTIBL) in Pre- and Postmenopausal Women with Early-Stage Breast Cancer. J. Bone Oncol. 2021, 28, 100355. [Google Scholar] [CrossRef]
- Watanabe, A.; Yoneyama, S.; Nakajima, M.; Sato, N.; Takao-Kawabata, R.; Isogai, Y.; Sakurai-Tanikawa, A.; Higuchi, K.; Shimoi, A.; Yamatoya, H.; et al. Osteosarcoma in Sprague-Dawley Rats after Long-Term Treatment with Teriparatide (Human Parathyroid Hormone (1-34)). J. Toxicol. Sci. 2012, 37, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Power, L.; Lefas, G.; Lambert, P.; Kim, D.; Evaniuk, D.; Lotocki, R.; Dean, E.; Nachtigal, M.W.; Altman, A.D. Hormone Use After Nonserous Epithelial Ovarian Cancer: Overall and Disease-Free Survival. Obstet. Gynecol. 2016, 127, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Guidozzi, F.; Daponte, A. Estrogen Replacement Therapy for Ovarian Carcinoma Survivors: A Randomized Controlled Trial. Cancer 1999, 86, 1013–1018. [Google Scholar] [CrossRef]
- Metcalf, S.; Pandha, H.S.; Morgan, R. Antiangiogenic Effects of Zoledronate on Cancer Neovasculature. Future Oncol. 2011, 7, 1325–1333. [Google Scholar] [CrossRef]
- Ottanelli, S. Prevention and Treatment of Bone Fragility in Cancer Patient. Clin. Cases Miner. Bone Metab. 2015, 12, 116–129. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, C.; Zhang, J.; Ji, X.; Liu, L.; Zhang, G.; Cao, X.; Wang, P. Safety of Denosumab in Postmenopausal Women with Osteoporosis or Low Bone Mineral Density: A Meta-Analysis. Int. J. Clin. Exp. Pathol. 2014, 7, 2113–2122. [Google Scholar]
- Rizzoli, R.; Chevalley, T. Bone Health: Biology and Nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 24–30. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]

| Mechanism | Biological/Pathophysiological Pathways | Clinical Evidence and Outcomes |
|---|---|---|
| Hormonal pathways (estrogen deficiency) | ↓ Estrogen → ↓ OPG/↑ RANKL → ↑osteoclastogenesis Inhibition of Fas/FasL-mediated osteoclast apoptosis ↑ TNF, IL-1 ↓ osteoblast survival & mechanotransduction | BMD decline within 1 year; 37–55% osteopenia/osteoporosis; more pronounced <45 yrs; fracture risk debated in prophylactic vs. oncologic cases |
| Tumor-driven pathways | Tumor-derived cytokines (TNF, IL-1, PTHrP, PGE2) ↑ RANKL/OPG ratio ↑ CTX | Accelerated bone turnover; reduced pre-operative BMD; hypercalcaemia in PTHrP-secreting tumors |
| Treatment-related mechanisms (chemotherapy) | Platinum/taxane regimens induce direct osteotoxicity and sarcopenia ↑ inflammation, oxidative stress | Additional BMD reduction (−10 to −16% at 1 yr); cumulative chemotherapy cycles predict osteoporosis; persistent loss beyond first year |
| Therapy | Mechanism of Action | Evidence in Ovarian Cancer (Preclinical/Clinical) | Clinical Relevance/Notes |
|---|---|---|---|
| Bisphosphonates (alendronate, risedronate, ibandronate, zoledronate) | Bind to hydroxyapatite → internalised by osteoclasts → induce apoptosis → ↓ bone resorption and turnover | Preclinical: Zoledronate ↓ RANKL/OPG ratio, preserved trabecular bone, improved muscle mass and strength; synergy with chemotherapy. Clinical: Zoledronate ↓ vertebral fracture risk by ~70% in general population; effective in oncology patients, widely used in bone metastases and hypercalcaemia | Zoledronate = most potent; preferred in premenopausal women; long clinical experience |
| Denosumab | Monoclonal antibody against RANKL → blocks RANK–RANKL pathway (mimics OPG) | Preclinical: preserved trabecular bone and partly muscle mass. Clinical: Meta-analysis of 11 RCTs showed ↓ vertebral fracture risk and maintained BMD with safety comparable to bisphosphonates | Useful alternative when bisphosphonates contraindicated; subcutaneous administration |
| Calcium + Vitamin D supplementation | Provide substrates for bone mineralization | Clinical: Meta-analyses show modest reduction in hip, vertebral and overall fragility fractures | Recommended in combination with anti-resorptives in both general and oncology populations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mineo, M.I.; Arnaldi, G.; Guarnotta, V. Osteoporosis and Fracture Risk in Ovarian Cancer: Beyond the Oncologic Burden. Diagnostics 2025, 15, 2966. https://doi.org/10.3390/diagnostics15232966
Mineo MI, Arnaldi G, Guarnotta V. Osteoporosis and Fracture Risk in Ovarian Cancer: Beyond the Oncologic Burden. Diagnostics. 2025; 15(23):2966. https://doi.org/10.3390/diagnostics15232966
Chicago/Turabian StyleMineo, Mariagrazia Irene, Giorgio Arnaldi, and Valentina Guarnotta. 2025. "Osteoporosis and Fracture Risk in Ovarian Cancer: Beyond the Oncologic Burden" Diagnostics 15, no. 23: 2966. https://doi.org/10.3390/diagnostics15232966
APA StyleMineo, M. I., Arnaldi, G., & Guarnotta, V. (2025). Osteoporosis and Fracture Risk in Ovarian Cancer: Beyond the Oncologic Burden. Diagnostics, 15(23), 2966. https://doi.org/10.3390/diagnostics15232966






