Clinicopathologic Determinants of Lymph Node Count and Prognostic Significance of Metastatic Lymph Node Ratio in Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRC | Colorectal cancer |
| AJCC | American Joint Committee on Cancer |
| OS | Overall survival |
| MLNR | Metastatic lymph node ratio |
| H&E | Hematoxylin and eosin |
| WHO | World Health Organization |
| LVI | Lymphovascular invasion |
| PNI | Perineural invasion |
| TILs | Tumor-infiltrating lymphocytes |
| LNC | Lymph node count |
| Forward LR | Forward stepwise elimination |
| MSI | Microsatellite instability |
| CLR | Crohn’s-like reaction |
| EMT | Epithelial–mesenchymal transition |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Johnson, K.; Ahmed, O.; Iqbal, N. Advances in the management of colorectal cancer: From biology to treatment. Int. J. Color. Dis. 2014, 29, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.J.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Moyer, V.A. Lymph node evaluation and survival after curative resection of colon cancer: Systematic review. J. Natl. Cancer Inst. 2007, 99, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.R. AJCC 8th Edition: Colorectal Cancer. Ann. Surg. Oncol. 2018, 25, 1454–1455. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, P.F.; Benson, A.B., 3rd; Chen, Y.J.; Choti, M.A.; Dilawari, R.A.; Enke, C.A.; Fakih, M.G.; Fuchs, C.; Kiel, K.; Knol, J.A.; et al. National Comprehensive Cancer Network. Colon cancer clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2005, 3, 468–491. [Google Scholar] [CrossRef] [PubMed]
- Trepanier, M.; Erkan, A.; Kouyoumdjian, A.; Nassif, G.; Albert, M.; Monson, J.; Lee, L. Examining the relationship between lymph node harvest and survival in patients undergoing colectomy for colon adenocarcinoma. Surgery 2019, 166, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.M.; Porter, G.A.; Ricciardi, R.; Baxter, N.N. Increasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancer. J. Clin. Oncol. 2006, 24, 3570–3575. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.L.; Gao, P.; Wang, Z.N.; Song, Y.X.; Xu, Y.Y.; Sun, Z.; Xing, C.Z.; Wang, X.; Xu, H.M. Can lymph node ratio take the place of pN categories in the UICC/AJCC TNM classification system for colorectal cancer? Ann. Surg. Oncol. 2011, 18, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Parnaby, C.N.; Scott, N.W.; Ramsay, G.; MacKay, C.; Samuel, L.; Murray, G.I.; Loudon, M.A. Prognostic value of lymph node ratio and extramural vascular invasion on survival for patients undergoing curative colon cancer resection. Br. J. Cancer 2015, 113, 212–219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carneiro, F.; Chan, J.; Cheung, N.Y.A. WHO Classification of Tumours. In Digestive System Tumours, 5th ed.; IARC Press: Lyon, France, 2019. [Google Scholar]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Yang, Y.; Wang, Y.; Wang, Z. Construction of a new clinical staging system for colorectal cancer based on the lymph node ratio: A validation study. Front. Surg. 2022, 9, 929576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Derwinger, K.; Carlsson, G.; Gustavsson, B. A study of lymph node ratio as a prognostic marker in colon cancer. Eur. J. Surg. Oncol. 2008, 34, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.C.; Sigurdson, E.R.; LeVoyer, T.; Hanlon, A.; Mayer, R.J.; Macdonald, J.S.; Catalano, P.J.; Haller, D.G. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J. Clin. Oncol. 2005, 23, 8706–8712. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Choi, H.J.; Park, K.J.; Shin, J.S.; Kwon, H.C.; Roh, M.S.; Kim, C. Prognostic significance of metastatic lymph node ratio in node-positive colon carcinoma. Ann. Surg. Oncol. 2007, 14, 1712–1717. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hassett, J.M.; Dayton, M.T.; Kulaylat, M.N. Lymph node ratio: Role in the staging of node-positive colon cancer. Ann. Surg. Oncol. 2008, 15, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- Mroczkowski, P.; Kim, S.; Otto, R.; Lippert, H.; Zajdel, R.; Zajdel, K.; Merecz-Sadowska, A. Prognostic Value of Metastatic Lymph Node Ratio and Identification of Factors Influencing the Lymph Node Yield in Patients Undergoing Curative Colon Cancer Resection. Cancers 2024, 16, 218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, S.L.; Steele, S.R.; Eberhardt, J.; Zhu, K.; Bilchik, A.; Stojadinovic, A. Lymph node ratio as a quality and prognostic indicator in stage III colon cancer. Ann. Surg. 2011, 253, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.L.; Stewart, K.E.; Garwe, T.; Sarwar, Z.; Morris, K.T. Retrospective Cohort Analysis of the Effect of Age on Lymph Node Harvest, Positivity, and Ratio in Colorectal Cancer. Cancers 2022, 14, 3817. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tekkis, P.P.; Smith, J.J.; Heriot, A.G.; Darzi, A.W.; Thompson, M.R.; Stamatakis, J.D.; Association of Coloproctology of Great Britain and Ireland. A national study on lymph node retrieval in resectional surgery for colorectal cancer. Dis. Colon Rectum 2006, 49, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Dai, Z.; Fu, J.; Liang, Z.; Du, P.; Wu, T. Prognostic significance of negative lymph node count in microsatellite instability-high colorectal cancer. World J. Surg. Oncol. 2024, 22, 186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chou, J.F.; Row, D.; Gonen, M.; Liu, Y.H.; Schrag, D.; Weiser, M.R. Clinical and pathologic factors that predict lymph node yield from surgical specimens in colorectal cancer: A population-based study. Cancer 2010, 116, 2560–2570. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Altintas, S.; Bayrak, M. Assessment of Factors Influencing Lymph Node Count in Colorectal Cancer. J. Coll. Physicians Surg. Pak. 2019, 29, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Betge, J.; Harbaum, L.; Pollheimer, M.J.; Lindtner, R.A.; Kornprat, P.; Ebert, M.P.; Langner, C. Lymph node retrieval in colorectal cancer: Determining factors and prognostic significance. Int. J. Color. Dis. 2017, 32, 991–998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yıldırım, F.; Sezak, M.; Yoldaş, T.; Karabulut, B.; Doğanavsargil, B. Frequency and Clinicopathologic Features of DNA Mismatch Repair Protein Deficiency in Colorectal Carcinoma in Turkish Population: Mismatch Repair Deficiency in Colorectal Carcinoma. Injector 2024, 3, 10–23. [Google Scholar] [CrossRef]
- Emile, S.H.; Horesh, N.; Garoufalia, Z.; Wignakumar, A.; Boutros, M.; Wexner, S.D. Association between lymphovascular invasion and lymph node metastases in colon cancer: A National Cancer Database analysis. Color. Dis. 2025, 27, e17256. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, W.B.; Dukes, C.; Bussey, H.J. Lymphatic Spread in Cancer of the Rectum. Br. J. Surg. 1935, 23, 395–413. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, N.P.; Webbink, L.; Haddad, T.S.; Rutgers, N.; van Vliet, S.; Wood, C.S.; Jansen, P.W.; Lafarge, M.W.; imCMS Consortium; de Wilt, J.H.; et al. Transcriptomics and proteomics reveal distinct biology for lymph node metastases and tumour deposits in colorectal cancer. J. Pathol. 2023, 261, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Bonnomet, A.; Brysse, A.; Tachsidis, A.; Waltham, M.; Thompson, E.W.; Polette, M.; Gilles, C. Epithelial-to-mesenchymal transitions and circulating tumor cells. J. Mammary Gland. Biol. Neoplasia 2010, 15, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.; Savagner, P.; Ortiz-Cuaran, S.; Mahjoubi, L.; Saintigny, P.; Thiery, J.P.; Chouaib, S. New insights into the role of EMT in tumor immune escape. Mol. Oncol. 2017, 11, 824–846. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Total (n = 989) | LNC < 12 (n = 346) | LNC ≥ 12 (n = 643) | OR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Age | 62.5 ± 12.7 | 64.9 ± 11.4 | 61.2 ± 13.2 | 0.977 (0.966–0.987) | <0.001 |
| Gender | |||||
| Male | 593 (60.0%) | 208 (60.1%) | 385 (59.9%) | 1.000 | - |
| Female | 396 (40.0%) | 138 (39.9%) | 258 (40.1%) | 1.010 (0.774–1.319) | 0.941 |
| Tumor localization | |||||
| Rectum | 446 (45.1%) | 153 (44.2%) | 293 (45.6%) | 1.056 (0.812–1.373) | 0.685 |
| Right colon | 221 (22.3%) | 52 (15.0%) | 169 (26.3%) | 2.016 (1.430–2.841) | <0.001 |
| Left colon | 326 (33.0%) | 141 (40.8%) | 185 (28.8%) | 0.587 (0.446–0.772) | <0.001 |
| Tumor size | 5.25 ± 2.37 | 4.71 ± 2.46 | 5.54 ± 2.27 | 1.196 (1.118–1.280) | <0.001 |
| Tumor type | |||||
| Adenocarcinoma | 885 (89.5%) | 312 (90.2%) | 573 (89.1%) | 1.000 | - |
| Mucinous | 98 (9.9%) | 33 (9.5%) | 65 (10.1%) | 1.073 (0.690–1.667) | 0.756 |
| Signet ring cell | 6 (0.6%) | 1 (0.3%) | 5 (0.8%) | 2.723 (0.317–23.406) | 0.362 |
| Tumor differentiation | |||||
| Well | 93 (10.5%) | 39 (12.5%) | 54 (9.4%) | 1.000 | - |
| Moderate | 716 (80.9%) | 254 (81.4%) | 462 (80.6%) | 1.314 (0.947–2.038) | 0.224 |
| Poor | 76 (8.6%) | 19 (6.1%) | 57 (10.0%) | 2.167 (1.117–4.204) | 0.022 |
| pT stage | |||||
| pT1 | 13 (1.3%) | 6 (1.7%) | 7 (1.1%) | 1.000 | - |
| pT2 | 96 (9.7%) | 46 (13.3%) | 50 (7.8%) | 0.932 (0.292–2.977) | 0.905 |
| pT3 | 794 (80.3%) | 268 (77.5%) | 526 (81.8%) | 1.682 (0.560–5.055) | 0.354 |
| pT4 | 86 (8.7%) | 26 (7.5%) | 60 (9.3%) | 1.978 (0.606–6.460) | 0.259 |
| pN stage | |||||
| pN0 | 476 (48.1%) | 171 (49.4%) | 305 (47.4%) | 1.000 | - |
| pN1 | 296 (29.9%) | 118 (34.1%) | 178 (27.7%) | 0.946 (0.627–1.140) | 0.272 |
| pN1c | 39 (3.9%) | 17 (4.9%) | 22 (3.4%) | 0.726 (0.375–1.404) | 0.341 |
| pN2 | 178 (18.0%) | 40 (11.6%) | 138 (21.5%) | 1.934 (1.298–2.882) | <0.001 |
| LVI | 248 (25.1%) | 86 (24.9%) | 162 (25.2%) | 1.018 (0.753–1.377) | 0.907 |
| PNI | 233 (23.6%) | 87 (25.1%) | 146 (22.7%) | 0.875 (0.645–1.186) | 0.389 |
| Satellite tumor deposit | 172 (17.4%) | 70 (20.2%) | 102 (15.9%) | 0.743 (0.531–1.041) | 0.085 |
| Presence of TIL | 103 (10.4%) | 28 (8.1%) | 75 (11.7%) | 1.500 (0.951–2.364) | 0.081 |
| Crohn’s-like reaction | 380 (38.4%) | 113 (32.7%) | 267 (41.5%) | 1.464 (1.113–1.926) | 0.006 |
| Mucinous tumor component | |||||
| Absent | 648 (65.5%) | 227 (65.6%) | 421 (65.5%) | 1.000 | - |
| <50% | 236 (23.9%) | 85 (24.6%) | 151 (23.5%) | 0.958 (0.702–1.307) | 0.786 |
| ≥50% | 105 (10.6%) | 34 (9.8%) | 71 (11.0%) | 1.126 (0.726–1.747) | 0.597 |
| Signet ring cell component | 39 (3.9%) | 11 (3.2%) | 28 (4.4%) | 1.387 (0.682–2.820) | 0.367 |
| Medullary tumor component | 49 (5.0%) | 11 (3.2%) | 38 (5.9%) | 1.913 (0.965–3.792) | 0.063 |
| Tumor budding | 234 (23.7%) | 84 (24.3%) | 150 (23.3%) | 0.949 (0.699–1.289) | 0.738 |
| Tumor border | |||||
| Expansive | 71 (7.2%) | 28 (8.1%) | 43 (6.7%) | 1.000 | - |
| Infiltrative | 918 (92.8) | 318 (91.9%) | 600 (93.3%) | 1.229 (0.749–2.016) | 0.415 |
| Odds Ratio | 95% Confidence Interval | Wald | p-Value | ||
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Age * | 1.236 | 1.106 | 1.384 | 14.408 | <0.001 |
| Right colon localization | 1.723 | 1.201 | 2.471 | 8.718 | 0.003 |
| Tumor size | 1.176 | 1.097 | 1.262 | 20.787 | <0.001 |
| N1 | 1.004 | 0.721 | 1.398 | 0.001 | 0.980 |
| N1c | 1.372 | 0.607 | 3.100 | 0.577 | 0.447 |
| N2 | 2.349 | 1.491 | 3.701 | 13.564 | <0.001 |
| Absence of satellite tumor deposit | 1.733 | 1.107 | 2.710 | 5.792 | 0.016 |
| Crohn’s-like reaction | 1.501 | 1.126 | 2.000 | 7.684 | 0.006 |
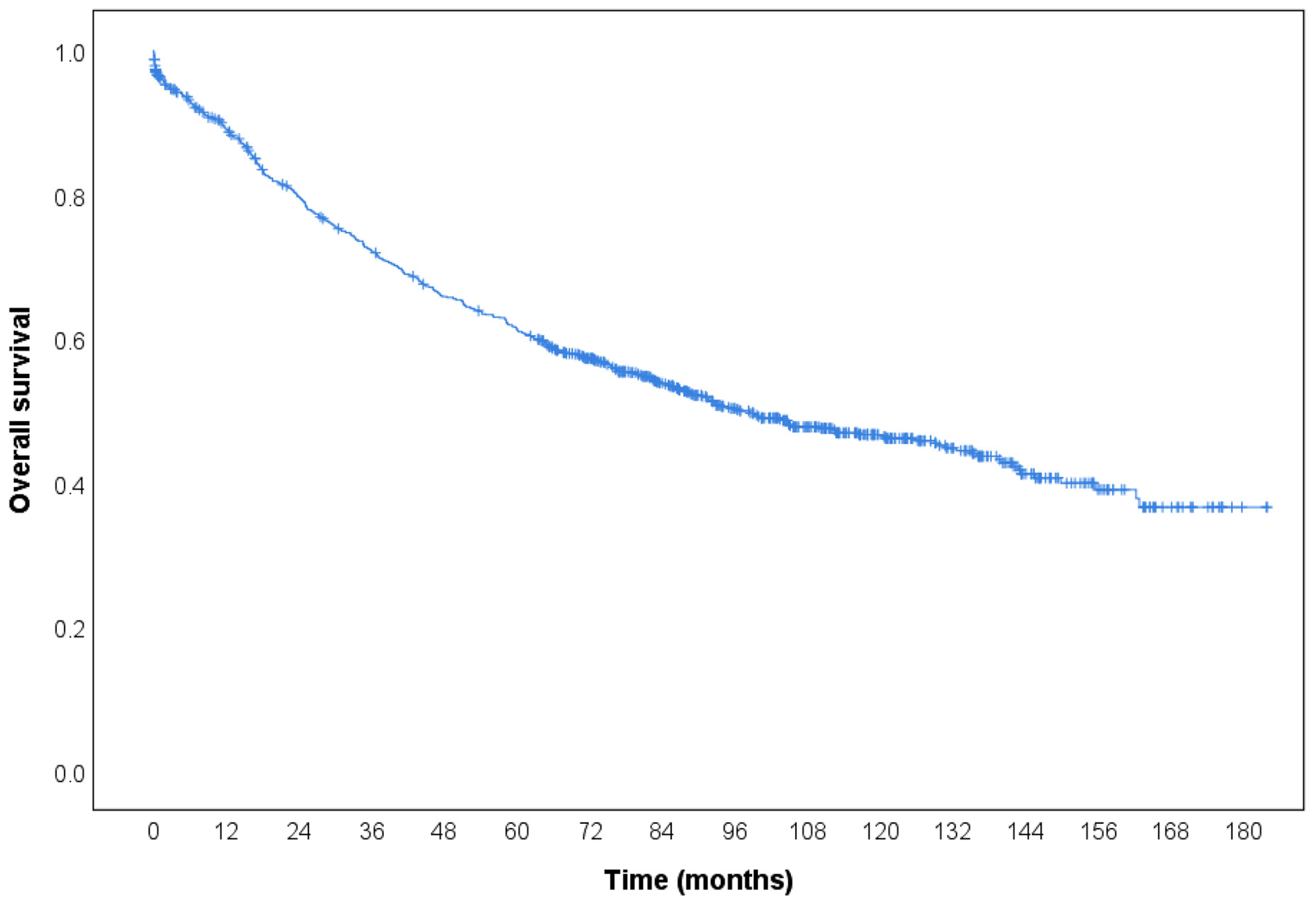
| Cases | Number of Deaths | Overall Survival Rate | Cumulative Survival Rate | Estimated Life Expectancy (Month) * | Log-Rank | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 year | 3 years | 5 years | |||||||
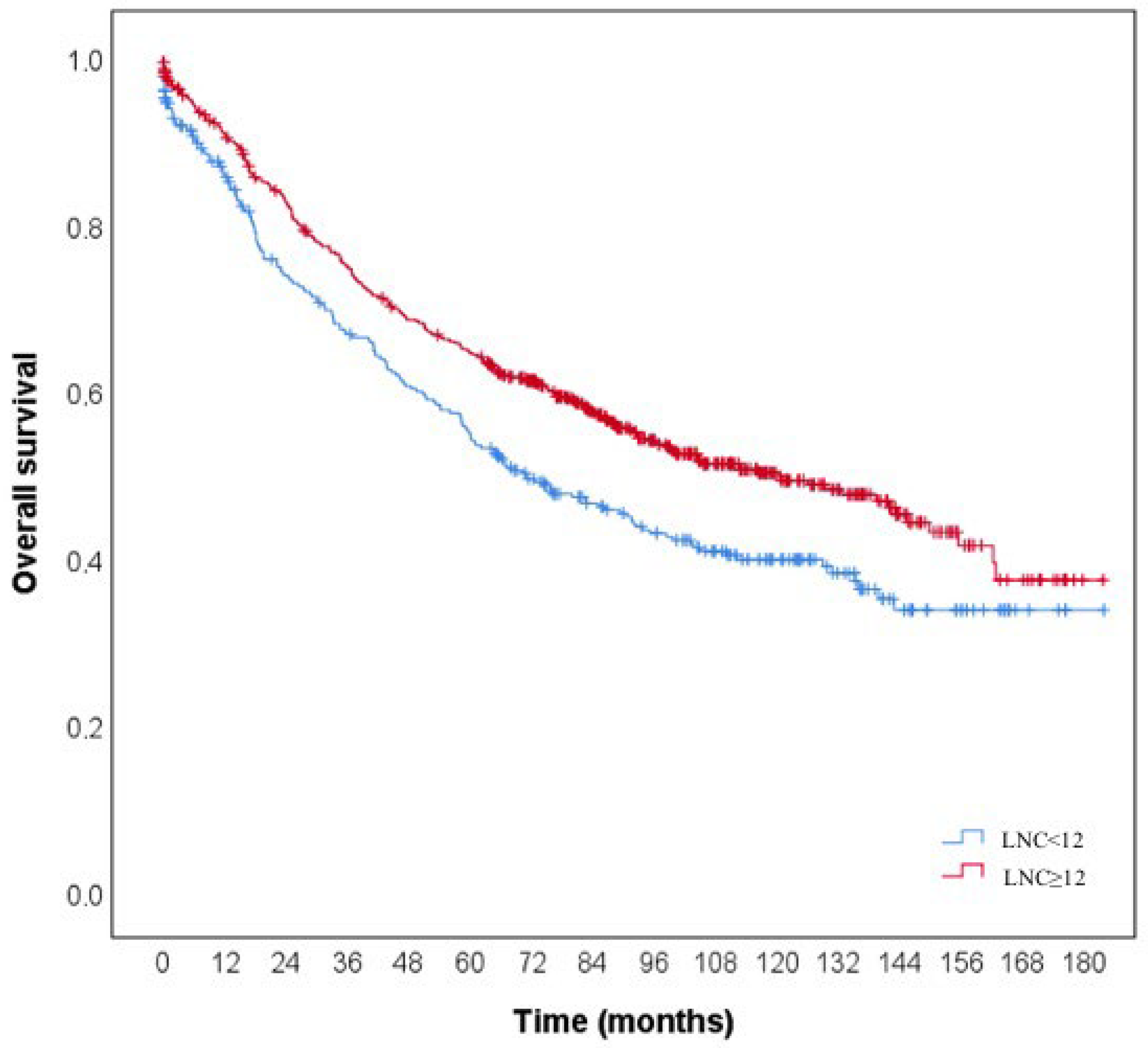
| LNC | 10.559 | <0.001 | |||||||
| <12 | 346 | 190 | 45.1% | 0.859 | 0.667 | 0.547 | 93.2 (84.9–101.6) | ||
| ≥12 | 643 | 299 | 53.5% | 0.908 | 0.749 | 0.645 | 108.7 (102.6–114.8) | ||
| MLNR | 135.141 | <0.001 | |||||||
| MLNR 0 | 518 | 201 | 61.2% | 0.921 | 0.835 | 0.743 | 122.8 (116.3–129.3) A | ||
| MLNR 1 (<0.20) | 218 | 114 | 47.7% | 0.911 | 0.725 | 0.616 | 100.0 (89.8–110.2) B | ||
| MLNR 2 (0.20–0.50) | 162 | 107 | 34.0% | 0.830 | 0.543 | 0.396 | 71.2 (60.6–81.7) C | ||
| MLNR 3 (>0.50) | 91 | 67 | 26.4% | 0.777 | 0.346 | 0.205 | 47.1 (34.9–59.3) D | ||
| LNC < 12 cases | 83.534 | <0.001 | |||||||
| MLN 0 | 190 | 81 | 57.4% | 0.903 | 0.823 | 0.721 | 118.3 (107.6–129.0) A | ||
| MLNR 1 (<0.20) | 45 | 31 | 31.1% | 0.909 | 0.602 | 0.445 | 68.6 (51.3–85.8) B | ||
| MLNR 2 (0.20–0.50) | 69 | 46 | 33.3% | 0.823 | 0.526 | 0.378 | 68.4 (52.3–84.5) B | ||
| MLNR 3 (>0.50) | 42 | 32 | 23.8% | 0.650 | 0.187 | 0.062 | 27.8 (14.7–41.0) C | ||
| LNC ≥ 12 cases | 64.552 | <0.001 | |||||||
| MLN 0 | 328 | 120 | 63.4% | 0.932 | 0.843 | 0.756 | 123.7 (115.9–131.5) A | ||
| MLNR 1 (<0.20) | 173 | 83 | 52.0% | 0.911 | 0.754 | 0.656 | 107.8 (96.2–119.4) B | ||
| MLNR 2 (0.20–0.50) | 93 | 61 | 34.4% | 0.835 | 0.556 | 0.408 | 68.9 (56.4–81.4) C | ||
| MLNR 3 (>0.50) | 49 | 35 | 28.6% | 0.874 | 0.460 | 0.307 | 58.1 (42.0–74.3) C | ||
| MLN Absent | 1.648 | 0.199 | |||||||
| LNC < 12 | 190 | 81 | 57.4% | 0.903 | 0.823 | 0.721 | 118.3 (107.6–129.0) | ||
| LNC ≥ 12 | 328 | 120 | 63.4% | 0.932 | 0.843 | 0.756 | 123.7 (115.9–131.5) | ||
| MLNR < 0.20 | 9.839 | 0.002 | |||||||
| LNC < 12 | 45 | 31 | 31.1% | 0.909 | 0.602 | 0.445 | 68.6 (51.3–85.8) | ||
| LNC ≥ 12 | 173 | 83 | 52.0% | 0.911 | 0.754 | 0.656 | 107.8 (96.2–119.4) | ||
| MLNR 0.20–0.50 | 0.181 | 0.670 | |||||||
| LNC < 12 | 69 | 46 | 33.3% | 0.823 | 0.526 | 0.378 | 68.4 (52.3–84.5) | ||
| LNC ≥ 12 | 93 | 61 | 34.4% | 0.835 | 0.556 | 0.408 | 68.9 (56.4–81.4) | ||
| MLNR > 0.50 | 10.042 | 0.002 | |||||||
| LNC < 12 | 42 | 32 | 23.8% | 0.650 | 0.187 | 0.062 | 27.8 (14.7–41.0) | ||
| LNC ≥ 12 | 49 | 35 | 28.6% | 0.874 | 0.460 | 0.307 | 58.1 (42.0–74.3) | ||
| Total | 989 | 489 | 50.6% | 0.891 | 0.721 | 0.612 | 103.6 (98.6–108.5) | - | - |
| HR (95% CI) | Wald | p-Value | |
|---|---|---|---|
| Age | 1.021 (1.013–1.029) | 29.302 | <0.001 |
| Male gender factor | 1.123 (0.936–1.348) | 1.563 | 0.211 |
| Right colon localization | 1.149 (0.929–1.422) | 1.634 | 0.201 |
| Tumor size | 1.010 (0.973–1.048) | 0.256 | 0.613 |
| Mucinous tumor type | 1.411 (1.075–1.853) | 6.144 | 0.013 |
| Signet ring cell tumor type | 1.867 (0.697–4.998) | 1.543 | 0.214 |
| Moderate differentiation | 1.485 (1.039–2.123) | 4.714 | 0.030 |
| Poor differentiation | 2.098 (1.330–3.311) | 10.136 | <0.001 |
| pT2 | 1.843 (0.437–7.765) | 0.694 | 0.405 |
| pT3 | 4.339 (1.081–17.412) | 4.287 | 0.038 |
| pT4 | 8.087 (1.972–33.164) | 8.427 | 0.004 |
| Lymph node count < 12 | 1.351 (1.126–1.620) | 10.476 | <0.001 |
| MLNR < 0.20 | 1.548 (1.230–1.949) | 13.871 | <0.001 |
| MLNR 0.20–0.50 | 2.510 (1.983–3.178) | 58.57 | <0.001 |
| MLNR > 0. 50 | 4.126 (3.117–5.462) | 98.065 | <0.001 |
| pN1 | 1.931 (1.562–2.386) | 36.999 | <0.001 |
| pN1c | 1.826 (1.161–2.872) | 6.802 | 0.009 |
| pN2 | 3.078 (2.442–3.880) | 90.528 | <0.001 |
| LVI | 2.201 (1.821–2.662) | 66.303 | <0.001 |
| PNI | 2.123 (1.752–2.573) | 59.044 | <0.001 |
| Satellite tumor deposit | 2.068 (1.676–2.552) | 45.888 | <0.001 |
| Absence of TILs | 1.787 (1.267–2.521) | 10.945 | <0.001 |
| Crohn’s-like reaction | 0.765 (0.635–0.922) | 7.916 | 0.005 |
| Medullary tumor component | 0.978 (0.637–1.500) | 0.011 | 0.918 |
| Tumor budding | 1.529 (1.254–1.863) | 17.684 | <0.001 |
| Infiltrative tumor border | 1.613 (1.094–2.379) | 5.828 | 0.016 |
| HR (95% CI) | Wald | p-Value | |
|---|---|---|---|
| Age | 1.026 (1.018–1.034) | 42.486 | <0.001 |
| pT2 | 1.650 (0.391–6.967) | 0.464 | 0.496 |
| pT3 | 3.304 (0.821–13.298) | 2.829 | 0.093 |
| pT4 | 5.410 (1.312–22.311) | 5.454 | 0.020 |
| Lymph node count < 12 | 1.326 (1.096–1.604) | 8.448 | 0.004 |
| MLNR < 0.20 | 1.331 (1.049–1.689) | 5.532 | 0.019 |
| MLNR 0.20–0.50 | 1.919 (1.488–2.475) | 25.204 | <0.001 |
| MLNR > 0.50 | 2.972 (2.193–4.026) | 49.392 | <0.001 |
| LVI | 1.525 (1.238–1.880) | 15.670 | <0.001 |
| PNI | 1.479 (1.201–1.821) | 13.557 | <0.001 |
| Crohn’s-like reaction | 0.801 (0.662–0.969) | 5.238 | 0.022 |
| Mucinous tumor component < 50% | 0.981 (0.788–1.220) | 0.030 | 0.862 |
| Mucinous tumor component ≥ 50% | 1.660 (1.258–2.192) | 12.794 | <0.001 |
| Tumor budding | 1.324 (1.078–1.626) | 7.140 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yildirim, F.; Sezak, M.; Bozbiyik, O.; Gursoy, P.; Doganavsargil, B. Clinicopathologic Determinants of Lymph Node Count and Prognostic Significance of Metastatic Lymph Node Ratio in Colorectal Cancer. Diagnostics 2025, 15, 2962. https://doi.org/10.3390/diagnostics15232962
Yildirim F, Sezak M, Bozbiyik O, Gursoy P, Doganavsargil B. Clinicopathologic Determinants of Lymph Node Count and Prognostic Significance of Metastatic Lymph Node Ratio in Colorectal Cancer. Diagnostics. 2025; 15(23):2962. https://doi.org/10.3390/diagnostics15232962
Chicago/Turabian StyleYildirim, Fatma, Murat Sezak, Osman Bozbiyik, Pinar Gursoy, and Basak Doganavsargil. 2025. "Clinicopathologic Determinants of Lymph Node Count and Prognostic Significance of Metastatic Lymph Node Ratio in Colorectal Cancer" Diagnostics 15, no. 23: 2962. https://doi.org/10.3390/diagnostics15232962
APA StyleYildirim, F., Sezak, M., Bozbiyik, O., Gursoy, P., & Doganavsargil, B. (2025). Clinicopathologic Determinants of Lymph Node Count and Prognostic Significance of Metastatic Lymph Node Ratio in Colorectal Cancer. Diagnostics, 15(23), 2962. https://doi.org/10.3390/diagnostics15232962






