Laser Confocal Microscopy May Be a Useful Tool in Neuropathological Intraoperative Examination
Abstract
1. Introduction
2. Materials and Methods
3. Results
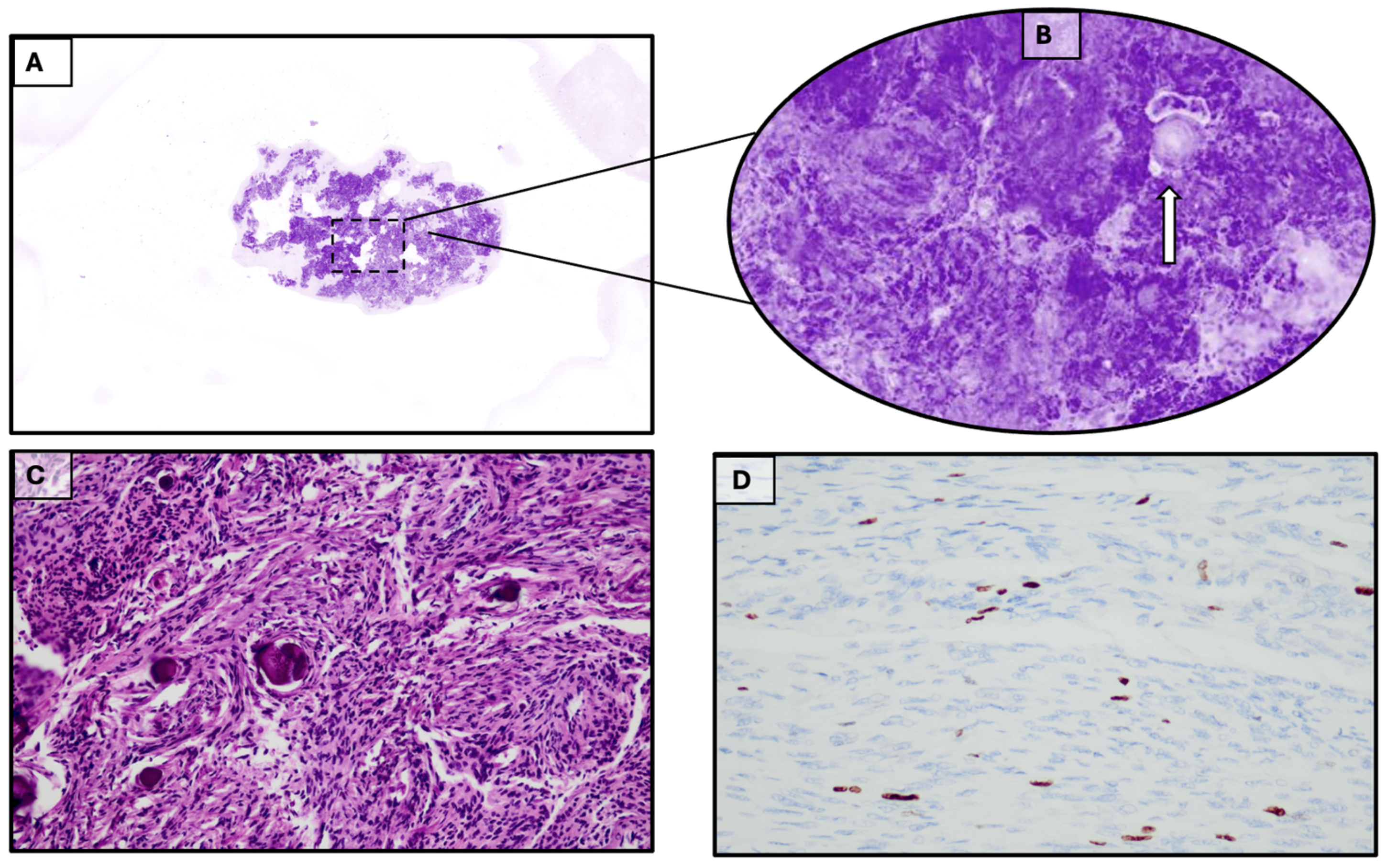
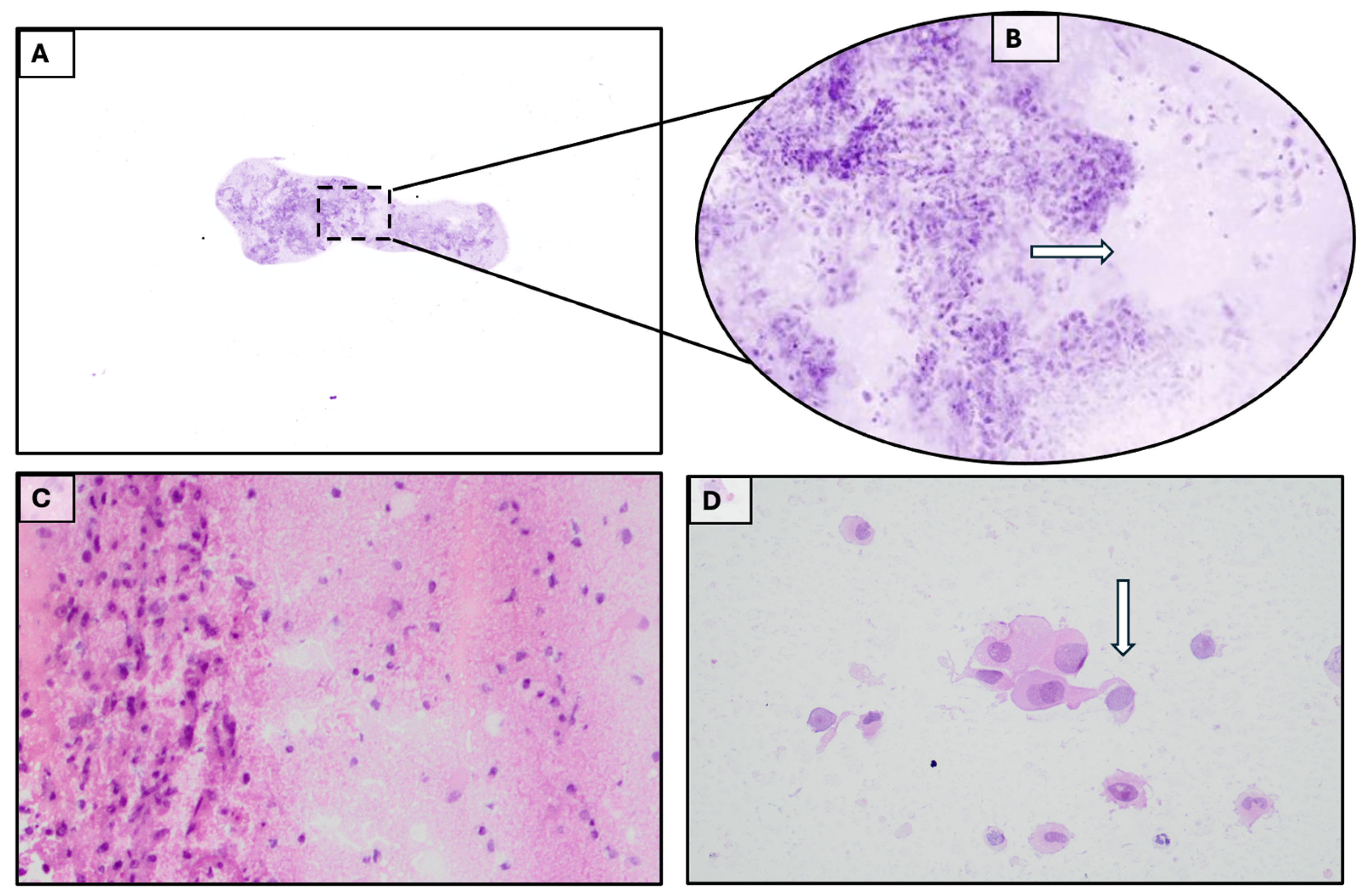
3.1. Case 1
3.2. Case 2
3.3. Case 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MRI | Magnetic Resonance Imaging |
| CT | Computed Tomography |
| H&E | Hematoxylin-eosin |
| NE | Neurological Examination |
| GCS | Glasgow Coma Scale |
| EOR | Extension Of Resection |
| SupTR | Supratotal Resection |
| GTR | Gross-Total Resection |
| STR | Subtotal Resection |
| NMR | Nuclear Magnetic Resonance |
| CRT | Chemoradiotherapy |
| SRS | Stereotactic RadioSurgery |
References
- Balsimelli, L.B.S.; Oliveira, J.C.; Adorno, F.Á.; Brites, C.A.; Bublitz, G.S.; Tavares, L.C.C.; Coelho, K.M.P.A.; Stall, J.; França, P.H.C. Accuracy of Intraoperative Examination in Central Nervous System Lesions: A Study of 133 Cases. Acta Cytol. 2019, 63, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Picart, T.; Gautheron, A.; Caredda, C.; Ray, C.; Mahieu-Williame, L.; Montcel, B.; Guyotat, J. Fluorescence-Guided Surgical Techniques in Adult Diffuse Low-Grade Gliomas: State-of-the-Art and Emerging Techniques: A Systematic Review. Cancers 2024, 16, 2698. [Google Scholar] [CrossRef]
- Ndirangu, B.; Bryan, K.; Nduom, E. Extent of Resection and Outcomes of Patients with Primary Malignant Brain Tumors. Curr. Treat Options Oncol. 2023, 24, 1948–1961. [Google Scholar] [CrossRef] [PubMed]
- Karschnia, P.; Tonn, J.C.; Cahill, D.P. The Infiltrative Margins in Glioblastoma: Important Is What Has Been Left behind. Clin. Cancer Res. 2024, 30, 4811–4812. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.; Schubert, M.; Metzler, G.; Geppert, J.P.; Moehrle, M. Diagnostic accuracy of a new ex vivo confocal laser scanning microscope compared to H&E-stained paraffin slides for micrographic surgery of basal cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 298–304. [Google Scholar] [PubMed]
- Elfgen, C.; Papassotiropoulos, B.; Varga, Z.; Moskovszky, L.; Nap, M.; Güth, U.; Baege, A.; Amann, E.; Chiesa, F.; Tausch, C. Comparative analysis of confocal microscopy on fresh breast core needle biopsies and conventional histology. Diagn. Pathol. 2019, 14, 58, Erratum in Diagn. Pathol. 2019, 14, 87. [Google Scholar] [CrossRef]
- Grizzetti, L.; Kuonen, F. Ex vivo confocal microscopy for surgical margin assessment: A histology-compared study on 109 specimens. Skin Health Dis. 2022, 2, e91. [Google Scholar] [CrossRef] [PubMed]
- Sandor, M.F.; Schwalbach, B.; Hofmann, V.; Istrate, S.E.; Schuller, Z.; Ionescu, E.; Heimann, S.; Ragazzi, M.; Lux, M.P. Imaging of lumpectomy surface with large field-of-view confocal laser scanning microscope for intraoperative margin assessment—POLARHIS study. Breast 2022, 66, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Cattacin, I.; Rochat, T.; Feki, A.; Fruscalzo, A.; Boulvain, M.; Guani, B. Confocal Microscopy for Intraoperative Margin Assessment of Lumpectomies by Surgeons in Breast Cancer: Training, Implementation in Routine Practice, and Two-Year Retrospective Analysis. Cancers 2025, 17, 2852. [Google Scholar] [CrossRef] [PubMed]
- Conversano, A.; Abbaci, M.; van Diest, P.; Roulot, A.; Falco, G.; Ferchiou, M.; Coiro, S.; Richir, M.; Genolet, P.M.; Clement, C.; et al. Breast carcinoma detection in ex vivo fresh human breast surgical specimens using a fast slide-free confocal microscopy scanner: HIBISCUSS project. BJS Open 2023, 7, zrad046. [Google Scholar] [CrossRef] [PubMed]
- Togawa, R.; Hederer, J.; Ragazzi, M.; Bruckner, T.; Fastner, S.; Gomez, C.; Hennigs, A.; Nees, J.; Pfob, A.; Riedel, F.; et al. Imaging of lumpectomy surface with large field-of-view confocal laser scanning microscopy ‘Histolog® scanner’ for breast margin assessment in comparison with conventional specimen radiography. Breast 2023, 68, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Wernly, D.; Beniere, C.; Besse, V.; Seidler, S.; Lachat, R.; Letovanec, I.; Huber, D.; Simonson, C. SENOSI Confocal Microscopy: A New and Innovating Way to Detect Positive Margins in Non-Palpable Breast Cancer? Life 2024, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Abbaci, M.; Villard, A.; Auperin, A.; Asmandar, S.; Moya-Plana, A.; Casiraghi, O.; Breuskin, I. Ultra-fast confocal fluorescence microscopy for neck lymph node imaging in head and neck cancer. Oral Oncol. 2024, 154, 106862. [Google Scholar] [CrossRef] [PubMed]
- Eissa, A.; Puliatti, S.; Rodriguez Peñaranda, N.; Resca, S.; Di Bari, S.; Vella, J.; Maggiorelli, S.; Bertoni, L.; Azzoni, P.; Reggiani Bonetti, L.; et al. Current applications of ex-vivo fluorescent confocal microscope in urological practice: A systematic review of literature. Chin. Clin. Oncol. 2024, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Lux, M.P.; Schuller, Z.; Heimann, S.; Reichert, V.M.C.; Kersting, C.; Buerger, H.; Sandor, M.F. Re-Operation Rate for Breast Conserving Surgery Using Confocal Histolog Scanner for Intraoperative Margin Assessment-SHIELD Study. Cancers 2025, 17, 1640. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Magana, R.; Au, M.; Al-Hammouri, T.; Mathew, M.; Dinneen, K.; Mendes, L.S.T.; Dinneen, E.; Vreuls, W.; Shaw, G.; Freeman, A.; et al. Accuracy of the LaserSAFE technique for detecting positive surgical margins during robot-assisted radical prostatectomy: Blind assessment and inter-rater agreement analysis. Histopathology 2025, 86, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Mayor, N.; Light, A.; Silvanto, A.; Cullen, E.; Ng, P.Y.; Badreldin, A.; Khoubehi, B.; Hellawell, G.; Fiorentino, F.; Connor, M.J.; et al. Fluorescence confocal microscopy for margin assessment in prostatectomy: IP8-FLUORESCE study protocol. BJU Int. 2025, 135, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Colard-Thomas, J.; Pialoux-Guibal, A.; Gemival, P.; Maran-Gonzalez, A.; Khellaf, L.; Leaha, C.; Verdanet, E.; Mourregot, A.; Gutowski, M.; Pourquier, D. Confocal Laser Microscopy for Intraoperative Margin Assessment in Breast-Conserving Surgery: A New Procedure in the Pathology Laboratory Workflow. Am. J. Surg. Pathol. 2025, 49, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Yoon, S.J.; Kim, K.H.; Jung, I.H.; Lim, S.H.; Kim, W.; Yoon, H.I.; Kim, S.H.; Sung, K.S.; Roh, T.H.; et al. Patterns of recurrence according to the extent of resection in patients with IDH-wild-type glioblastoma: A retrospective study. J. Neurosurg. 2021, 137, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Nagayasu, M.A.; Fukushima, T.; Matsumoto, F.; Takeshima, H.; Sato, Y.; Kataoka, H. Supratentorial extra-axial RELA fusion-positive ependymoma misdiagnosed as meningioma by intraoperative histological and cytological examinations: A case report. J. Med. Case Rep. 2022, 16, 312. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Jiang, J.H.; Tian, X.Y.; Li, Z. Isolated spinal Rosai-Dorfman disease misdiagnosed as lymphoplasmacyte-rich meningioma by intraoperative histological examination. Brain Tumor Pathol. 2015, 32, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Tamada, T.; Enatsu, R.; Kikuchi, N.; Mikuni, N. Meningioma mimicking an intraparenchymal cystic tumor. Nagoya J. Med. Sci. 2018, 80, 431–434. [Google Scholar] [PubMed]
- Shi, H.J.; Zhao, S.L.; Tian, X.Y.; Li, Z.; Huang, Q.; Li, B. Meningioangiomatosis-associated meningioma misdiagnosed as glioma by radiologic and intraoperative histological examinations. Brain Tumor Pathol. 2011, 28, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Haslund-Vinding, J.; Skjoth-Rasmussen, J.; Poulsgaard, L.; Fugleholm, K.; Mirian, C.; Maier, A.D.; Santarius, T.; Rom Poulsen, F.; Meling, T.; Bartek, J.J.; et al. Proposal of a new grading system for meningioma resection: The Copenhagen Protocol. Acta Neurochir. 2022, 164, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Norden, A.D.; Wen, P.Y.; Kesari, S. Brain metastases. Curr. Opin. Neurol. 2005, 18, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, D.; Shaked, Z.; Fuchs, A.; Roohani, S.; Xu, R.; Schlaak, M.; Frost, N.; Misch, M.; Capper, D.; Kaul, D.; et al. Re-resection of brain metastases-outcomes of an institutional cohort study and literature review. BMC Cancer 2025, 25, 973. [Google Scholar] [CrossRef] [PubMed]


| Authors and Date of Publication | Organ Studied | Type of Surgical Specimen | Results | Reference in This Paper |
|---|---|---|---|---|
| Peters N, Schubert M, Metzler G et al., 2019 | Skin | Basal cell carcinoma excision | In the comparison of the HS digital images with the H&E-stained slides, sensitivity was 73%, and specificity was 96%. | [5] |
| Elfgen C, Papassotiropoulos B, Varga Z et al., 2019 | Breast | Fresh breast core needle biopsy | HS provides a visualization of cellular details equivalent to the H&E standards, which could permit rapid diagnosis of malignant and benign breast lesions. | [6] |
| Grizzetti L, Kuonen F., 2022 | Skin | Biopsies and surgical specimens of cutaneous basal cell and squamous cell carcinomas | Ex vivo Confocal Laser Scanning Microscopy (CLSM) is a fast and accurate alternative to analyze surgical margins and ex vivo CLSM using HS is mostly suited for the assessment of non-infiltrative BCCs margins. Especially when conventional histopathological analysis (prone to false negative because of limited margin assessment) is used. | [7] |
| Sandor MF, Schwalbach B, Hofmann V et al., 2022 | Breast | Lumpectomy | Invasive breast cancer and/or in situ-carcinoma lesions can be detected in HS images of surgical margins leading to a potential reduction of 30% and 75% of the re-operations. | [8] |
| Conversano A, Abbaci M, van Diest P et al., 2023 | Breast | Lumpectomy or mastectomy | Ultra-fast confocal microscopy by HS could represent a practical alternative for rapid intraoperative margin assessment of the surface of the whole surgical specimen with conservation of tissue integrity for further histologic and immunologic investigations. | [10] |
| Togawa R, Hederer J, Ragazzi M et al., 2023 | Breast | Lumpectomy | The study demonstrates feasibility of the HS showing similar detection rates for breast cancer compared to the intraoperative standard of care. | [11] |
| Wernly D, Beniere C, Besse V et al., 2024 | Breast | Lumpectomy | Results indicate that the HS is a reliable and time-efficient method for margin assessment | [12] |
| Abbaci M, Villard A, Auperin A, 2024 | Lymph node | Neck lymph node imaging in head and neck surgery | UFCM images by pathologists have a good accuracy (95.5%) for the detection of metastatic lymph nodes in operated patients undergoing sentinel lymph node biopsy or neck dissection with very high specificity (98.8%) but a sensitivity of 76.7% due to the lack of detection of small metastases (<3 mm) and micro metastases (≤2 mm). | [13] |
| Eissa A, Puliatti S, Rodriguez Peñaranda N, 2024 | Prostate biopsies, surgical margins (prostatectomy and cystectomy), kidney biopsies | Review on prostate cancer (15 articles), bladder cancer (1 article), and renal biopsy (1 article). | Ex vivo confocal microscopy (FCM) has been shown to be 85–95% accurate in distinguishing between cancerous and healthy prostate tissue and has been used for intraoperative margin assessment during prostatectomy, reducing the need for frozen sections. | [14] |
| Cattacin I, Rochat T, Feki A et al., 2025 | Breast | Lumpectomy | Training of a surgeon and HS integration into clinical practice are described. When using the training program, 81% and 100% sensitivities were achieved by surgeons. | [9] |
| Lux MP, Schuller Z, Heimann S, Reichert VMC et al., 2025 | Breast | Lumpectomy | The intraoperative use of the HS provides a significant increase in detection rates of lumpectomy positive margins resulting in a substantial reduction in the re-operation rate. | [15] |
| Almeida-Magana R, Au M, Al-Hammouri T, Mathew M et al., 2025 | Prostate | Robot-assisted radical prostatectomy | HS shows a sensitivity of 73–91 and specificity of 94–100% for the presence of Positive Surgical Margins | [16] |
| Mayor N, Light A, Silvanto A et al., 2025 | Prostate | Robot-assisted radical prostatectomy | Study-protocol in progress on margins assessment | [17] |
| Colard-Thomas J, Pialoux-Guibal A, Gemival P et al., 2025 | Breast | Lumpectomy | In Breast Conservative Surgical specimens, the combination of HS and macroscopic examination allowed the accurate assessment of surgical margins and led to intraoperative re-excision in cases where macroscopic examination alone would not have provided an adequate assessment. | [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dardano, D.; Bilotta, A.; Gallucci, G.; Gentile, C.; Riganati, G.; Veraldi, A.; Policicchio, D.; Nevolo, M.T.; Filardo, A.V.; Lavecchia, A.M.; et al. Laser Confocal Microscopy May Be a Useful Tool in Neuropathological Intraoperative Examination. Diagnostics 2025, 15, 2936. https://doi.org/10.3390/diagnostics15222936
Dardano D, Bilotta A, Gallucci G, Gentile C, Riganati G, Veraldi A, Policicchio D, Nevolo MT, Filardo AV, Lavecchia AM, et al. Laser Confocal Microscopy May Be a Useful Tool in Neuropathological Intraoperative Examination. Diagnostics. 2025; 15(22):2936. https://doi.org/10.3390/diagnostics15222936
Chicago/Turabian StyleDardano, Deborah, Anna Bilotta, Gianmarco Gallucci, Carlo Gentile, Giuseppe Riganati, Antonio Veraldi, Domenico Policicchio, Maria Teresa Nevolo, Alberto V. Filardo, Anna Maria Lavecchia, and et al. 2025. "Laser Confocal Microscopy May Be a Useful Tool in Neuropathological Intraoperative Examination" Diagnostics 15, no. 22: 2936. https://doi.org/10.3390/diagnostics15222936
APA StyleDardano, D., Bilotta, A., Gallucci, G., Gentile, C., Riganati, G., Veraldi, A., Policicchio, D., Nevolo, M. T., Filardo, A. V., Lavecchia, A. M., & Donato, G. (2025). Laser Confocal Microscopy May Be a Useful Tool in Neuropathological Intraoperative Examination. Diagnostics, 15(22), 2936. https://doi.org/10.3390/diagnostics15222936





