Active Matrixmetalloproteinase-8 in Periodontal Diagnosis: A Scoping Review
Abstract
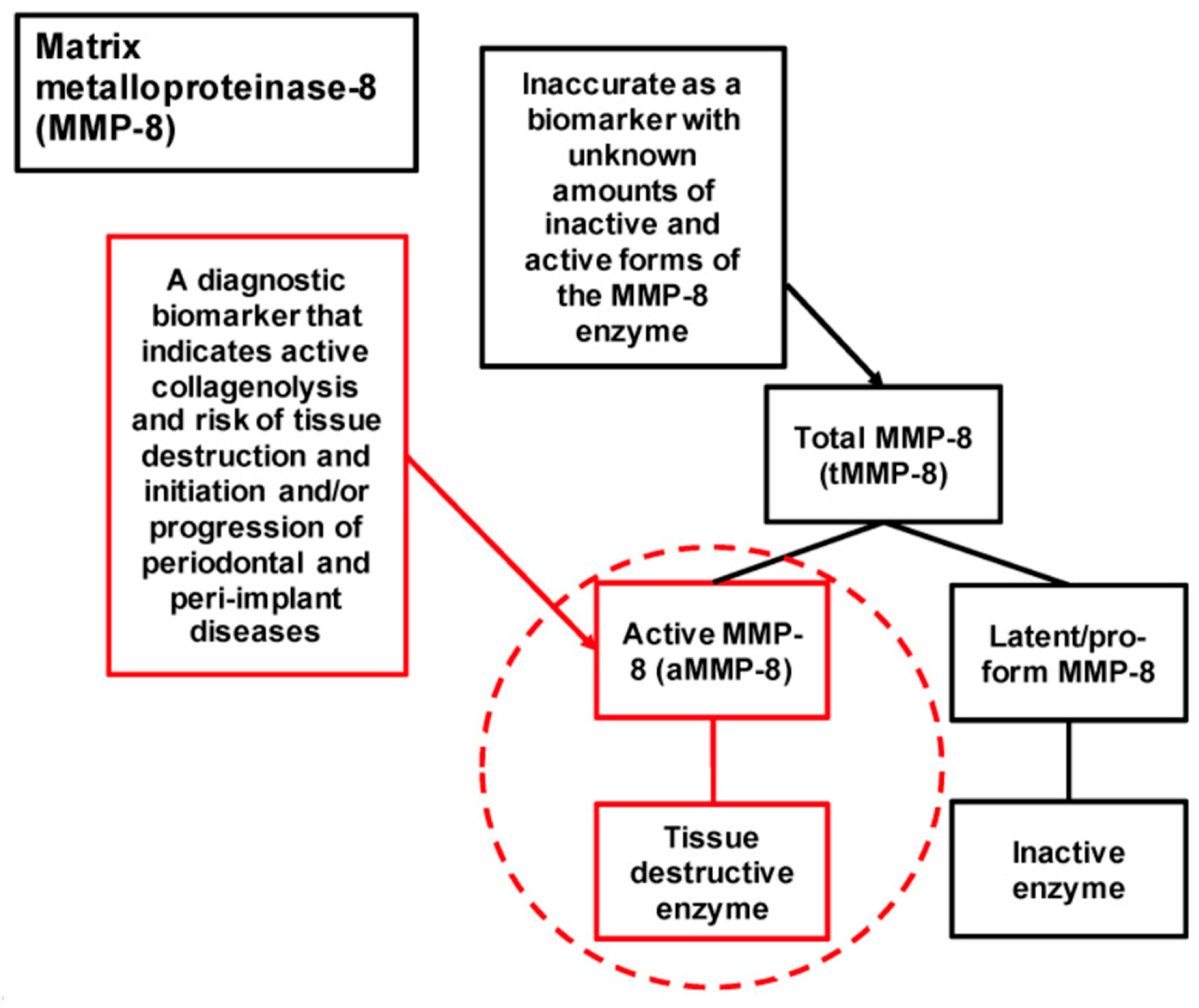
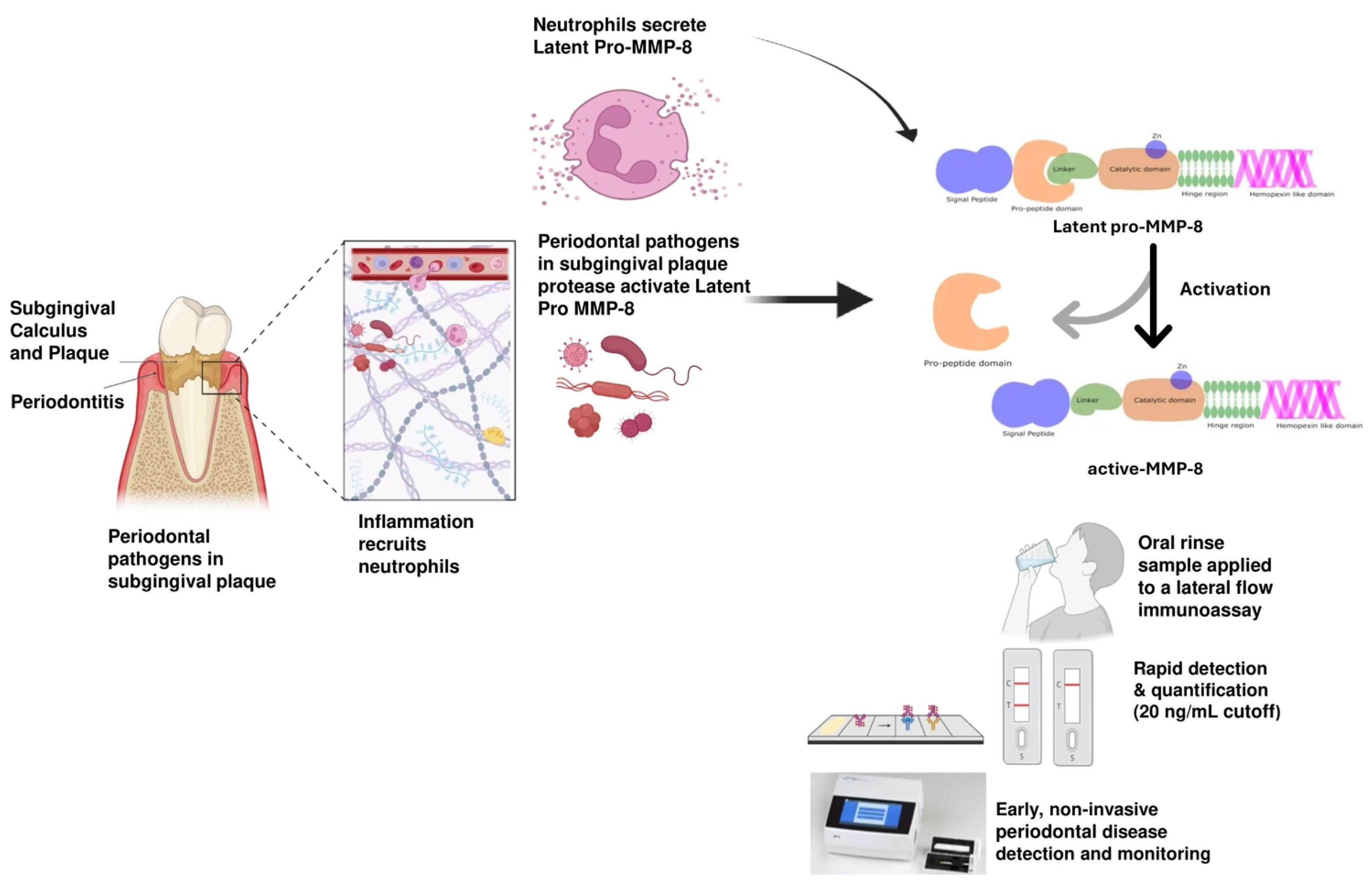
1. Introduction
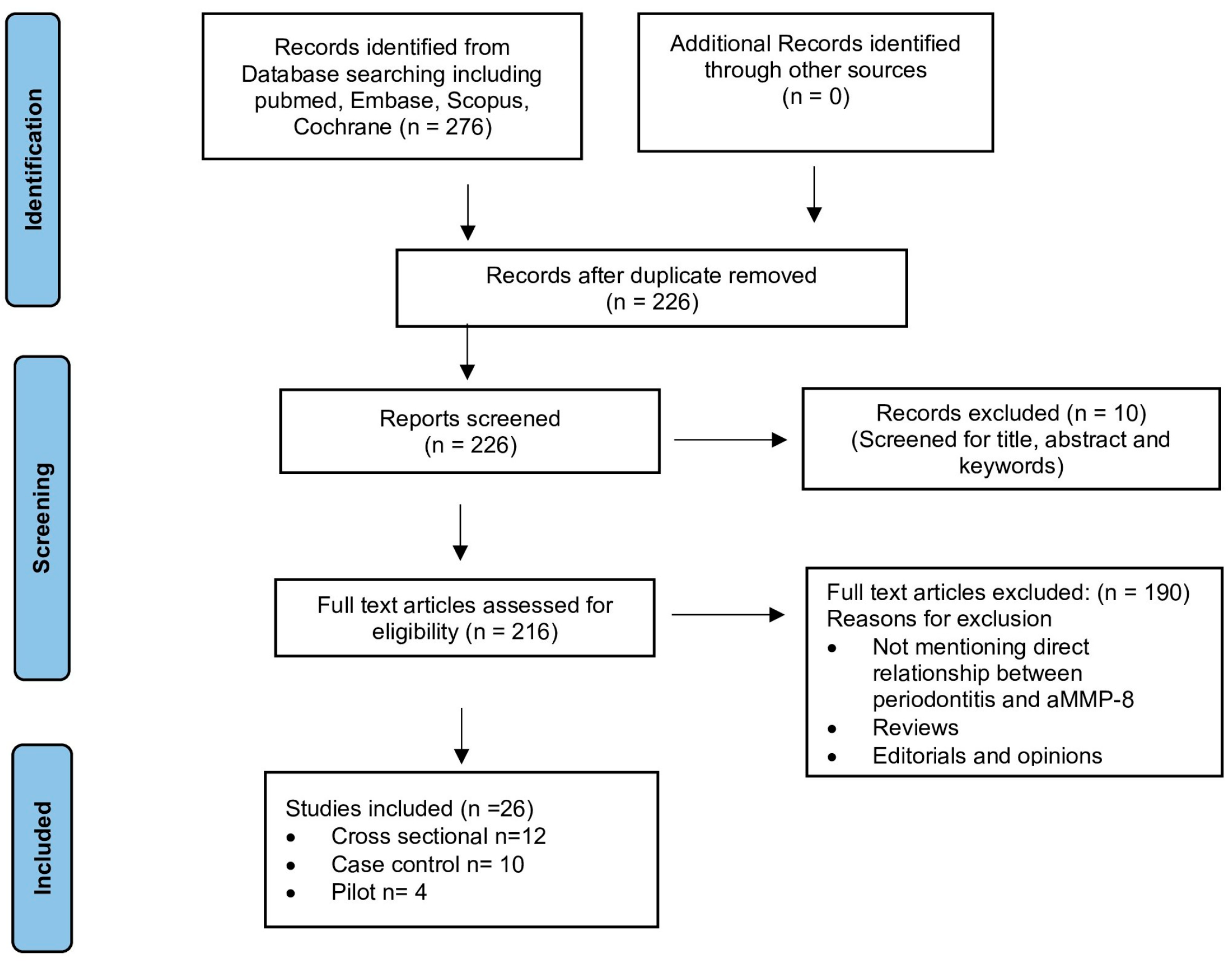
2. Materials and Methods
3. Results
4. Discussion
5. Limitations
6. Conclusions
7. Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MMP | Matrix metalloproteinase |
| PoC | Point of care |
| IFMA | Immunofluorometric assay |
References
- Löe, H.; Anerud, A.; Boysen, H.; Smith, M. The natural history of periodontal disease in man. Study design and baseline data. J. Periodontal Res. 1978, 13, 550–562. [Google Scholar] [CrossRef]
- Yin, L.; Li, X.; Hou, J. Macrophages in periodontitis: A dynamic shift between tissue destruction and repair. Jpn. Dent. Sci. Rev. 2022, 58, 336–347. [Google Scholar] [CrossRef] [PubMed]
- de Morais, E.F.; Pinheiro, J.C.; Leite, R.B.; Santos, P.P.A.; Barboza, C.A.G.; Freitas, R.A. Matrix metalloproteinase-8 levels in periodontal disease patients: A systematic review. J. Periodontal Res. 2018, 53, 156–163. [Google Scholar] [CrossRef]
- Albandar, J.M.; Rams, T.E. Risk factors for periodontitis in children and young persons. Periodontol. 2000 2002, 29, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Van der Velden, U.; Abbas, F.; Armand, S.; Loos, B.G.; Timmerman, M.F.; Van der Weijden, G.A.; Van Winkelhoff, A.J.; Winkel, E.G. Java project on periodontal diseases. The natural development of periodontitis: Risk factors, risk predictors and risk determinants. J. Clin. Periodontol. 2006, 33, 540–548. [Google Scholar] [CrossRef]
- Oh, T.-J.; Eber, R.; Wang, H.-L. Periodontal diseases in the child and adolescent. J. Clin. Periodontol. 2002, 29, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Mombelli, A. Early-onset periodontitis. Ann. Periodontol. 1999, 4, 39–53. [Google Scholar] [CrossRef]
- Van Dyke, T.E.; Sima, C. Understanding resolution of inflammation in periodontal diseases: Is chronic inflammatory periodontitis a failure to resolve? Periodontol. 2000 2020, 82, 205–213. [Google Scholar] [CrossRef]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef]
- Usui, M.; Onizuka, S.; Sato, T.; Kokabu, S.; Ariyoshi, W.; Nakashima, K. Mechanism of alveolar bone destruction in periodontitis—Periodontal bacteria and inflammation. Jpn. Dent. Sci. Rev. 2021, 57, 201–208. [Google Scholar] [CrossRef]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef]
- López, R.; Frydenberg, M.; Baelum, V. Clinical features of early periodontitis. J. Periodontol. 2009, 80, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.; Fontenele, R.C.; Lahoud, P.; Shujaat, S.; Bornstein, M.M. Radiographic diagnosis of periodontal diseases—Current evidence versus innovations. Periodontol. 2000 2024, 95, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Tugnait, A.; Clerehugh, V.; Hirschmann, P.N. The usefulness of radiographs in diagnosis and management of periodontal diseases: A review. J. Dent. 2000, 28, 219–226. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef]
- Hoare, A.; Soto, C.; Rojas-Celis, V.; Bravo, D. Chronic Inflammation as a Link between Periodontitis and Carcinogenesis. Mediat. Inflamm. 2019, 2019, 1029857. [Google Scholar] [CrossRef]
- Darby, I. Risk factors for periodontitis & peri-implantitis. Periodontol. 2000 2022, 90, 9–12. [Google Scholar] [CrossRef]
- Dewan, M.; Pandit, A.K.; Goyal, L. Association of periodontitis and gingivitis with stroke: A systematic review and meta-analysis. Dent. Med. Probl. 2024, 61, 407–415. [Google Scholar] [CrossRef]
- Jagannathachary, S.; Kamaraj, D. Obesity and periodontal disease. J. Indian Soc. Periodontol. 2010, 14, 96–100. [Google Scholar] [CrossRef]
- Said-Sadier, N.; Sayegh, B.; Farah, R.; Abbas, L.A.; Dweik, R.; Tang, N.; Ojcius, D.M. Association between Periodontal Disease and Cognitive Impairment in Adults. Int. J. Environ. Res. Public Health 2023, 20, 4707. [Google Scholar] [CrossRef]
- Baeza, M.; Morales, A.; Cisterna, C.; Cavalla, F.; Jara, G.; Isamitt, Y.; Pino, P.; Gamonal, J. Effect of periodontal treatment in patients with periodontitis and diabetes: Systematic review and meta-analysis. J. Appl. Oral Sci. Rev. FOB 2020, 28, e20190248. [Google Scholar] [CrossRef]
- Neurath, N.; Kesting, M. Cytokines in gingivitis and periodontitis: From pathogenesis to therapeutic targets. Front. Immunol. 2024, 15, 1435054. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef]
- Ramadan, D.E.; Hariyani, N.; Indrawati, R.; Ridwan, R.D.; Diyatri, I. Cytokines and Chemokines in Periodontitis. Eur. J. Dent. 2020, 14, 483–495. [Google Scholar] [CrossRef]
- Bhuyan, R.; Bhuyan, S.K.; Mohanty, J.N.; Das, S.; Juliana, N.; Juliana, I.F. Periodontitis and Its Inflammatory Changes Linked to Various Systemic Diseases: A Review of Its Underlying Mechanisms. Biomedicines 2022, 10, 2659. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wu, J.; Wang, M.; Hu, B.; Qu, H.; Zhang, J.; Li, Q. The global burden of periodontal diseases in 204 countries and territories from 1990 to 2019. Oral Dis. 2024, 30, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C. Time to take periodontitis seriously. BMJ 2014, 348, g2645. [Google Scholar] [CrossRef] [PubMed]
- Buset, S.L.; Walter, C.; Friedmann, A.; Weiger, R.; Borgnakke, W.S.; Zitzmann, N.U. Are periodontal diseases really silent? A systematic review of their effect on quality of life. J. Clin. Periodontol. 2016, 43, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M. Detection and diagnosis of periodontal conditions amenable to prevention. BMC Oral Health 2015, 15, S5. [Google Scholar] [CrossRef]
- Batchelor, P. Is periodontal disease a public health problem? Br. Dent. J. 2014, 217, 405–409. [Google Scholar] [CrossRef]
- Gellibolian, R.; Miller, C.S.; Markaryan, A.N.; Weltman, R.L.; Van Dyke, T.E.; Ebersole, J.L. Precision periodontics: Quantitative measures of disease progression. J. Am. Dent. Assoc. 2022, 153, 826–828. [Google Scholar] [CrossRef]
- Gürsoy, U.K.; Kantarci, A. Molecular biomarker research in periodontology: A roadmap for translation of science to clinical assay validation. J. Clin. Periodontol. 2022, 49, 556–561. [Google Scholar] [CrossRef]
- Shaddox, L.M.; Walker, C.B. Treating chronic periodontitis: Current status, challenges, and future directions. Clin. Cosmet. Investig. Dent. 2010, 2, 79–91. [Google Scholar] [CrossRef]
- Knight, E.T.; Liu, J.; Seymour, G.J.; Faggion, C.M.; Cullinan, M.P. Risk factors that may modify the innate and adaptive immune responses in periodontal diseases. Periodontol. 2000 2016, 71, 22–51. [Google Scholar] [CrossRef]
- Hooshiar, M.H.; Moghaddam, M.A.; Kiarashi, M.; Al-Hijazi, A.Y.; Hussein, A.F.; A Alrikabi, H.; Salari, S.; Esmaelian, S.; Mesgari, H.; Yasamineh, S. Recent advances in nanomaterial-based biosensor for periodontitis detection. J. Biol. Eng. 2024, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Cafiero, C.; Spagnuolo, G.; Marenzi, G.; Martuscelli, R.; Colamaio, M.; Leuci, S. Predictive Periodontitis: The Most Promising Salivary Biomarkers for Early Diagnosis of Periodontitis. J. Clin. Med. 2021, 10, 1488. [Google Scholar] [CrossRef]
- Taba, M.; Kinney, J.; Kim, A.S.; Giannobile, W.V. Diagnostic biomarkers for oral and periodontal diseases. Dent. Clin. N. Am. 2005, 49, 551–571. [Google Scholar] [CrossRef] [PubMed]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Khiste, S.V.; Ranganath, V.; Nichani, A.S.; Rajani, V. Critical analysis of biomarkers in the current periodontal practice. J. Indian Soc. Periodontol. 2011, 15, 104–110. [Google Scholar] [CrossRef]
- Gürsoy, U.K.; Özdemir Kabalak, M.; Gürsoy, M. Advances in periodontal biomarkers. Adv. Clin. Chem. 2024, 120, 145–168. [Google Scholar] [CrossRef]
- Kc, S.; Wang, X.Z.; Gallagher, J.E. Diagnostic sensitivity and specificity of host-derived salivary biomarkers in periodontal disease amongst adults: Systematic review. J. Clin. Periodontol. 2020, 47, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef]
- Baidya, S.K.; Banerjee, S.; Guti, S.; Jha, T.; Adhikari, N. Matrix metalloproteinase-8 (MMP-8) and its inhibitors: A minireview. Eur. J. Med. Chem. Rep. 2024, 10, 100130. [Google Scholar] [CrossRef]
- Murphy, G.; Reynolds, J.J.; Bretz, U.; Baggiolini, M. Collagenase is a component of the specific granules of human neutrophil leucocytes. Biochem. J. 1977, 162, 195–197. [Google Scholar] [CrossRef]
- Checchi, V.; Maravic, T.; Bellini, P.; Generali, L.; Consolo, U.; Breschi, L.; Mazzoni, A. The Role of Matrix Metalloproteinases in Periodontal Disease. Int. J. Environ. Res. Public Health 2020, 17, 4923. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Fingleton, B. Matrix metalloproteinases as regulators of inflammatory processes. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The Nexus Between Periodontal Inflammation and Dysbiosis. Front. Immunol. 2020, 11, 511. [Google Scholar] [CrossRef]
- Uriarte, S.M.; Hajishengallis, G. Neutrophils in the periodontium: Interactions with pathogens and roles in tissue homeostasis and inflammation. Immunol. Rev. 2023, 314, 93–110. [Google Scholar] [CrossRef]
- Lee, W.; Aitken, S.; Sodek, J.; McCulloch, C.A. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: Role of active enzyme in human periodontitis. J. Periodontal Res. 1995, 30, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, R.; Mancini, S.; Laschinger, C.; Overall, C.M.; Sodek, J.; McCulloch, C.A.G. Activation of Neutrophil Collagenase in Periodontitis. Infect. Immun. 1999, 67, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Le, N.T.V.; Xue, M.; Castelnoble, L.A.; Jackson, C.J. The dual personalities of matrix metalloproteinases in inflammation. Front. Biosci. J. Virtual Libr. 2007, 12, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Mauk, M.G.; Ziober, B.L.; Chen, Z.; Thompson, J.A.; Bau, H.H. Lab-on-a-chip technologies for oral-based cancer screening and diagnostics: Capabilities, issues, and prospects. Ann. N. Y. Acad. Sci. 2007, 1098, 467–475. [Google Scholar] [CrossRef]
- Gul, S.S.; Zardawi, F.M.; Abdulkareem, A.A.; Shaikh, M.S.; Al-Rawi, N.H.; Zafar, M.S. Efficacy of MMP-8 Level in Gingival Crevicular Fluid to Predict the Outcome of Nonsurgical Periodontal Treatment: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 3131. [Google Scholar] [CrossRef]
- Atanasova, T.; Stankova, T.; Bivolarska, A.; Vlaykova, T. Matrix Metalloproteinases in Oral Health-Special Attention on MMP-8. Biomedicines 2023, 11, 1514. [Google Scholar] [CrossRef]
- Kasuma, N.; Oenzil, F.; Darwin, E.; Sofyan, Y. The analysis of matrix metalloproteinase-8 in gingival crevicular fluid and periodontal diseases. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2018, 29, 450–454. [Google Scholar] [CrossRef]
- Bastos, M.F.; Tucci, M.A.; de Siqueira, A.; de Faveri, M.; Figueiredo, L.C.; Vallim, P.C.; Duarte, P.M. Diabetes may affect the expression of matrix metalloproteinases and their inhibitors more than smoking in chronic periodontitis. J. Periodontal Res. 2017, 52, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, F.; Faramarzi, M.; Salimnezhad, S.; Jafari, B.; Eslami, H.; MohammadPourTabrizi, B. Comparing the activity level of salivary matrix metalloproteinase-8 in patients with diabetes and moderate to severe chronic generalized periodontitis. Clin. Exp. Dent. Res. 2024, 10, e865. [Google Scholar] [CrossRef]
- Van Doren, S.R. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. J. Int. Soc. Matrix Biol. 2015, 44–46, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, I.T.; Aji, N.R.A.S.; Sakellari, D.; Grigoriadis, A.; Rantala, I.; Pätilä, T.; Heikkilä, P.; Gupta, S.; Sorsa, T. Active Matrix Metalloproteinase-8 (aMMP-8) Versus Total MMP-8 in Periodontal and Peri-Implant Disease Point-of-Care Diagnostics. Biomedicines 2023, 11, 2885. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sorsa, T.; Alassiri, S.; Grigoriadis, A.; Räisänen, I.T.; Pärnänen, P.; Nwhator, S.O.; Gieselmann, D.-R.; Sakellari, D. Active MMP-8 (aMMP-8) as a Grading and Staging Biomarker in the Periodontitis Classification. Diagnostics 2020, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Radzki, D.; Negri, A.; Kusiak, A.; Obuchowski, M. Matrix Metalloproteinases in the Periodontium-Vital in Tissue Turnover and Unfortunate in Periodontitis. Int. J. Mol. Sci. 2024, 25, 2763. [Google Scholar] [CrossRef] [PubMed]
- Keles Yucel, Z.P.; Afacan, B.; Emingil, G.; Tervahartiala, T.; Kose, T.; Sorsa, T. Local and systemic levels of aMMP-8 in gingivitis and stage 3 grade C periodontitis. J. Periodontal Res. 2020, 55, 887–894. [Google Scholar] [CrossRef]
- Räisänen, I.T.; Lähteenmäki, H.; Gupta, S.; Grigoriadis, A.; Sahni, V.; Suojanen, J.; Seppänen, H.; Tervahartiala, T.; Sakellari, D.; Sorsa, T. An aMMP-8 Point-of-Care and Questionnaire Based Real-Time Diagnostic Toolkit for Medical Practitioners. Diagnostics 2021, 11, 711. [Google Scholar] [CrossRef]
- Ramenzoni, L.L.; Hofer, D.; Solderer, A.; Wiedemeier, D.; Attin, T.; Schmidlin, P.R. Origin of MMP-8 and Lactoferrin levels from gingival crevicular fluid, salivary glands and whole saliva. BMC Oral Health 2021, 21, 385. [Google Scholar] [CrossRef] [PubMed]
- Aji, N.R.A.S.; Räisänen, I.T.; Rathnayake, N.; Lundy, F.T.; Mc Crudden, M.T.C.; Goyal, L.; Sorsa, T.; Gupta, S. aMMP-8 POCT vs. Other Potential Biomarkers in Chair-Side Diagnostics and Treatment Monitoring of Severe Periodontitis. Int. J. Mol. Sci. 2024, 25, 9421. [Google Scholar] [CrossRef]
- Bartold, P.M.; Van Dyke, T.E. Host modulation: Controlling the inflammation to control the infection. Periodontol. 2000 2017, 75, 317–329. [Google Scholar] [CrossRef]
- Öztürk, V.Ö.; Emingil, G.; Umeizudike, K.; Tervahartiala, T.; Gieselmann, D.-R.; Maier, K.; Köse, T.; Sorsa, T.; Alassiri, S. Evaluation of active matrix metalloproteinase-8 (aMMP-8) chair-side test as a diagnostic biomarker in the staging of periodontal diseases. Arch. Oral Biol. 2021, 124, 104955. [Google Scholar] [CrossRef]
- Deng, K.; Pelekos, G.; Jin, L.; Tonetti, M.S. Diagnostic accuracy of a point-of-care aMMP-8 test in the discrimination of periodontal health and disease. J. Clin. Periodontol. 2021, 48, 1051–1065. [Google Scholar] [CrossRef]
- Hernández, M.; Baeza, M.; Räisänen, I.T.; Contreras, J.; Tervahartiala, T.; Chaparro, A.; Sorsa, T.; Hernández-Ríos, P. Active MMP-8 Quantitative Test as an Adjunctive Tool for Early Diagnosis of Periodontitis. Diagnostics 2021, 11, 1503. [Google Scholar] [CrossRef]
- Deng, K.; Wei, S.; Xu, M.; Shi, J.; Lai, H.; Tonetti, M.S. Diagnostic accuracy of active matrix metalloproteinase-8 point-of-care test for the discrimination of periodontal health status: Comparison of saliva and oral rinse samples. J. Periodontal Res. 2022, 57, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sahni, V.; Räisänen, I.T.; Grigoriadis, A.; Sakellari, D.; Gieselmann, D.-R.; Sorsa, T. Linking oral microbial proteolysis to aMMP-8 PoC diagnostics along with the stage and grade of periodontitis: A cross-sectional study. Oral Dis. 2023, 29, 285–289. [Google Scholar] [CrossRef]
- Yilmaz, D.; Niskanen, K.; Gonullu, E.; Tervahartiala, T.; Gürsoy, U.K.; Sorsa, T. Salivary and serum levels of neutrophil proteases in periodontitis and rheumatoid arthritis. Oral Dis. 2024, 30, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.T.; Joseph, B.; Varghese, S.; Thomas, N.G.; Kamalasanan Vijayakumary, B.; Sorsa, T.; Anil, S.; Waltimo, T. Association between metabolic syndrome and salivary MMP-8, myeloperoxidase in periodontitis. Oral Dis. 2025, 31, 225–238. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, X.; Li, Y.; Cai, Y.; Kang, N.; Duan, J.; Chen, F.; Xue, F.; Chen, X. Evaluation of salivary interleukin-17 and developmental endothelial locus-1 in patients with periodontitis with and without type 2 diabetes mellitus. J. Periodontol. 2024, 96, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Umeizudike, K.A.; Aji, N.R.A.S.; Niskanen, K.; Rantala, I.; Sakellari, D.; Grigoriadis, A.; Pätilä, T.; Gupta, S.; Sorsa, T.; Räisänen, I.T. Prediabetes Associates with Matrix Metalloproteinase-8 Activation and Contributes to the Rapid Destruction of Periodontal Tissues. Eur. J. Dent. 2024, 19, 305–314. [Google Scholar] [CrossRef]
- Raivisto, T.; Sorsa, T.; Räisänen, I.T.; Kauppila, T.; Ruokonen, H.; Tervahartiala, T.; Haukka, J.; Heikkinen, A.M. Active matrix metalloproteinase-8 chair side mouth rinse test, health behaviour and oral health in Finnish adolescent cohort. J. Clin. Diagn. Res. 2020, 14, ZC35–ZC39. [Google Scholar] [CrossRef]
- Mauramo, M.; Mauramo, E.; Sorsa, T.; Tervahartiala, T.; Räisänen, I.T.; Waltimo, T. Human leukocyte antigens are associated with salivary level of active MMP-8. Clin. Exp. Dent. Res. 2021, 7, 833–839. [Google Scholar] [CrossRef]
- Gupta, S.; Mohindra, R.; Singla, M.; Khera, S.; Kumar, A.; Rathnayake, N.; Sorsa, T.; Pfützner, A.; Räisänen, I.T.; Soni, R.K.; et al. Validation of a noninvasive aMMP-8 point-of-care diagnostic methodology in COVID-19 patients with periodontal disease. Clin. Exp. Dent. Res. 2022, 8, 988–1001. [Google Scholar] [CrossRef]
- Umeizudike, K.A.; Lähteenmäki, H.; Räisänen, I.T.; Taylor, J.J.; Preshaw, P.M.; Bissett, S.M.; Tervahartiala, T.; O Nwhator, S.; Pärnänen, P.; Sorsa, T. Ability of matrix metalloproteinase-8 biosensor, IFMA, and ELISA immunoassays to differentiate between periodontal health, gingivitis, and periodontitis. J. Periodontal Res. 2022, 57, 558–567. [Google Scholar] [CrossRef]
- Keskin, M.; Rintamarttunen, J.; Gülçiçek, E.; Räisänen, I.T.; Gupta, S.; Tervahartiala, T.; Pätilä, T.; Sorsa, T. A Comparative Analysis of Treatment-Related Changes in the Diagnostic Biomarker Active Metalloproteinase-8 Levels in Patients with Periodontitis. Diagnostics 2023, 13, 903. [Google Scholar] [CrossRef]
- Brandt, E.; Keskin, M.; Räisänen, I.T.; Tervahartiala, T.; Mäkitie, A.; Harmankaya, İ.; Karaçetin, D.; Hagström, J.; Rautava, J.; Sorsa, T. Induction of Collagenolytic MMP-8 and -9 Tissue Destruction Cascade in Mouth by Head and Neck Cancer Radiotherapy: A Cohort Study. Biomedicines 2023, 12, 27. [Google Scholar] [CrossRef]
- Yilmaz, M.; Sorsa, T.; Demir, E.; Gürsoy, M.; Suominen, A.; Tervahartiala, T.; Räisänen, I.T.; Gürsoy, U.K. Accuracy of aMMP-8 point-of-care test in indicating periodontal treatment outcomes in stage III/IV periodontitis: A 24-week follow-up study. J. Periodontal Res. 2023, 58, 325–335. [Google Scholar] [CrossRef]
- Aji, N.R.A.S.; Yucel-Lindberg, T.; Räisänen, I.T.; Kuula, H.; Nieminen, M.T.; Mc Crudden, M.T.C.; Listyarifah, D.; Lundmark, A.; Lundy, F.T.; Gupta, S.; et al. In Vivo Regulation of Active Matrix Metalloproteinase-8 (aMMP-8) in Periodontitis: From Transcriptomics to Real-Time Online Diagnostics and Treatment Monitoring. Diagnostics 2024, 14, 1011. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, R.; Reda, R.; Di Nardo, D.; Miccoli, G.; Pagnoni, F.; Zanza, A.; Testarelli, L. Expression of IL-1β, IL-6, TNF-α, and a-MMP-8 in sites with healthy conditions and with periodontal and peri-implant diseases: A case-control study. J. Dent. Res. Dent. Clin. Dent. Prospect. 2024, 18, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Keskin, M.; Lähteenmäki, H.; Rathnayake, N.; Räisänen, I.T.; Tervahartiala, T.; Pärnänen, P.; Şenışık, A.M.; Karaçetin, D.; Yentek Balkanay, A.; Heikkilä, P.; et al. Active matrix metalloproteinase-8 and interleukin-6 detect periodontal degeneration caused by radiotherapy of head and neck cancer: A pilot study. Expert Rev. Proteom. 2020, 17, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Kallio, E.; Puolakkainen, T.; Tervahartiala, T.; Snäll, J.; Marttila, E.; Sorsa, T.; Uittamo, J. Applicability of an active matrix metalloproteinase-8 point-of-care test in an oral and maxillofacial surgery clinic: A pilot study. Odontology 2024, 112, 250–255. [Google Scholar] [CrossRef]
- Brandt, E.; Keskin, M.; Tervahartiala, T.; Yılmaz, M.; Harmankaya, İ.; Karaçetin, D.; İpek, T.; Gürsoy, U.K.; Rautava, J.; Gupta, S.; et al. Radiotherapy Increases aMMP-8-Levels and Neutrophil/Lymphocyte Ratio Rapidly in Head and Neck Cancer Patients: A Pilot Study. Cancer Control J. Moffitt Cancer Cent. 2023, 30, 10732748231163653. [Google Scholar] [CrossRef]
- Heikkinen, A.M.; Sokka, T.T.; Torppa-Saarinen, E.; Pimiä, E.; Jokinen, M.; Maijala, M.; Rantala, I.; Tervahartiala, T.; Sorsa, T.; Kauppila, T. aMMP-8 Point-of-Care Test (POCT) Identifies Reliably Periodontitis in Patients with Type 2 Diabetes as well as Monitors Treatment Response. Diagnostics 2023, 13, 2224. [Google Scholar] [CrossRef]
- Mancini, S.; Romanelli, R.; Laschinger, C.A.; Overall, C.M.; Sodek, J.; McCulloch, C.A. Assessment of a novel screening test for neutrophil collagenase activity in the diagnosis of periodontal diseases. J. Periodontol. 1999, 70, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Lin, T.; Sáenz-Ravello, G.; Gao, H.; Zhang, Y.; Tonetti, M.S.; Deng, K. Diagnostic accuracy of salivary active matrix metalloproteinase (aMMP)-8 point-of-care test for detecting periodontitis in adults: A systematic review and meta-analysis. J. Clin. Periodontol. 2024, 51, 1093–1108. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kung, J.C.K.; Shi, J.; Wu, X.; Lam, S.L.T.; Deng, K.; Zhang, X.; Lai, H.; Pelekos, G.; Jin, L.; et al. Diagnostic Accuracy of a Point-Of-Care aMMP-8 Test for Discriminating Periodontal Health Status in Adults: Validation Trials and Updated Meta-Analysis. J. Clin. Periodontol. 2025, 52, 510–529. [Google Scholar] [CrossRef]
- Griffith, A.; Chande, C.; Kulkarni, S.; Morel, J.; Cheng, Y.-H.; Shimizu, E.; Cugini, C.; Basuray, S.; Kumar, V. Point-of-care diagnostic devices for periodontitis—Current trends and urgent need. Sens. Diagn. 2024, 3, 1119–1134. [Google Scholar] [CrossRef]
- Ramseier, C.A.; Kinney, J.S.; Herr, A.E.; Braun, T.; Sugai, J.V.; Shelburne, C.A.; Rayburn, L.A.; Tran, H.M.; Singh, A.K.; Giannobile, W.V. Identification of pathogen and host-response markers correlated with periodontal disease. J. Periodontol. 2009, 80, 436–446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boynes, S.G.; Sofiyeva, N.; Saw, T.; Nieto, V.; Palomo, L. Assessment of salivary matrix metalloproteinase (MMP8) and activated salivary matrix metalloproteinase (aMMP8) in periodontitis patients: A systematic review and meta-analysis. Front. Oral Health 2025, 6, 1444399. [Google Scholar] [CrossRef]
- Frankenberger, R.; Arweiler, N.B.; Sorsa, T.A.; Volland, G.; Gaßmann, G.; Fietz, C.; Thiem, H.; Rychlik, R.P.T. Health economic potential of oral aMMP-8 biomarker diagnostics for personalised prevention of periodontal and peri-implant diseases. J. Pharm. Health Serv. Res. 2022, 13, 52–60. [Google Scholar] [CrossRef]
| S.No | Authors/Year/Place of Study | Participants (N)/Groups (n) | Sample/Technique Used for aMMP-8 and tMMP-8 Quantification | Findings | Clinical Implication |
|---|---|---|---|---|---|
| 1 | Sorsa T et al., 2020, Greece [64] | 150 adult participants which include healthy controls and periodontitis. | Mouthrinse/aMMP-8 PerioSafe® test and ORALyzer®. | High diagnostic accuracy using aMMP-8 to discriminate healthy vs. periodontitis sites: AUC 0.90 (95% CI 0.83–0.96), p < 0.01. Levels classify progression: Grade A < 20 ng/mL, Grade B ≥ 20 ng/mL, Grade C > 30 ng/mL | aMMP-8 cut-off 20 ng/mL effectively distinguishes periodontitis from health, improving early diagnosis and monitoring. |
| 2 | Keles Yucel ZP et al., 2020, Turkey [66] | 83 (Healthy 23, Gingivitis 20, Stage 3 periodontitis 40) | GCF, serum and saliva/Immunofluorometric assay (IFMA) | Elevated aMMP-8 levels in periodontitis vs. gingivitis and healthy controls aMMP-8 Levels (ng/mL) GCF Healthy: 16.83 ± 9.28 Gingivitis: 44.13 ± 10.6 Peridontitis: 49.42 ± 15.21 Saliva Healthy: 625.74 ± 163.10 Gingivitis: 609.77 ± 174.13 Peridontitis: 779.32 ± 87.26 Serum Healthy: 45.98 ± 39.74 Gingivitis: 106.04 ± 85.27 Periodontitis: 116.05 ± 107.28 | MMP-8 is a potential biomarker at both the local and systemic levels for differentiating severe stages of periodontitis. |
| 3 | Deng K et al., 2021, Hong Kong [72] | 408 (healthy, gingivitis, periodontitis) | Mouthrinse/aMMP-8 PerioSafe® + ORALyzer® | aMMP-8 showed a sensitivity of 33.2% and specificity of 93.0% for detecting periodontitis at a cut-off >10 ng/mL. Adjusting aMMP-8 by the number of teeth improved performance with sensitivity 67.1% and specificity 68.8%. The aMMP-8/number of teeth ratio strongly predicted Stage IV periodontitis with sensitivity 89.7%, specificity 73.6%, and AUROC 0.856. | aMMP-8 POCT test has better specificity than sensitivity and is particularly useful for screening or self-assessment rather than ruling out disease. |
| 4 | Räisänen IT et al., 2021, Greece [67] | 150 patients with periodontitis | Mouthrinse/aMMP-8 POCT lateral-flow immunotest; Saliva/tMMP-8, tMMP-9 ELISA; Saliva/aMMP-9 gelatin zymography | AUC (95% CI) aMMP-8: 0.834 (0.761–0.906) tMMP-8: 0.800 (0.722–0.878) aMMP-9: 0.787 (0.704–0.870) tMMP-9: 0.767 (0.680–0.855) | aMMP-8 offers better screening ability among the tested biomarkers to indicate periodontal tissue breakdown, aiding early detection and monitoring. |
| 5 | Öztürk VÖ et al., 2021, Turkey [71] | 80 individuals (18 stage III, 19 stage IV periodontitis, 21 gingivitis, 22 health) | GCF, saliva/IFMA; Mouthrinse/aMMP-8 POCT lateral-flow immunotest | aMMP-8 POCT demonstrated Sensitivity 83.9%, specificity 79.2% at 20 ng/mL | aMMP-8 POCT with cut-off 20 ng/mL can be used as non-invasive real-time diagnosis and monitoring tool. |
| 6 | Hernandez M et al., 2021, Chile [73] | 31 individuals with mild and severe periodontitis. | GCF/ aMMP-8 analysis by IFMA tMMP-8 levels analysis by ELISA. | aMMP-8 demonstrated AUC 0.89 (95% CI 0.83–0.96), sensitivity 98%, specificity 67% at 6.04 ng/mL; tMMP-8 discriminates mild/severe with AUC ≥ 0.80 (95% CI 0.72–0.92), sensitivity 58%, and specificity 96% at a cut-off of 52.79 ng/mL. | aMMP-8 POCT can be a useful adjunct for early diagnosis, severity assessment, and monitoring of periodontitis. |
| 7 | Deng K et al., 2022, Hong Kong [74] | 95 (61 periodontitis, 34 health/gingivitis) | Unstimulated saliva, oral rinse/lateral flow immunoassay | The aMMP-8 test with cut-off—10 ng/mL on the 1st oral rinse exhibited the best diagnostic accuracy for detecting periodontitis, with a sensitivity of 80.3%, specificity of 67.8%, and an AUROC of 0.740. | The first oral rinse (30 s rinse) provides rapid and reliable discrimination between periodontal health and disease. |
| 8 | Gupta S et al., 2023, Greece [75] | 150 (periodontitis, gingivitis, healthy) | Mouthrinse, GCF, saliva/aMMP-8 POCT lateral flow, tMMP ELISA | aMMP-8 in oral fluids correlates with clinical parameters. Oral rinse: BOP (r = 0.559), PPD (r = 0.684), CAL (r = 0.770); Saliva: BOP (r = 0.601), PPD (r = 0.705), CAL (r = 0.776); GCF: BOP (r = 0.546), PPD (r = 0.642), CAL (r = 0.743). Elevated aMMP-8 (>20 ng/mL) effectively distinguished periodontitis from health. | aMMP-8 can be a reliable biomarker reflecting tissue inflammation and destruction. |
| 9 | Yilmaz D et al., 2024, Turkey [76] | 97 (rheumatoid arthritis (RA) periodontitis 26, RA healthy 23, SH periodontitis 24, controls 24) | Serum, saliva/ELISA, immunofluorescence assay | Higher levels of aMMP-8, aMMP-8/TIMP-1 ratio, tMMP-9, MPO, and HNE in periodontitis patients compared to healthy control. Salivary TIMP-1 was more elevated in patients with RA with or without periodontitis. (p-value < 0.001 Significant) | aMMP-8 and neutrophil elastase serve as potential biomarkers for both RA and periodontitis. |
| 10 | Thomas JT et al., 2025, India [77] | 120 (metabolic syndrome with periodontitis 40, healthy with PD 40, healthy 40) | Saliva/aMMP-8 and tMMP-8/ELISA | Levels of aMMP-8, tMMP-8—highest in patients with systemic disease and periodontitis aMMP-8 values (ng/mL) MetS-PD:26.26 ± 3 SH-PD: 24.1 ± 2.56 SH-PH: 14.366 ± 1.89 | aMMP-8 could be a potential screening biomarker for periodontitis with metabolic syndrome. |
| 11 | Zhang Y et al., 2024, China [78] | 80 (healthy 27, periodontitis 29, periodontitis with diabetes 24) | Saliva/ aMMP-8, TNF-alpha, IL-6, IL-8, IL-17 and developmental endothelial locus-1 (Del-1)/ELISA. | aMMP-8 levels were higher in periodontitis (P) and periodontitis with diabetes (PDM) groups compared to the healthy (H) group (p < 0.05), with PDM showing higher levels than p (p < 0.05). aMMP-8 positively correlated with IL-17 (r = 0.77; p < 0.01) and negatively correlated with Del-1 (r = −0.69; p < 0.01). | Monitoring aMMP-8 levels enables early detection and targeted intervention to prevent progression of both periodontitis and diabetes. |
| 12 | Umeizudike KA et al. 2024, Greece [79] | 150 individuals with periodontitis and prediabetes | Mouthrinse, saliva/aMMP-8 POCT lateral flow; tMMP-8, elastase, MMP-9/ELISA. | Among stage III grade C periodontitis patients, aMMP-8 levels correlated significantly with prediabetes status (HbA1c ≥ 5.7%) with Spearman’s rho = 0.646, p = 0.044. p- value—0.001 (Significant) | Elevated aMMP-8 rapidly identifies periodontitis patients with or at risk for prediabetes, enabling integrated management. |
| S.No | Authors/Year/Place of Study | Participants (N)/Groups n | Sample/Technique used for aMMP-8 and tMMP-8 Quantification | Findings | Clinical Implication |
|---|---|---|---|---|---|
| 1 | Raivisto T et al., 2020, Finland [80] | 125 participants; gingivitis/ subclinical periodontitis group and healthy controls | Saliva/aMMP-8 by POCT lateral flow immunoassay | Elevated salivary aMMP-8 in 34% of adolescents significantly linked with higher visible plaque index (VPI) % (p = 0.005); responsive to treatment with VPI and RC reduction (p < 0.05) | Non-surgical therapy reduces aMMP-8; aMMP-8 useful to rule out early inflammation including in orthodontic treatment |
| 2 | Mauramo M et al., 2021, Switzerland [81] | 202 individuals; controls (86), mild/moderate periodontitis (83), severe periodontitis (33) | Saliva/HLA determination and aMMP-8 IFMA | HLA-A11 allele linked to increased salivary aMMP-8; levels reflect severity and genetic modulation | Measuring aMMP-8 aids personalized risk assessment and targeted management |
| 3 | Gupta S et al., 2022, India [82] | 102 participants; COVID-19-positive (72), negative (30) | GCF and Mouthrinse/aMMP-8 POCT lateral flow immunoassay | Adjusting for age, gender, and smoking improved test accuracy to 82.35% sensitivity 76.47% specificity (mouthrinse) and 73.53% sensitivity/88.24% specificity (site-specific). AUC from 0.746 to 0.872 (p < 0.001) indicates good diagnostic performance. | aMMP-8 POCT useful for screening active periodontal disease in COVID-19 patients |
| 4 | Umeizudike KA et al., 2022, UK [83] | 189 participants; periodontal health (59), gingivitis (63), periodontitis (67) | Saliva/MMP-8 biosensor, IFMA, ELISA | AUC for differentiating periodontitis and gingivitis from health was 0.81, with diagnostic accuracy of 74.2%. For periodontitis versus health and gingivitis, the AUC was 0.86 with 82.8% diagnostic accuracy. | POCT biosensors and IFMA as effective diagnostic tools to discriminate periodontal disease status and monitor treatment response. |
| 5 | Keskin M et al., 2023, Turkey & Finland [84] | 52 individuals; controls (25), periodontitis (27) | Mouthrinse/aMMP-8 POCT lateral flow, IFMA, Western immunoblot | The PoC aMMP-8 test demonstrated sensitivity 85.2%, specificity 100%; correlation between aMMP-8 reduction and clinical improvement (p < 0.05) | Oral rinse aMMP-8 tests are accurate, responsive, and effective tools for diagnosing and monitoring periodontitis. |
| 6 | Brandt E et al., 2023, Turkey [85] | 21 periodontitis patients undergoing head and neck radiotherapy | Mouthrinse/PerioSafe®, tMMP ELISA | Radiotherapy (RT) for head and neck cancer (HNC) increased aMMP-8 from 21.6 to 54.6 ng/mL (p < 0.05) during and after therapy. | aMMP-8, aMMP-9, and IL-6 are potential non-invasive indicators of oral tissue response during cancer therapy. |
| 7 | Yilmaz M et al., 2023, Turkey [86] | 42 Stage III/IV periodontitis patients | Mouthrinse/POCT, immunofluorescence assay | Sensitivity of the aMMP-8 test reduced from 71.4% baseline to 28.6–42.9% post-treatment; Specificity ranged from 64.3% to 80% at week 6, decreasing to 40–57.1% at week 12, and then slightly increased to 56–64.3% at week 24. | aMMP-8 testing is effective for baseline periodontitis diagnosis, but its accuracy for monitoring treatment outcomes or residual disease declines over time, so clinical assessments must complement biomarker testing. |
| 8 | Aji N et al., 2024, Finland [87] | 57 participants; Stage III/IV Grade B/C periodontitis (27), healthy (30) | Mouthrinse POCT, gingival tissue/IHC, transcriptomics | IHC demonstrated more Td dentilisin and MMP-8 in CP patients than healthy control. Significant reductions in aMMP-8 were observed after scaling and root planing. | The upregulation of aMMP-8 in response to inflammation makes it a key target for assessing the severity of periodontitis. |
| 9 | Aji N et al., 2024, Finland [69] | 26 participants; Stage III/IV Grade B/C periodontitis and healthy controls. | Mouthrinse POCT with digital reader; ELISA for tMMP-8 | aMMP-8 POCT demonstrated sensitivity 92.3%, specificity 100%; best biomarker with 20 ng/mL cutoff. | aMMP-8 is found to be a precise biomarker for diagnosis and monitoring periodontitis. |
| 10 | Guarnieri R et al., 2024, Rome [88] | 112 participants; periodontal health, peri-implant health, periodontitis (PER), peri-implantitis (PIM) | GCF, PISF/aMMP-8 POCT mouthrinse with digital reader | Sites with PER and PIM have a higher level of aMMP-8 which positively correlated with other biomarkers aMMP-8 (ng/mL): Periodontal health: 11.58 ± 3.1 Periodontitis: 17.51 ± 9.3 Peri-implant health: 12.42 ± 2.9 Peri-implantitis: 29.8 ± 10.6 | aMMP-8 is responsible for destruction of periodontal and peri-implant tissues. Altered neutrophil maturation in periodontitis increases activated neutrophils, driving inflammation and tissue damage. |
| S.No | Authors/Year/Place of Study | Participants (N)/Groups (n) | Sample/Technique used for aMMP-8 and tMMP-8 Quantification | Findings | Clinical Implication |
|---|---|---|---|---|---|
| 1 | Keskin M et al., 2020, Finland [89] | 11 head and neck cancer patients | Mouthrinse POCT for aMMP-8. Samples analyzed at 3 points of time (pre-radiotherapy, after 6 weeks of radiotherapy and 1 month after radiotherapy) | Significant changes in aMMP-8 levels with cut-off 20 ng/mL after radiotherapy. aMMP-8 levels (ng/mL) Pre-radiotherapy: 17.99 6 weeks: 75.12 1 month: 38.00 | aMMP-8 can be a potential biomarker for identifying periodontal destruction induced by radiotherapy. |
| 2 | Kallio E et al., 2024, Finland [90] | 112 individuals | Mouthrinse POCT quantified by digital reader. | Patients at increased infection risk had 35.5% higher aMMP-8 values (37.6 ± 49.58) than healthy (27.8 ± 23.3) | aMMP-8 testing is valuable in managing periodontal health in patients undergoing oral surgery, as it enables real-time assessment of inflammation and tissue breakdown. |
| 3 | Brandt E et al., 2023, Turkey [91]. | 13 head and neck cancer patients undergoing radiotherapy | Oral rinse POCT quantified using digital reader | Elevation of aMMP-8 with cut-off 20 ng/mL and fragmented MMP-8 were observed after radiotherapy. aMMP-8 levels (ng/mL) Before radiotherapy: 20.1 3 weeks after radiotherapy: 59.4 1 month after radiotherapy: 38.00 | Elevated aMMP-8 in oral rinse can increase the risk of periodontitis after radiotherapy. |
| 4. | Heikkinen et al., 2023, Finland [92] | 51 participants with type II diabetes mellitus. | Mouthrinse/aMMP-8 POCT at baseline and post-therapy | At baseline, aMMP-8 levels positively correlated with probing pocket depth 5 mm (r = 0.308, p < 0.05), and bleeding on probing (r = 0.298, p < 0.05), but not with GHbA1c. Changes in aMMP-8 and GHbA1c during treatment were positively correlated (p < 0.01). | The aMMP-8 POCT reliably detects periodontitis and monitors treatment response in type 2 diabetes patients, enabling timely and integrated care. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goyal, L.; Gupta, M.; Sareen, S.; Aji, N.R.A.S.; Sahni, V.; Thomas, J.T.; Pätilä, T.; Penttala, M.; Pärnänen, P.; Sorsa, T.; et al. Active Matrixmetalloproteinase-8 in Periodontal Diagnosis: A Scoping Review. Diagnostics 2025, 15, 2932. https://doi.org/10.3390/diagnostics15222932
Goyal L, Gupta M, Sareen S, Aji NRAS, Sahni V, Thomas JT, Pätilä T, Penttala M, Pärnänen P, Sorsa T, et al. Active Matrixmetalloproteinase-8 in Periodontal Diagnosis: A Scoping Review. Diagnostics. 2025; 15(22):2932. https://doi.org/10.3390/diagnostics15222932
Chicago/Turabian StyleGoyal, Lata, Mehak Gupta, Shubham Sareen, Nur Rahman Ahmad Seno Aji, Vaibhav Sahni, Julie Toby Thomas, Tommi Pätilä, Miika Penttala, Pirjo Pärnänen, Timo Sorsa, and et al. 2025. "Active Matrixmetalloproteinase-8 in Periodontal Diagnosis: A Scoping Review" Diagnostics 15, no. 22: 2932. https://doi.org/10.3390/diagnostics15222932
APA StyleGoyal, L., Gupta, M., Sareen, S., Aji, N. R. A. S., Sahni, V., Thomas, J. T., Pätilä, T., Penttala, M., Pärnänen, P., Sorsa, T., Gupta, S., Räisänen, I. T., & Leone, P. (2025). Active Matrixmetalloproteinase-8 in Periodontal Diagnosis: A Scoping Review. Diagnostics, 15(22), 2932. https://doi.org/10.3390/diagnostics15222932







