Diagnostic Value of Opportunistic CT-Based Bone Density Assessment in Patients with and Without Sacral Insufficiency Fractures
Abstract
1. Introduction
2. Materials and Methods
2.1. Detection of Fractures
2.2. Determination of Bone Density
2.3. Treatment of the Patient
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. Fracture Morphology and Fracture Distribution
3.2.1. Pelvis: Classification of SIFs According to FFP (Figure 4)

3.2.2. Hip Fractures (Figure 5)
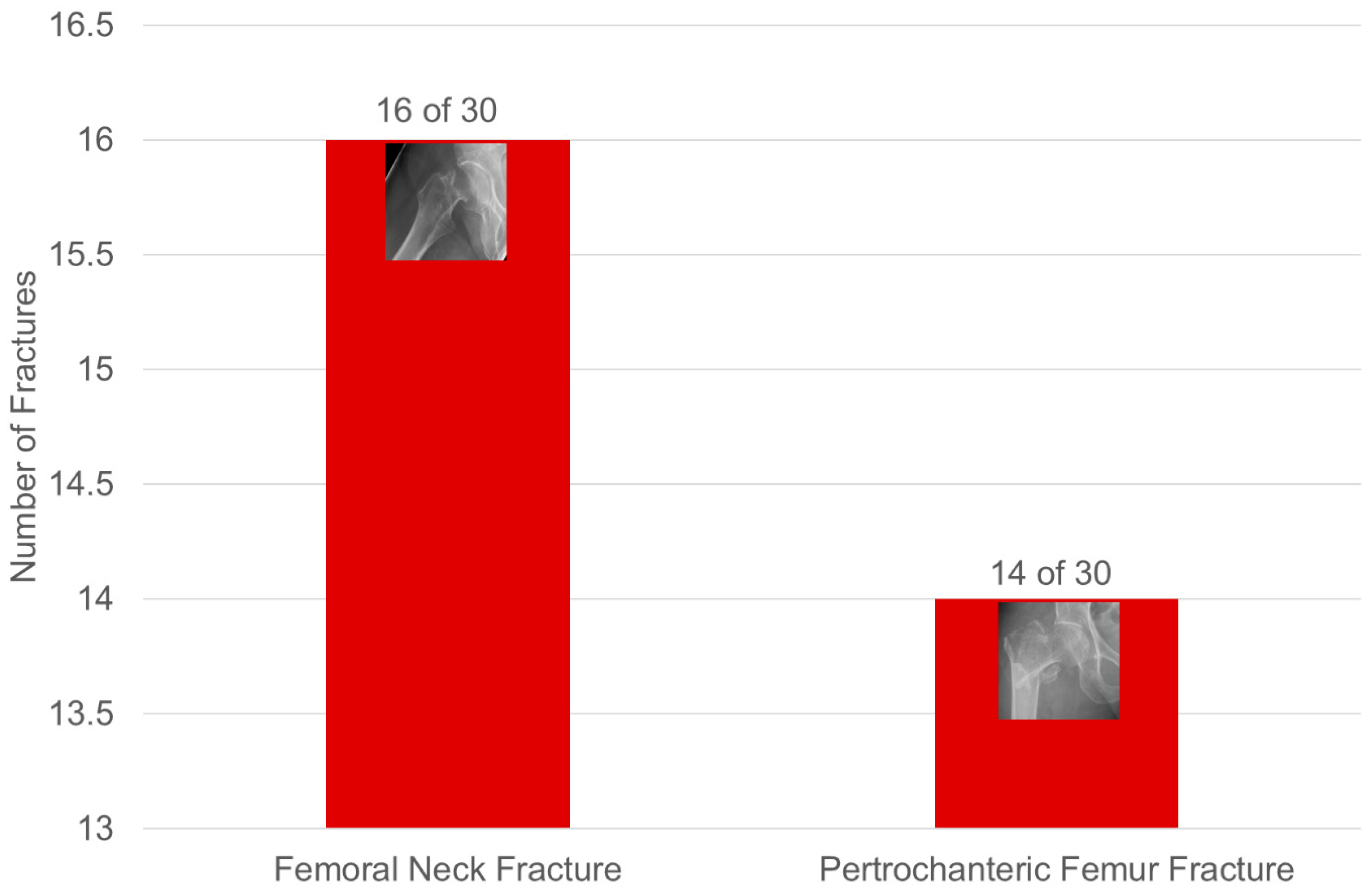
3.2.3. Fractures of the Axial Skeleton (Figure 6, Table 2)

| Patients (n = 98) | Men (n = 14) | Women (n = 84) | |
|---|---|---|---|
| Age (in years) | Ø 78.95 | Ø 74.14 | Ø 79.75 |
| (min.: 61; max.: 92) | (min.: 61; max.: 85) | (min.: 61; max.: 92) | |
| BMI (kg/m2) | Ø 24.92 | Ø 27.97 | Ø 24.41 |
| (min.: 17.9; max.: 34.4) | (min.: 20; max.: 32.4) | (min.: 17.9; max.: 34.4) | |
| Patients with at least one fracture (n) | 98 | 14 | 84 |
| Average OVF accumulation per patient | 2.37 | 2.71 | 2.31 |
| Affected vertebral bodies (Figure 6) (n) with at least one grade 1 vertebral body deformity according to Genant et al. [36] | 232 | 38 | 194 |
| SIF | 98 (100%) | 14 (100%) | 84 (100%) |
| Unilateral | 14 (14.29%) | 0 | 14 (16.67%) |
| on both sides | 84 (85.71%) | 14 (100%) | 70 (83.33%) |
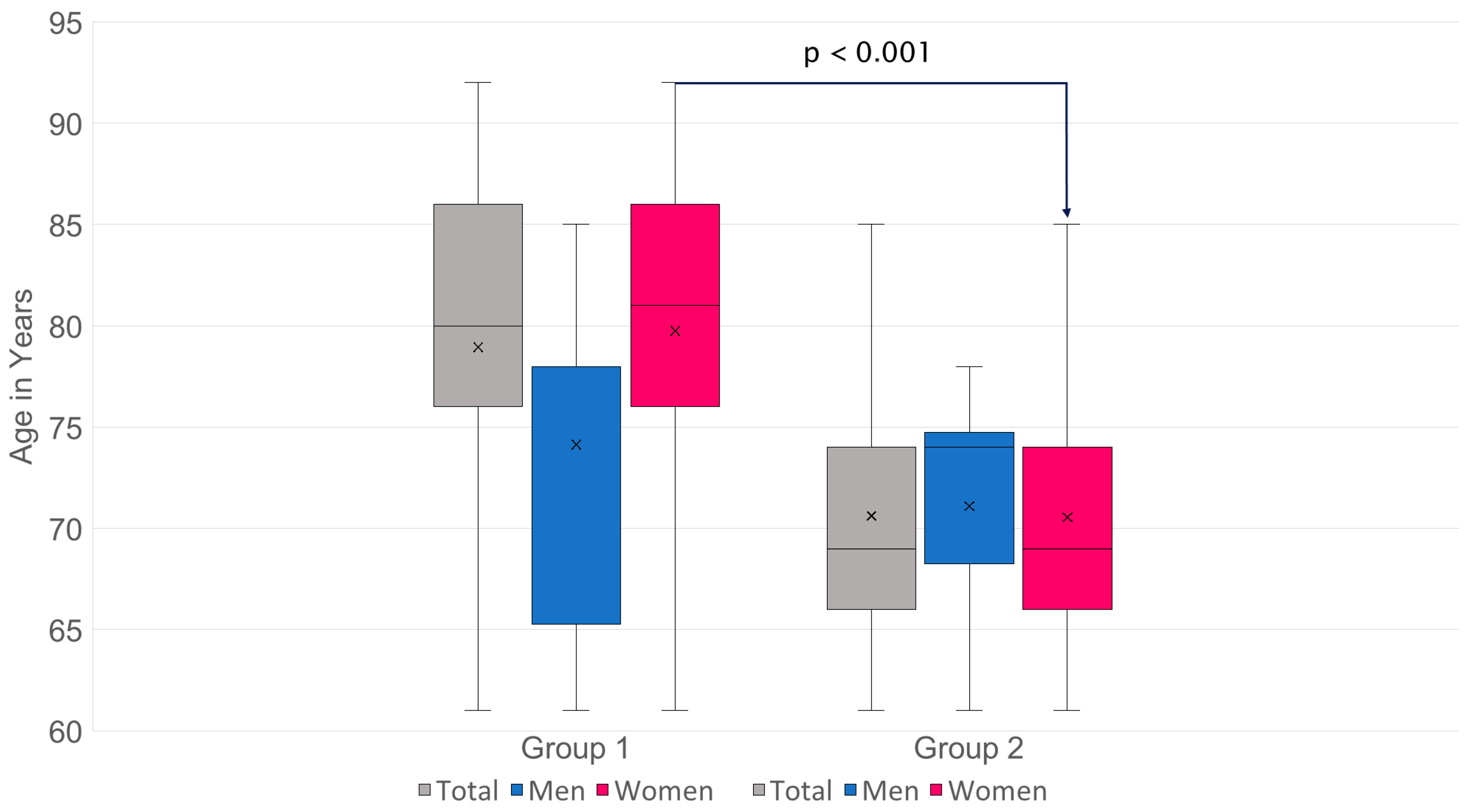
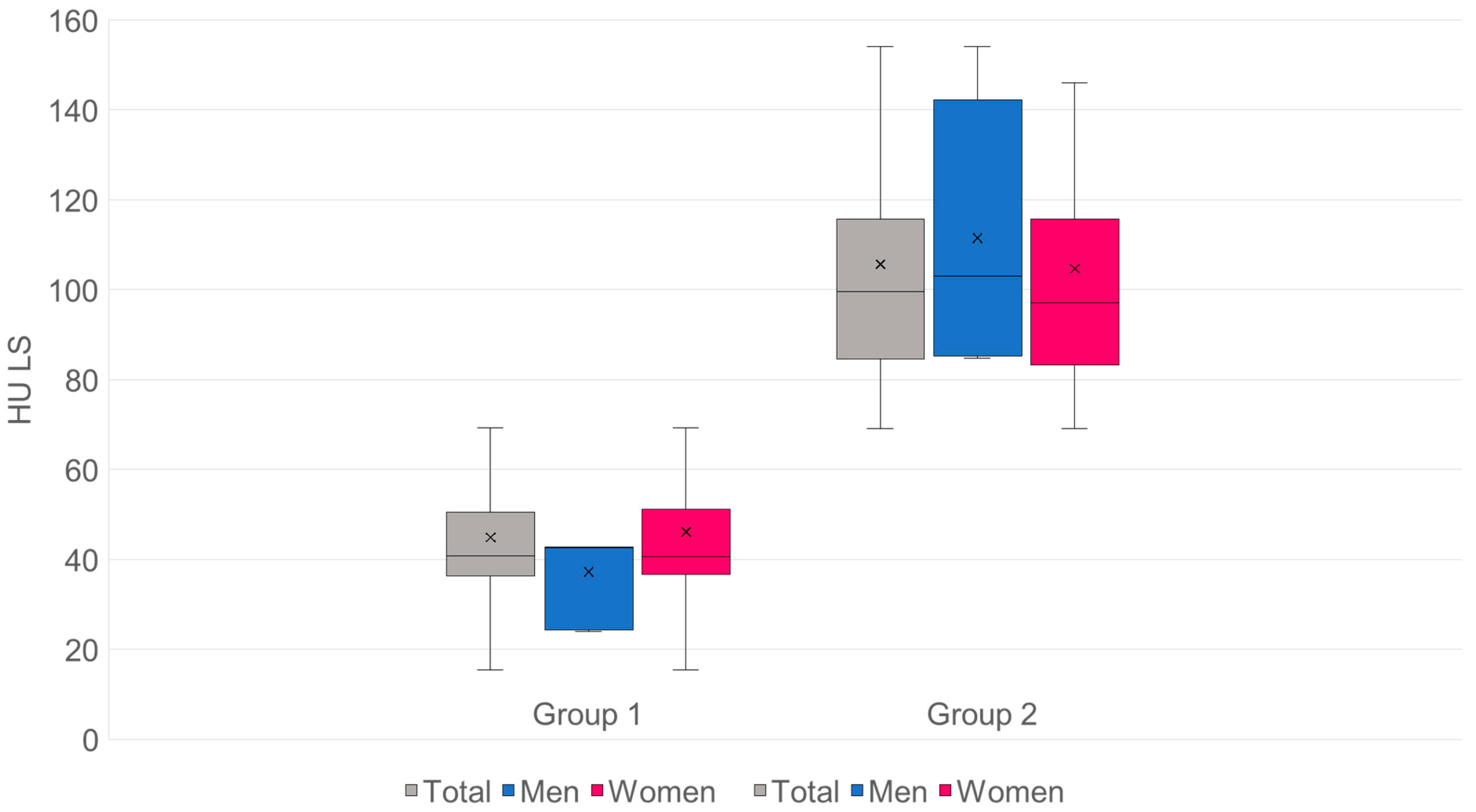
3.3. Comparison of Cancellous Bone Density in HU of the Lumbar Spine and Proximal Femur in Group 1 and Group 2
- n: G1 = 98·G2 = 155.
- Mean values (SD): G1 44.84 (18.28) HU·G2 105.66 (27.67) HU.
- Mean difference (G2−G1): +60.82 HU·95% CI: [+55.13; +66.51].
- Welch’s t-test: t = −21.05, df ≈ 250.5, p = 2.50 × 10−57.
- Mann–Whitney U: U = 417, p = 1.04 × 10−37.
- Hedges’ g = 2.48.
- Cliff’s δ = 0.96 → approx. 96% of pair comparisons: value from G2 > G1 (Figure 7).
- The mean HU value for group 1 is 44.84, which corresponds to 37.16 mg/cm3.
- The mean HU value for group 2 is 105.66, which corresponds to 86.42 mg/cm3.
- The threshold for osteoporosis of 80 mg/cm3 is 97.73 HU.
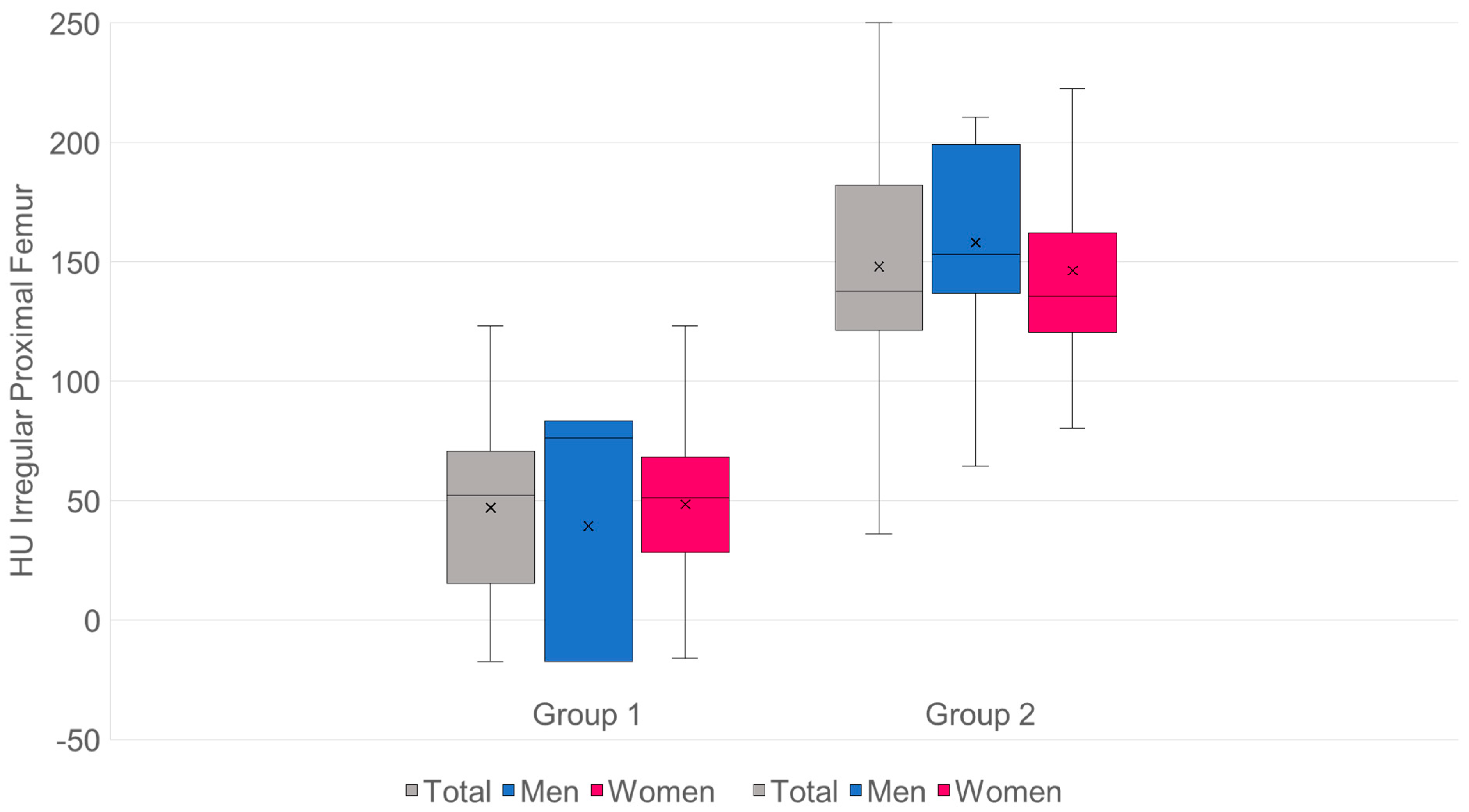
- n: G1 = 98·G2 = 155.
- Mean values (SD): G1 47 (34.5) HU·G2 148.0 (49.3) HU.
- Mean difference (G2−G1): +101.0 HU·95% CI: [+90.6; +111.4].
- Welch’s t-test: t = −19.13, df = 248.4, p = 9.09 × 10−51.
- Mann–Whitney U: U = 520, p = 9.95 × 10−36.
- Hedges’ g = 2.28.
- Cliff’s δ = 0.93 → approx. 93% of pair comparisons: value from G2 > G1 (Figure 8).
- The mean HU value for group 1 is 47, which corresponds to a T-value of −3.58.
- The mean HU value for group 2 is 148, which corresponds to a T-value of −1.35.
- The threshold for osteoporosis with a T-value of −2.5 is 95.93 HU.
Spongy Bone Density of Patients with and without Hip Fractures in Group 1 (Figure 9)

3.4. Conversion of HU Values into Equivalent Quantitative QCT Values in mg/cm3 for the Lumbar Spine and Equivalent Quantitative CTXA Values in mg/cm2 (Table 3) and Comparison to a Previous Study on the Hip (Table 4)
| Conversion Values from Different Studies | |||
|---|---|---|---|
| Studies | Average Age (in Years) | Conversion Formula from HU to QCT Values (mg/cm3) | Absolute Value and Percentage Deviation at 100 HU Compared to Own Results |
| Buenger et al., 2021 [40] | 66.98 | QCT value | 87.8 mg/cm3 |
| min.: 28; max.: 92 | = 17.8 + (0.7 × HU) | 9.6% greater than the values from [37] | |
| Schröder et al., 2024 [41] | 81.1 | QCT value | 73.7 mg/cm3 |
| min.: 66; max.: 102 | = 13.7 + (0.6 × HU) | 8.7% lower than the values from [37] | |
| Andresen et al., 2025 [37] | 65.9 | QCT value | 81.8 mg/cm3 |
| min.: 24; max.: 91 | = 0.84 + (0.8127 × HU) | ||
| Conversion Values from a Previous Study | |||
|---|---|---|---|
| Study | Average Age (in Years) | Conversion Formula from HU to CTXA Values T-Score Values | At 100 HU, the Following T-Value Is Obtained |
| Andresen et al., 2025 [38] | 65.9 min.: 24; max.: 91 | CTXA value = −4.62 + (0.0221 × HU) | −2.41 |
3.5. Vitamin D Levels
3.6. Pain at the Onset of SIF
3.7. Additional Disease Profile
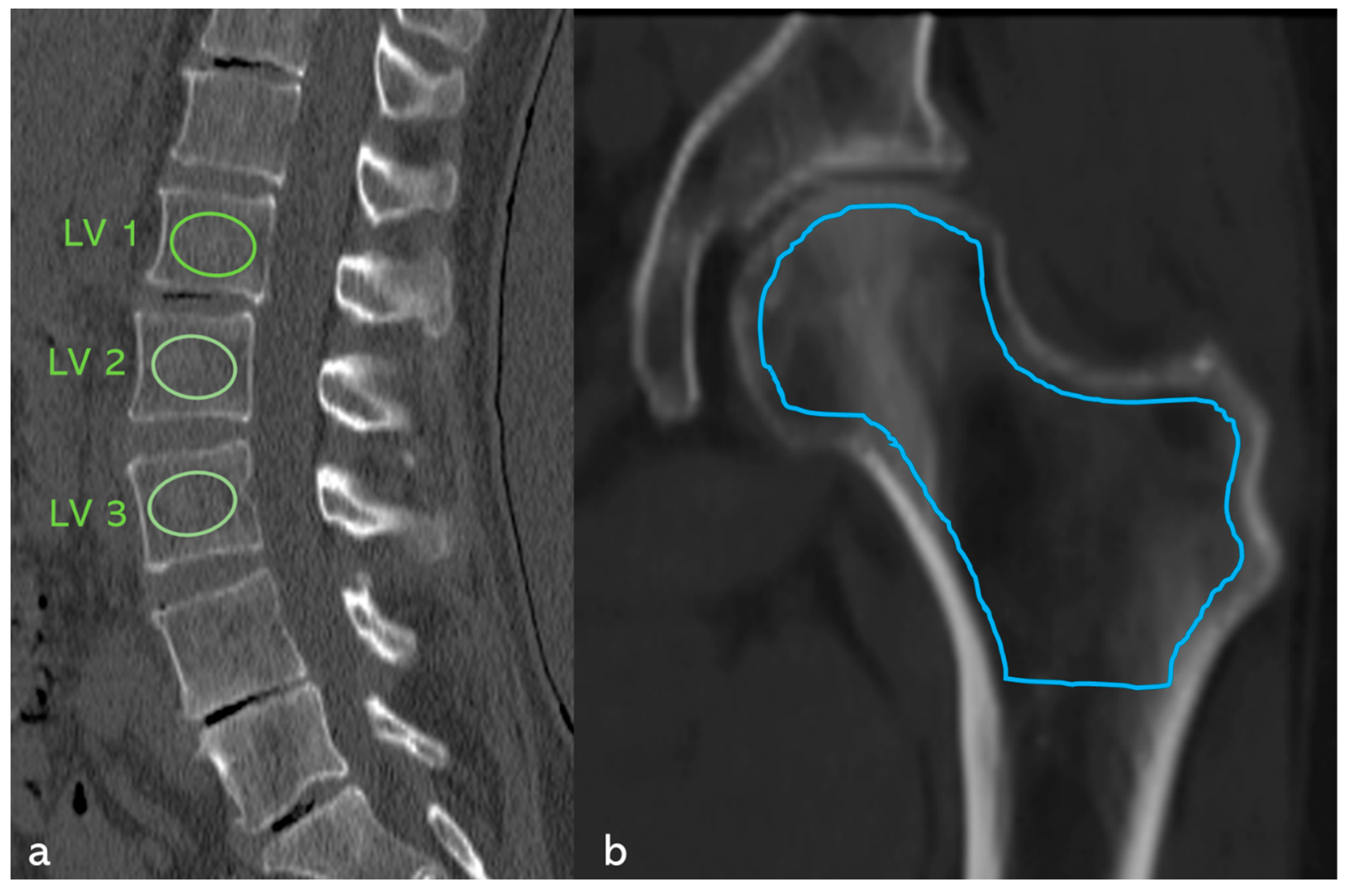
3.8. Case Study (Figure 11)
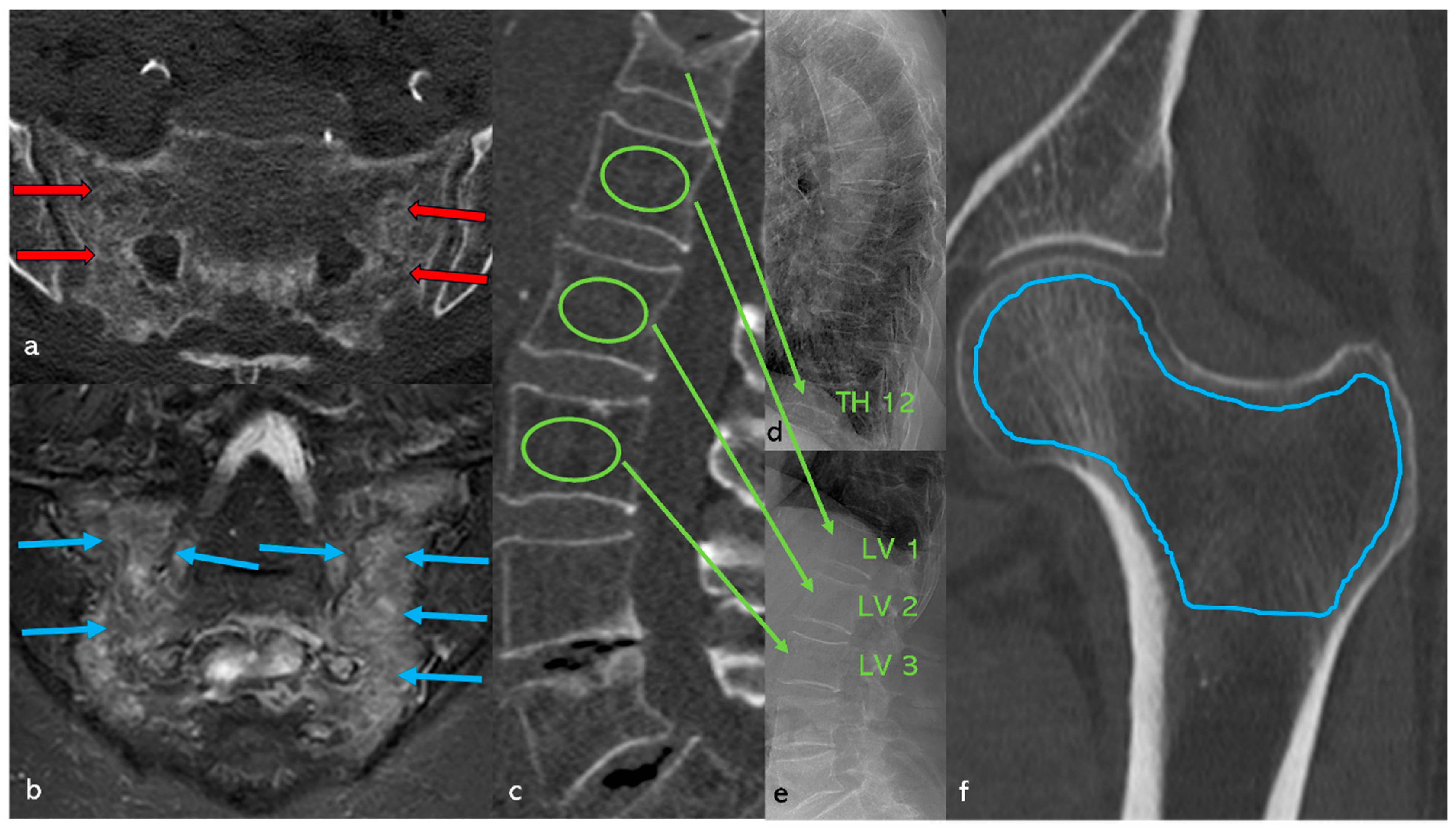
4. Discussion
4.1. The Axial Skeleton
4.2. The Proximal Femur
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| BMD | Bone mineral density |
| BMI | Body mass index |
| CTXA | Computed tomography X-ray absorptiometry |
| DEXA | Dual-energy X-ray absorptiometry |
| FFP | Fragility fractures of the pelvis |
| FFS | Fragility fractures of the sacrum |
| GE | General Electric |
| HU | Hounsfield unit |
| LS | Lumbar spine |
| LV | Lumbar vertebrae |
| MA | Mean age |
| MRI | Magnetic resonance imaging |
| n | Number |
| OVF | Osteoporotic vertebral fracture |
| prox. | proximal |
| Q1-Q3 | First and third quartile |
| QCT | Quantitative computed tomography |
| ROC | Receiver operating characteristic |
| ROI | Region of interest |
| SD | Standard deviation |
| SIF | Sacral insufficiency fracture |
| STIR | Short tau inversion recovery |
| TEP | Total endoprosthesis |
| TH | Thoracic vertebrae |
References
- Gotis-Graham, I.; McGuigan, L.; Diamond, T.; Portek, I.; Quinn, R.; Sturgess, A.; Tulloch, R. Sacral Insufficiency Fractures in the Elderly. J. Bone Joint Surg. Br. 1994, 76, 882–886. [Google Scholar] [CrossRef] [PubMed]
- West, S.G.; Troutner, J.L.; Baker, M.R.; Place, H.M. Sacral Insufficiency Fractures in Rheumatoid Arthritis. Spine 1994, 19, 2117–2121. [Google Scholar] [CrossRef]
- Lips, P. Vitamin D Deficiency and Secondary Hyperparathyroidism in the Elderly: Consequences for Bone Loss and Fractures and Therapeutic Implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef]
- Maier, G.S.; Kolbow, K.; Lazovic, D.; Horas, K.; Roth, K.E.; Seeger, J.B.; Maus, U. Risk Factors for Pelvic Insufficiency Fractures and Outcome after Conservative Therapy. Arch. Gerontol. Geriatr. 2016, 67, 80–85. [Google Scholar] [CrossRef]
- Kim, H.J.; Boland, P.J.; Meredith, D.S.; Lis, E.; Zhang, Z.; Shi, W.; Yamada, Y.J.; Goodman, K.A. Fractures of the Sacrum After Chemoradiation for Rectal Carcinoma: Incidence, Risk Factors, and Radiographic Evaluation. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 694–699. [Google Scholar] [CrossRef]
- Lin, J.T.; Lane, J.M. Sacral Stress Fractures. J. Women’s Health 2003, 12, 879–888. [Google Scholar] [CrossRef]
- Andrich, S.; Haastert, B.; Neuhaus, E.; Neidert, K.; Arend, W.; Ohmann, C.; Grebe, J.; Vogt, A.; Jungbluth, P.; Rösler, G.; et al. Epidemiology of Pelvic Fractures in Germany: Considerably High Incidence Rates among Older People. PLoS ONE 2015, 10, e0139078. [Google Scholar] [CrossRef] [PubMed]
- Schindler, O.; Watura, R.; Cobby, M. Sacral Insufficiency Fractures. J. Orthop. Surg. 2007, 15, 339–346. [Google Scholar] [CrossRef]
- Lourie, H. Spontaneous Osteoporotic Fracture of the Sacrum. An Unrecognized Syndrome of the Elderly. JAMA 1982, 248, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Orhurhu, V.; Callan, J.; Maganty, N.V.; Pousti, S.; Simopoulos, T.; Yazdi, C.; Kaye, R.J.; Eng, L.K.; Kaye, A.D.; et al. Sacral Insufficiency Fractures: A Review of Risk Factors, Clinical Presentation, and Management. Curr. Pain Headache Rep. 2020, 24, 10. [Google Scholar] [CrossRef]
- Grasland, A. Sacral Insufficiency Fractures: An Easily Overlooked Cause of Back Pain in Elderly Women. Arch. Intern. Med. 1996, 156, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Sudhir, G.K.L.K.; Kalra, K.L.; Acharya, S.; Chahal, R. Sacral Insufficiency Fractures Mimicking Lumbar Spine Pathology. Asian Spine J. 2016, 10, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Population Pyramid: Age Structure of Germany from 1950 to 2070. Available online: https://service.destatis.de/bevoelkerungspyramide/ (accessed on 7 September 2025).
- Kandziora, F.; Yildiz, U. Sacral insufficiency fractures: Surgical/conservative treatment. Die Wirbelsäule 2017, 1, 41–50. [Google Scholar] [CrossRef]
- Kannus, P.; Palvanen, M.; Niemi, S.; Parkkari, J.; Järvinen, M. Epidemiology of Osteoporotic Pelvic Fractures in Elderly People in Finland: Sharp Increase in 1970–1997 and Alarming Projections for the New Millennium. Osteoporos. Int. 2000, 11, 443–448. [Google Scholar] [CrossRef]
- Nanninga, G.L.; De Leur, K.; Panneman, M.J.M.; Van Der Elst, M.; Hartholt, K.A. Increasing Rates of Pelvic Fractures among Older Adults: The Netherlands, 1986–2011. Age Ageing 2014, 43, 648–653. [Google Scholar] [CrossRef]
- Lyders, E.M.; Whitlow, C.T.; Baker, M.D.; Morris, P.P. Imaging and Treatment of Sacral Insufficiency Fractures. Am. J. Neuroradiol. 2010, 31, 201–210. [Google Scholar] [CrossRef]
- Nüchtern, J.V.; Hartel, M.J.; Henes, F.O.; Groth, M.; Jauch, S.Y.; Haegele, J.; Briem, D.; Hoffmann, M.; Lehmann, W.; Rueger, J.M.; et al. Significance of Clinical Examination, CT and MRI Scan in the Diagnosis of Posterior Pelvic Ring Fractures. Injury 2015, 46, 315–319. [Google Scholar] [CrossRef]
- Hackenbroch, C.; Riesner, H.-J.; Lang, P.; Stuby, F.; Beer, M.; Friemert, B.; Palm, H.-G.; Becken, A.G., III. Dual Energy Computed Tomography in Musculoskeletal Imaging, with Focus on Fragility Fractures of the Pelvis. Z. Orthop. Unf. 2017, 155, 708–715. [Google Scholar] [CrossRef]
- Inagaki, N.; Nakata, N.; Ichimori, S.; Udaka, J.; Mandai, A.; Saito, M. Detection of Sacral Fractures on Radiographs Using Artificial Intelligence. JBJS Open Access 2022, 7, e22.00030. [Google Scholar] [CrossRef]
- Rommens, P.M.; Hofmann, A. Comprehensive Classification of Fragility Fractures of the Pelvic Ring: Recommendations for Surgical Treatment. Injury 2013, 44, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Rommens, P.M.; Drees, P.; Thomczyk, S.; Betz, U.; Wagner, D.; Hofmann, A. Fragility fractures of the pelvis are an indicator of osteoporosis. Osteology 2018, 27, 144–153. [Google Scholar] [CrossRef]
- Cosker, T.D.A.; Ghandour, A.; Gupta, S.K.; Tayton, K.J.J. Pelvic Ramus Fractures in the Elderly: 50 Patients Studied with MRI. Acta Orthop. 2005, 76, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Alnaib, M.; Waters, S.; Shanshal, Y.; Caplan, N.; Jones, S.; St Clair Gibson, A.; Kader, D. Combined Pubic Rami and Sacral Osteoporotic Fractures: A Prospective Study. J. Orthop. Traumatol. 2012, 13, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Aretxabala, I.; Fraiz, E.; Pérez-Ruiz, F.; Ríos, G.; Calabozo, M.; Alonso-Ruiz, A. Sacral insufficiency fractures. High association with pubic rami fractures. Clin. Rheumatol. 2000, 19, 399–401. [Google Scholar] [CrossRef]
- Morris, R.O.; Sonibare, A.; Green, D.J.; Masud, T. Closed Pelvic Fractures: Characteristics and Outcomes in Older Patients Admitted to Medical and Geriatric Wards. Postgrad. Med. J. 2000, 76, 646–650. [Google Scholar] [CrossRef]
- Josten, C.; Höch, A. Sacral insufficiency fractures: Surgical/conservative. Die Wirbelsäule 2017, 1, 31–40. [Google Scholar] [CrossRef]
- Holderread, B.M.; Shin, C.P.; Syed, I.Y.; Avramis, I.; Rizkalla, J.M. Sacral insufficiency fracture after lumbosacral decompression and fusion. Bayl. Univ. Med. Cent. Proc. 2022, 35, 451–454. [Google Scholar] [CrossRef]
- De Smet, A. Neff Pubic and Sacral Insufficiency Fractures: Clinical Course and Radiologic Findings. Am. J. Roentgenol. 1985, 145, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Peh, W.C.; Khong, P.L.; Yin, Y.; Ho, W.Y.; Evans, N.S.; Gilula, L.A.; Yeung, H.W.; Davies, A.M. Imaging of Pelvic Insufficiency Fractures. RadioGraphics 1996, 16, 335–348. [Google Scholar] [CrossRef]
- Muthukumar, T.; Butt, S.H.; Cassar-Pullicino, V.N.; McCall, I.W. Cauda Equina Syndrome Presentation of Sacral Insufficiency Fractures. Skelet. Radiol 2007, 36, 309–313. [Google Scholar] [CrossRef]
- Babayev, M.; Lachmann, E.; Nagler, W. The Controversy Surrounding Sacral Insufficiency Fractures: To Ambulate or Not to Ambulate? Am. J. Phys. Med. Rehabil. 2000, 79, 404–409. [Google Scholar] [CrossRef]
- Andresen, R.; Radmer, S.; Lüdtke, C.W.; Kamusella, P.; Görmez, M.; Wissgott, C.; Schober, H.-C. Comparison of conservative therapy vs. CT-guided balloon sacroplasty in the treatment of insufficiency fractures of the sacrum. Osteology 2015, 24, 92–98. [Google Scholar] [CrossRef]
- Andresen, J.R.; Radmer, S.; Andresen, R.; Prokop, A.; Schröder, G.; Nissen, U.; Schober, H.-C. Comparative Outcome of Different Treatment Options for Fragility Fractures of the Sacrum. BMC Musculoskelet. Disord. 2022, 23, 1106. [Google Scholar] [CrossRef]
- Briggs, P.; King, S.W.; Staniland, T.; Gopal, S.; Shah, R.; Chimutengwende-Gordon, M. A Systematic Review of Sacral Insufficiency Fractures: Treatment Modalities and Outcomes. Cureus 2023, 15, e41745. [Google Scholar] [CrossRef] [PubMed]
- Genant, H.K.; Wu, C.Y.; Van Kuijk, C.; Nevitt, M.C. Vertebral Fracture Assessment Using a Semiquantitative Technique. J. Bone Miner. Res. 1993, 8, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Andresen, J.R.; Schröder, G.; Haider, T.; Andresen, R. Opportunistic Osteoporosis Assessment and Fracture Risk Determination Using Cancellous Density Measurement in Hounsfield Units of Native Lumbar Computed Tomography Images—A Comparative Study with Conventional Bone Density Evaluation. JCM 2025, 14, 1226. [Google Scholar] [CrossRef] [PubMed]
- Andresen, J.R.; Schröder, G.; Haider, T.; Schober, H.-C.; Andresen, R. Osteoporosis Assessment Using Bone Density Measurement in Hounsfield Units in the Femoral Native CT Cross-Section: A Comparison with Computed Tomography X-Ray Absorptiometry of the Hip. Diagnostics 2025, 15, 1014. [Google Scholar] [CrossRef]
- Dachverband Osteologie, e.V. S3-Leitlinie Prophylaxe, Diagnostik und Therapie der Osteoporose bei Postmenopausalen Frauen und bei Männern ab dem 50. Lebensjahr. Available online: https://register.awmf.org/de/leitlinien/detail/183-001 (accessed on 4 February 2025).
- Buenger, F.; Eckardt, N.; Sakr, Y.; Senft, C.; Schwarz, F. Correlation of Bone Density Values of Quantitative Computed Tomography and Hounsfield Units Measured in Native Computed Tomography in 902 Vertebral Bodies. World Neurosurg. 2021, 151, e599–e606. [Google Scholar] [CrossRef]
- Schröder, G.; Andresen, J.R.; Hiepe, L.; Schulze, M.; Kullen, C.M.; Kopetsch, C.; Burmeister, J.; Schober, H.-C.; Andresen, R. Interobserver Variability in the Determination of Bone Mineral Density in Hounsfield Units from Differently Configured Fields of Measurement in the Cancellous Bone of Vertebral Bodies from Elderly Body Donors. J. Orthop. 2024, 49, 48–55. [Google Scholar] [CrossRef]
- Gleich, J.; Steiner, E.; Ehrnthaller, C.; Degen, N.; Lampert, C.; Böcker, W.; Neuerburg, C.; Linhart, C. CT-Based Evaluation of Hounsfield Units—A Novel Screening Tool for Undiagnosed Osteoporosis in Patients with Fragility Fractures of the Pelvis. JCM 2025, 14, 3346. [Google Scholar] [CrossRef]
- Kelly, M.A.; McCabe, E.; Bergin, D.; Kearns, S.R.; McCabe, J.P.; Armstrong, C.; Heaney, F.; Carey, J.J. Osteoporotic Vertebral Fractures Are Common in Hip Fracture Patients and Are Under-Recognized. J. Clin. Densitom. 2021, 24, 183–189. [Google Scholar] [CrossRef]
- Wagner, D.; Kamer, L.; Sawaguchi, T.; Richards, R.G.; Noser, H.; Rommens, P.M. Sacral Bone Mass Distribution Assessed by Averaged Three-Dimensional CT Models: Implications for Pathogenesis and Treatment of Fragility Fractures of the Sacrum. J. Bone Joint Surg. Am. 2016, 98, 584–590. [Google Scholar] [CrossRef]
- Klauke, F.; Zänker, K.; Schenk, P.; Kobbe, P.; Muhl, C.; Mendel, T. Comparison of the Zonal Distribution of Calcium Salt Density and Fat Marrow in Bone-Healthy and Osteoporotic Sacra: An Image Data Analysis Using Quantitative Computed Tomography and Magnetic Resonance Imaging. Eur. J. Trauma Emerg. Surg. 2024, 50, 1765–1773. [Google Scholar] [CrossRef]
- Henes, F.O.; Groth, M.; Bley, T.A.; Regier, M.; Nüchtern, J.V.; Ittrich, H.; Treszl, A.; Adam, G.; Bannas, P. Quantitative Assessment of Bone Marrow Attenuation Values at MDCT: An Objective Tool for the Detection of Bone Bruise Related to Occult Sacral Insufficiency Fractures. Eur. Radiol. 2012, 22, 2229–2236. [Google Scholar] [CrossRef]
- Delsmann, M.M.; Leonhardt, L.-G.; Alimy, A.-R.; Hoenig, T.; Beil, F.T.; Püschel, K.; Von Brackel, F.N.; Amling, M.; Viezens, L.; Thiesen, D.M.; et al. Biopsies from Patients with Sacral Insufficiency Fracture Are Characterized by Low Bone Matrix Mineralization and High Turnover. JBMR Plus 2024, 8, ziae094. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, C.; Lee, E.; Lee, J.W. Coronal Plane in Opportunistic Screening of Osteoporosis Using Computed Tomography: Comparison with Axial and Sagittal Planes. Skelet. Radiol. 2024, 53, 1103–1109. [Google Scholar] [CrossRef]
- Working Group on Osteoporotic Fractures of the Spine Section of the German Society for Orthopedics and Trauma Surgery; Scheyerer, M.J.; Ullrich, B.; Osterhoff, G.; Spiegl, U.A.; Schnake, K.J. “Hounsfield units” as a measure of bone density—Possible applications in spinal surgery. Unfallchirurg 2019, 122, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.L.; Nappo, K.E.; Wolfe, J.A.; Wade, S.M.; Brooks, D.I.; Potter, B.K.; Forsberg, J.A.; Tintle, S.M. Proximal Femur Hounsfield Units on CT Colonoscopy Correlate with Dual-Energy X-Ray Absorptiometry. Clin. Orthop. Relat. Res. 2019, 477, 850–860. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kwon, S.-S.; Kim, H.S.; Yoo, J.H.; Kim, J.; Kim, J.Y.; Min, B.C.; Moon, S.J.; Sung, K.H. Reliability and Validity of Lower Extremity Computed Tomography as a Screening Tool for Osteoporosis. Osteoporos. Int. 2015, 26, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, A.; Cai, A.; Xi, Y.; Maalouf, N.M.; Rubin, C.; Chhabra, A. CT Bone Density Analysis of Low-Impact Proximal Femur Fractures Using Hounsfield Units. Clin. Imaging 2019, 57, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K.; Ha, H.I.; Park, S.-Y.; Lee, K. Comparison of the Diagnostic Performance of CT Hounsfield Unit Histogram Analysis and Dual-Energy X-Ray Absorptiometry in Predicting Osteoporosis of the Femur. Eur. Radiol. 2019, 29, 1831–1840. [Google Scholar] [CrossRef]
- Kılınc, R.M.; Açan, A.E.; Türk, G.; Kılınç, C.Y.; Yeniçeri, İ.Ö. Evaluation of Femoral Head Bone Quality by Hounsfield Units: A Comparison with Dual-Energy X-Ray Absorptiometry. Acta Radiol 2022, 63, 933–941. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Z.; Ren, Q.; Nie, G.; Zhao, D. Measurement of Hounsfield Units on Proximal Femur Computed Tomography for Predicting Regional Osteoporosis. Radiologie 2023, 63, 90–97. [Google Scholar] [CrossRef]
- Fan, J.; Lv, Y.; Xu, X.; Zhou, F.; Zhang, Z.; Tian, Y.; Ji, H.; Guo, Y.; Yang, Z.; Hou, G. Evaluation of Femoral Head Bone Quality by Hounsfield Units: A Predictor of Implant Failure for Intertrochanteric Fractures after Intramedullary Nail Fixation. Front. Surg. 2023, 9, 816742. [Google Scholar] [CrossRef]
- Imren, Y.; Karslioglu, B.; Semih Dedeoğlu, S.; Keskin, A.; Firat Berkay, A.; Cagri Tekin, A. Feasibility of Diagnosing Osteoporosis Using Routine Computed Tomography Scans for Hip Fractures: Correlation with Histopathological Diagnosis of Head and Neck Regions. Acta Orthop. Traumatol. Turc. 2024, 57, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Andresen, J.R.; Haider, T.; Andresen, R. Trabecular bone density measurement in Hounsfield units in the proximal femur for osteoporosis assessment and fracture risk determination. Die Orthopädie 2025. Epub ahead of print. [Google Scholar] [CrossRef]
- Felsenberg, D.; Kalender, W.; Banzer, D.; Schmilinsky, G.; Heyse, M.; Fischer, E.; Schneider, U. Quantitative computed tomography bone mineral content determination. Fortschr Röntgenstr 1988, 148, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Felsenberg, D.; Gowin, W. Bone density measurement using dual-spectrum methods. Der Radiologe 1999, 39, 186–193. [Google Scholar] [CrossRef]
- Schmidmaier, R.; Hadji, P.; Kern, P.; Drey, M.; Jakob, F.; Thomasius, F. Recommendations for the Pharmacological Treatment of Osteoporosis—Update 2023 of the German Osteoporosis Guideline. Osteology 2023, 32, 115–122. [Google Scholar] [CrossRef]
- Choi, M.K.; Kim, S.M.; Lim, J.K. Diagnostic Efficacy of Hounsfield Units in Spine CT for the Assessment of Real Bone Mineral Density of Degenerative Spine: Correlation Study between T-Scores Determined by DEXA Scan and Hounsfield Units from CT. Acta Neurochir. 2016, 158, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Li, W.; Deng, C.; Du, G.; Xu, N. The Use of CT Hounsfield Unit Values to Identify the Undiagnosed Spinal Osteoporosis in Patients with Lumbar Degenerative Diseases. Eur. Spine J. 2019, 28, 1758–1766. [Google Scholar] [CrossRef]
- Chanbour, H.; Steinle, A.M.; Chen, J.W.; Waddell, W.H.; Vickery, J.; LaBarge, M.E.; Longo, M.; Gardocki, R.J.; Abtahi, A.M.; Stephens, B.F.; et al. The Importance of Hounsfield Units in Adult Spinal Deformity Surgery: Finding an Optimal Threshold to Minimize the Risk of Mechanical Complications. J. Spine Surg. 2023, 9, 149–158. [Google Scholar] [CrossRef]
- Ye, K.; Zou, D.; Zhou, F.; Li, W.; Tian, Y. Low Vertebral CT Hounsfield Units: A Risk Factor for New Osteoporotic Vertebral Fractures after the Treatment of Percutaneous Kyphoplasty. Arch. Osteoporos. 2022, 17, 137. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Xie, T.; Song, Z.; Song, Y.; Zeng, J. The Preoperative Hounsfield Unit Value at the Position of the Future Screw Insertion Is a Better Predictor of Screw Loosening than Other Methods. Eur. Radiol. 2022, 33, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, K.; Zhao, B.; Du, H.; Zhang, T.; Han, W.; Wang, J. A Retrospective Study on the Relationship Between Calcar Femorale Injury and Postoperative Complications of Femoral Neck Fracture in Young and Middle-Aged Patients Treated with Three Cannulated Screws. Orthop. Surg. 2025, 17, 2653–2661. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.; Robinson, J.; Gendelberg, D.; Jimenez, J.; Anand, A.; Rao, A.; Khandehroo, B.; Khandehroo, B.; Kahwaty, S.; Anand, N. Do Peri-Operative Parathyroid Hormone (PTH) Analogues Improve Bone Density and Decrease Mechanical Complications in Spinal Deformity Correction?—A Minimum 2-Year Radiological Study Measuring Hounsfield Units. Eur. Spine J. 2023, 32, 3651–3658. [Google Scholar] [CrossRef]
- Uemura, K.; Otake, Y.; Takashima, K.; Hamada, H.; Imagama, T.; Takao, M.; Sakai, T.; Sato, Y.; Okada, S.; Sugano, N. Development and Validation of an Open-Source Tool for Opportunistic Screening of Osteoporosis from Hip CT Images: A Multicenter Study of 978 Hips. Bone Joint Res. 2023, 12, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.H. Incorporating Artificial Intelligence into Fracture Risk Assessment: Using Clinical Imaging to Predict the Unpredictable. Endocrinol. Metab. 2025, 40, 499–507. [Google Scholar] [CrossRef]
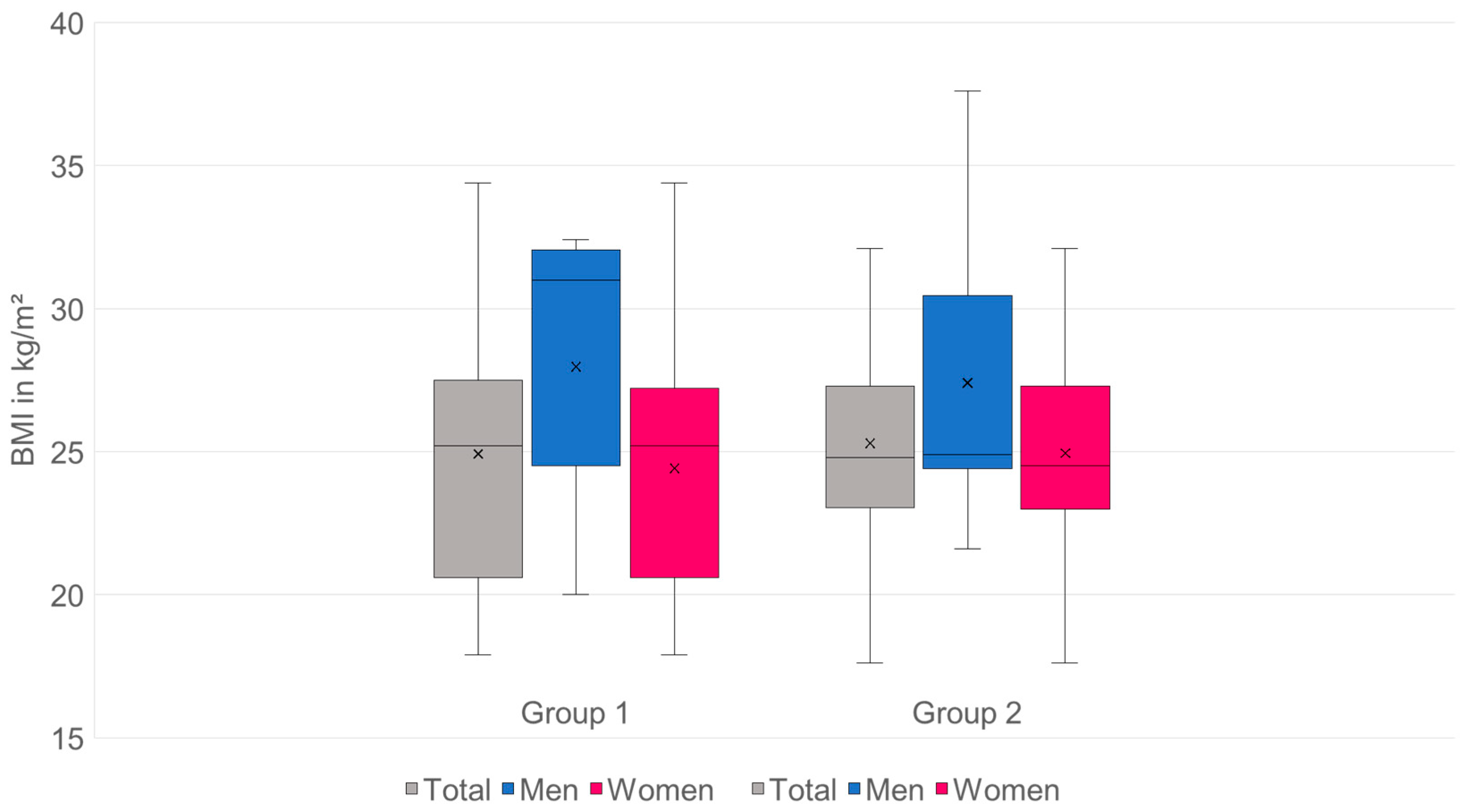

| Characterization of the total sample | |||
| Patients (n = 253) | Men (n = 36) | Women (n = 217) | |
| Age | Ø 73.85 | Ø 72.28 | Ø 74.11 |
| (in years) | (min.: 61; max.: 92) | (min.: 61; max.: 85) | (min.: 61; max.: 92) |
| BMI | Ø 25.15 | Ø 27.62 | Ø 24.74 |
| (kg/m2) | (min.: 17.6; max.: 37.6) | (min.: 20; max.: 37.6) | (min.: 17.6; max.: 34.4) |
| Characterization of group 1 (sacrum fractures) | |||
| Patients (n = 98) | Men (n = 14) | Women (n = 84) | |
| Age | Ø 78.95 | Ø 74.14 | Ø 79.75 |
| (in years) | (min.: 61; max.: 92) | (min.: 61; max.: 85) | (min.: 61; max.: 92) |
| BMI | Ø 24.92 | Ø 27.97 | Ø 24.41 |
| (kg/m2) | (min.: 17.9; max.: 34.4) | (min.: 20; max.: 32.4) | (min.: 17.9; max.: 34.4) |
| Characterization of group 2 (control group without fractures) | |||
| Patients (n = 155) | Men (n = 22) | Women (n = 133) | |
| Age | Ø 70.62 | Ø 71.09 | Ø 70.54 |
| (in years) | (min.: 61; max.: 85) | (min.: 61; max.: 78) | (min.: 61; max.: 85) |
| BMI | Ø 25.3 | Ø 27.4 | Ø 24.95 |
| (kg/m2) | (min.: 17.6; max.: 37.6) | (min.: 21.6; max.: 37.6) | (min.: 17.6; max.: 32.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andresen, J.R.; Schröder, G.; Haider, T.; Schober, H.-C.; Andresen, R. Diagnostic Value of Opportunistic CT-Based Bone Density Assessment in Patients with and Without Sacral Insufficiency Fractures. Diagnostics 2025, 15, 2926. https://doi.org/10.3390/diagnostics15222926
Andresen JR, Schröder G, Haider T, Schober H-C, Andresen R. Diagnostic Value of Opportunistic CT-Based Bone Density Assessment in Patients with and Without Sacral Insufficiency Fractures. Diagnostics. 2025; 15(22):2926. https://doi.org/10.3390/diagnostics15222926
Chicago/Turabian StyleAndresen, Julian Ramin, Guido Schröder, Thomas Haider, Hans-Christof Schober, and Reimer Andresen. 2025. "Diagnostic Value of Opportunistic CT-Based Bone Density Assessment in Patients with and Without Sacral Insufficiency Fractures" Diagnostics 15, no. 22: 2926. https://doi.org/10.3390/diagnostics15222926
APA StyleAndresen, J. R., Schröder, G., Haider, T., Schober, H.-C., & Andresen, R. (2025). Diagnostic Value of Opportunistic CT-Based Bone Density Assessment in Patients with and Without Sacral Insufficiency Fractures. Diagnostics, 15(22), 2926. https://doi.org/10.3390/diagnostics15222926






