Development and Evaluation of a Smartphone App-Based Rapid 25-Hydroxy Vitamin D Test
Abstract
1. Introduction
2. Materials and Methods
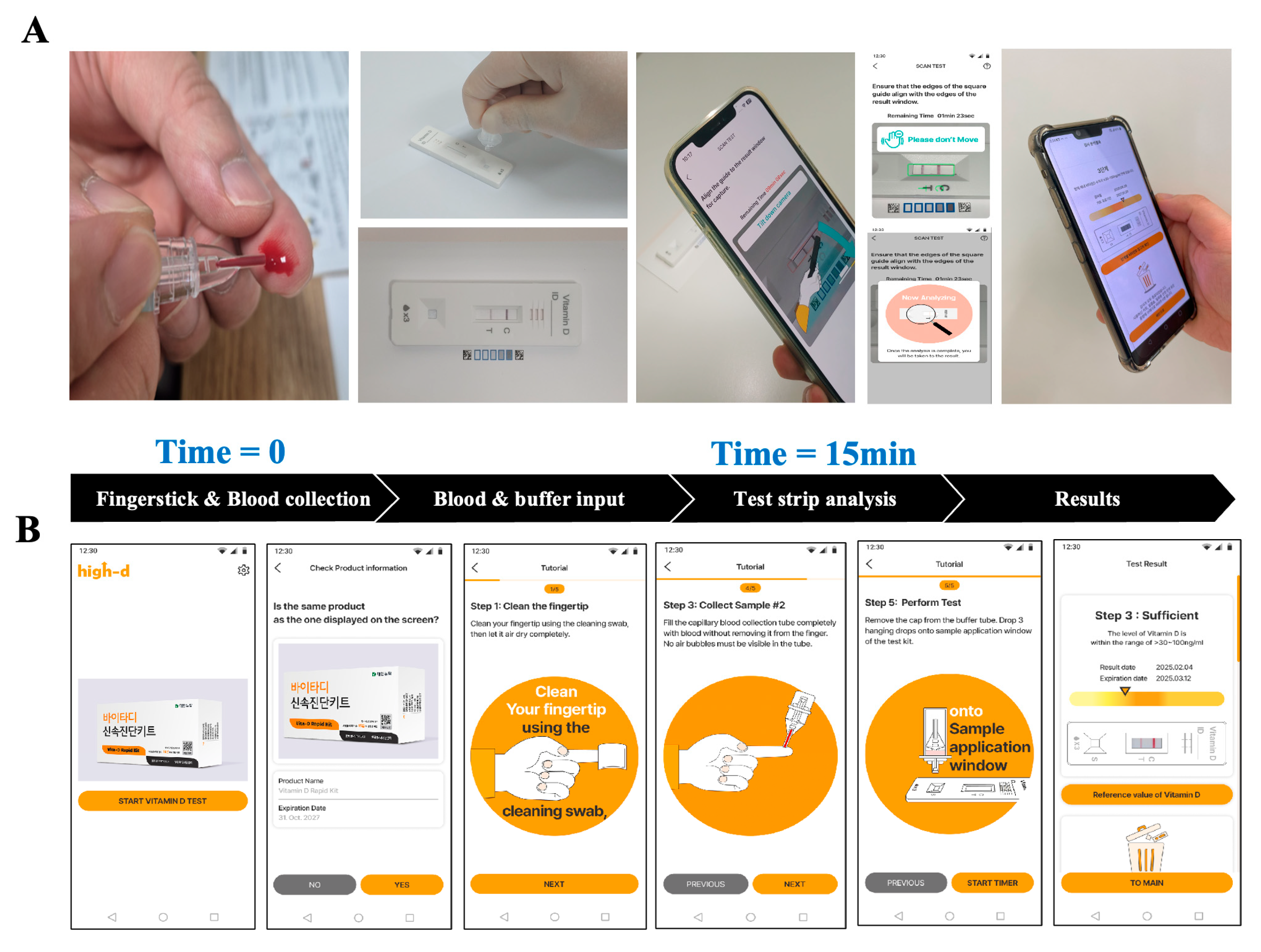
2.1. Development of the Smartphone-Based Vitamin D Rapid Assay
2.2. Image-Based Quantification Using Smartphone Application
2.3. Evaluation of Reproducibility and Repeatability
2.4. Evaluation of Dynamic Range and Limit of Detection (LoD)
2.5. Cross-Reactivity and Interference Assessment
2.6. Correlation with a Commercial Vitamin D Analyzer
2.7. Specimen Type Equivalence
2.8. Statistical Analysis
3. Results
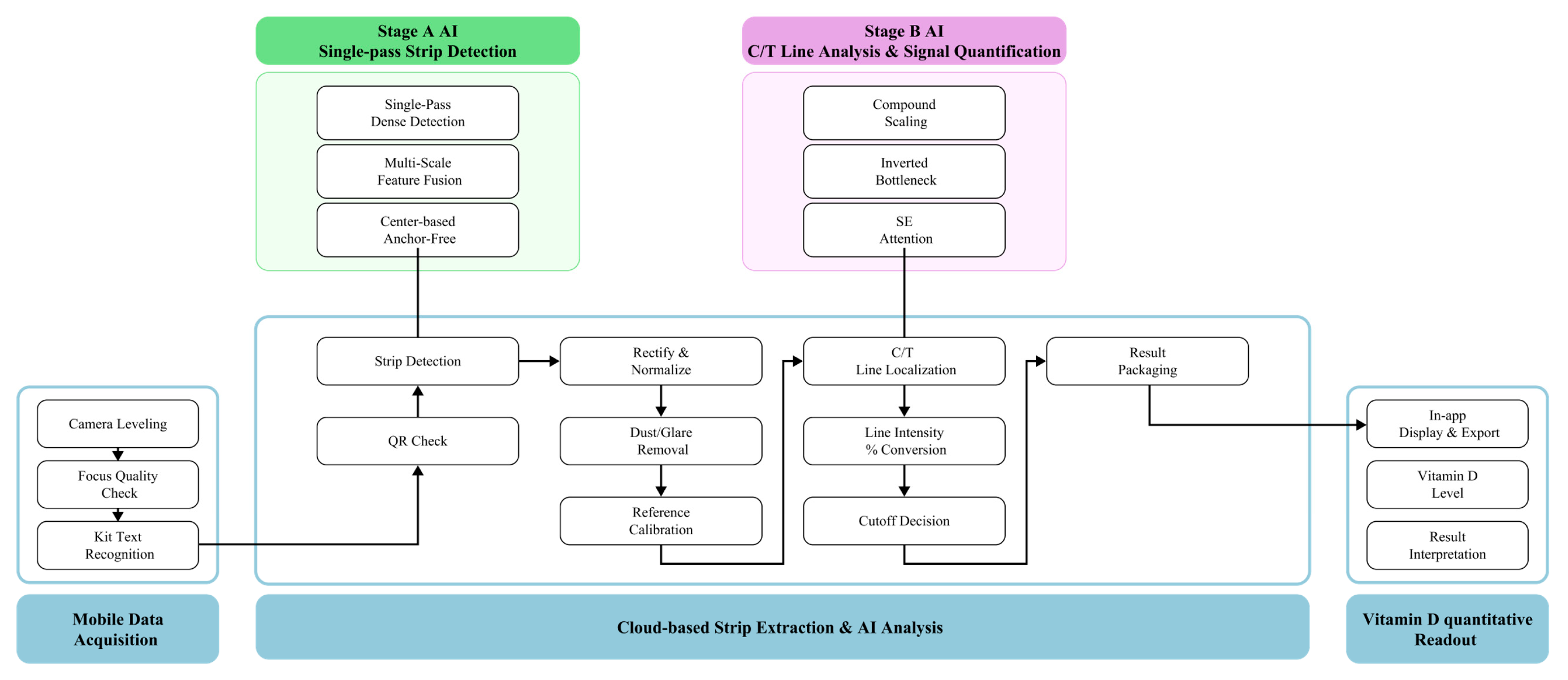
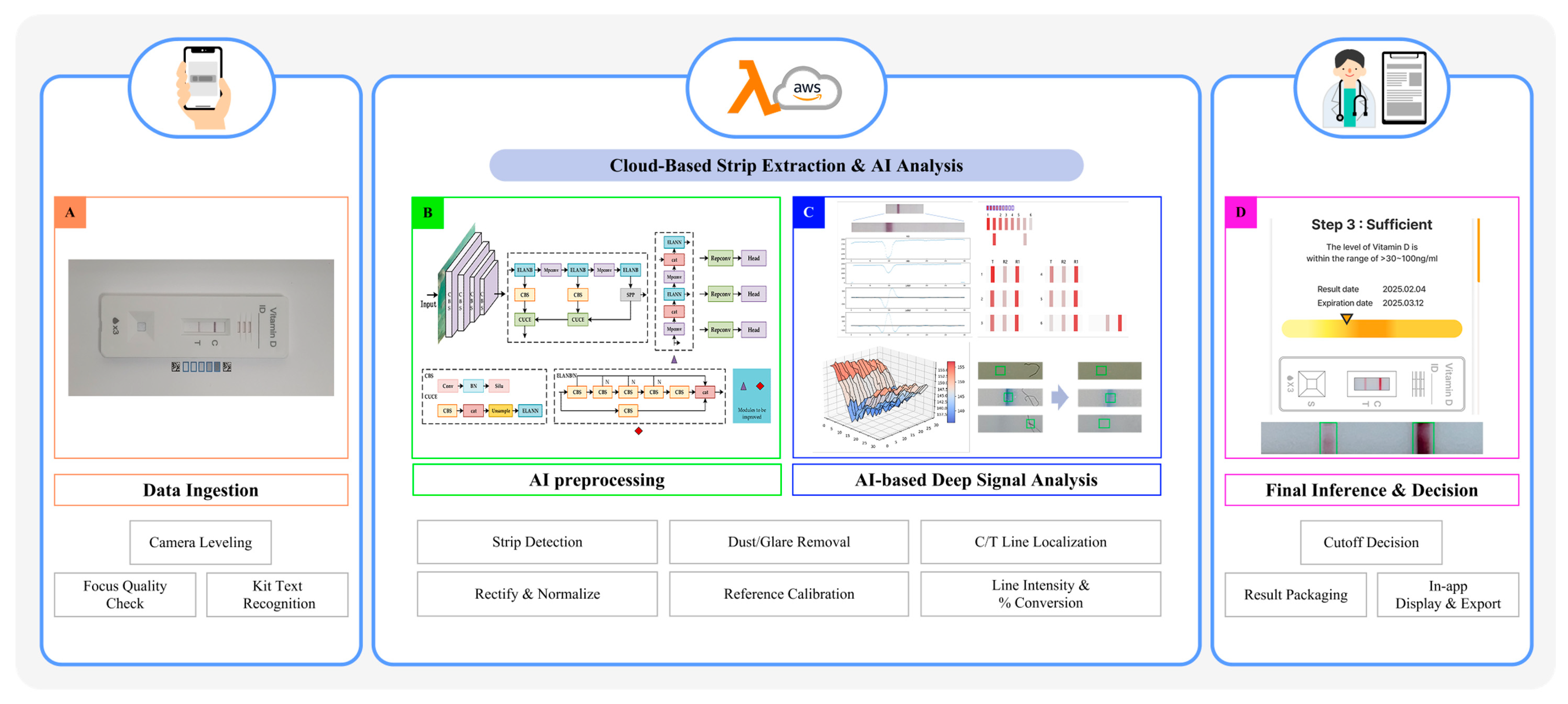
3.1. System Architecture and Analytical Design
3.2. Semi-Quantitative Detection of 25(OH)D via Smartphone-Based Image Analysis
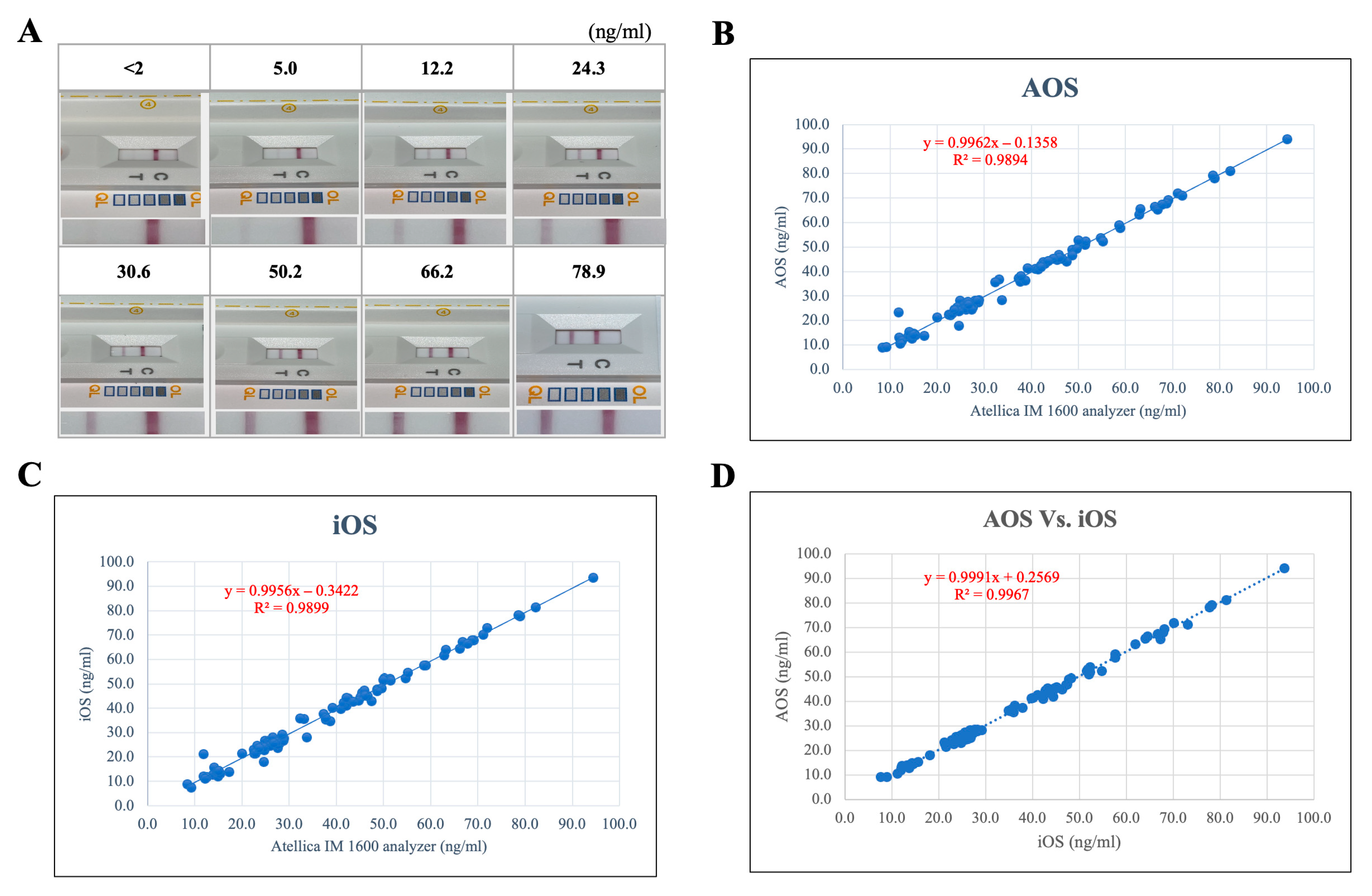
3.3. Analytical Sensitivity and Cross-Platform Performance
3.4. Cross-Platform Analytical Reproducibility
3.5. Comparative Analytical Accuracy with a Commercial Vitamin D Assay
3.6. Matrix Equivalence Between Capillary Blood and Serum
4. Discussions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consents Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellavia, D.; Costa, V.; De Luca, A.; Maglio, M.; Pagani, S.; Fini, M.; Giavaresi, G. Vitamin D Level Between Calcium-Phosphorus Homeostasis and Immune System: New Perspective in Osteoporosis. Curr. Osteoporos. Rep. 2024, 22, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C. The role of vitamin D in the endocrinology controlling calcium homeostasis. Mol. Cell. Endocrinol. 2017, 453, 36–45. [Google Scholar] [CrossRef]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef]
- Ismailova, A.; White, J.H. Vitamin D, infections and immunity. Rev. Endocr. Metab. Disord. 2022, 23, 265–277. [Google Scholar] [CrossRef]
- Ringe, J.D. Plain vitamin D or active vitamin D in the treatment of osteoporosis: Where do we stand today? Arch. Osteoporos. 2020, 14, 182. [Google Scholar] [CrossRef]
- O’Brien, M.A.; Jackson, M.W. Vitamin D and the immune system: Beyond rickets. Vet. J. 2012, 194, 27–33. [Google Scholar] [CrossRef]
- Kawahara, T.; Okada, Y.; Tanaka, Y. Vitamin D efficacy in type 1 and type 2 diabetes. J. Bone Miner. Metab. 2024, 42, 438–446. [Google Scholar] [CrossRef]
- Gouni-Berthold, I.; Berthold, H.K. Vitamin D and Vascular Disease. Curr. Vasc. Pharmacol. 2021, 19, 250–268. [Google Scholar] [CrossRef] [PubMed]
- Teymoori-Rad, M.; Shokri, F.; Salimi, V.; Marashi, S.M. The interplay between vitamin D and viral infections. Rev. Med. Virol. 2019, 29, e2032. [Google Scholar] [CrossRef]
- Moukayed, M.; Grant, W.B. The roles of UVB and vitamin D in reducing risk of cancer incidence and mortality: A review of the epidemiology, clinical trials, and mechanisms. Rev. Endocr. Metab. Disord. 2017, 18, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the Prevention of Disease: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947. [Google Scholar] [CrossRef]
- Vieth, R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am. J. Clin. Nutr. 1999, 69, 842–856. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Misra, M.; Pacaud, D.; Petryk, A.; Collett-Solberg, P.F.; Kappy, M. Vitamin D deficiency in children and its management: Review of current knowledge and recommendations. Pediatrics 2008, 122, 398–417. [Google Scholar] [CrossRef] [PubMed]
- Nikooyeh, B.; Samiee, S.M.; Farzami, M.R.; Alavimajd, H.; Zahedirad, M.; Kalayi, A.; Shariatzadeh, N.; Boroumand, N.; Golshekan, E.; Gholamian, Y.; et al. Harmonization of serum 25-hydroxycalciferol assay results from high-performance liquid chromatography, enzyme immunoassay, radioimmunoassay, and immunochemiluminescence systems: A multicenter study. J. Clin. Lab. Anal. 2017, 31, e22117. [Google Scholar] [CrossRef] [PubMed]
- Polli, F.; D’Agostino, C.; Zumpano, R.; De Martino, V.; Favero, G.; Colangelo, L.; Minisola, S.; Mazzei, F. ASu@MNPs-based electrochemical immunosensor for vitamin D3 serum samples analysis. Talanta 2023, 1, 123755. [Google Scholar] [CrossRef]
- Palermiti, A.; Manca, A.; Mastrantonio, F.; Maiese, D.; Curatolo, A.; Antonucci, M.; Simiele, M.; De Nicolò, A.; D’Avolio, A. Comparative Performance Assessment of Novel Fluorescence Immunoassay POCTs for Measuring Circulating Levels of Vitamin-D. Molecules 2024, 5, 1636. [Google Scholar] [CrossRef]
- McLean, G.R.; Soyemi, S.; Ajayi, O.P.; Fernando, S.; Sowinski-Mydlarz, W.; Stewart, D.; Illingworth, S.; Atkins, M.; Bhakta, D. Comparative Analysis of a Rapid Quantitative Immunoassay to the Reference Methodology for the Measurement of Blood Vitamin D Levels. Methods Protoc. 2025, 1, 85. [Google Scholar] [CrossRef]
- Jalal, U.M.; Jin, G.J.; Shim, J.S. Paper-Plastic Hybrid Microfluidic Device for Smartphone-Based Colorimetric Analysis of Urine. Anal. Chem. 2017, 19, 13160–13166. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Lamb, K.V.; Wu, P.; Chang, P.; Cui, Y.; Wu, Y. Smartphone-Based Self-Empowerment App on Secondary Prevention of Patients with Cardiovascular Disease. Stud. Health Technol. Inform. 2019, 21, 1712–1713. [Google Scholar]
- Wongvibulsin, S.; Yan, M.J.; Pahalyants, V.; Murphy, W.; Daneshjou, R.; Rotemberg, V. Current State of Dermatology Mobile Applications With Artificial Intelligence Features. JAMA Dermatol. 2024, 1, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Rau, P.P. A Mobile Phone-Based Gait Assessment App for the Elderly: Development and Evaluation. JMIR Mhealth Uhealth 2020, 8, e14453. [Google Scholar] [CrossRef]
- Gruwez, H.; Verbrugge, F.H.; Proesmans, T.; Evens, S.; Vanacker, P.; Rutgers, M.P.; Vanhooren, G.; Bertrand, P.; Pison, L.; Haemers, P.; et al. Smartphone-based atrial fibrillation screening in the general population: Feasibility and impact on medical treatment. Eur. Heart J. Digit. Health 2023, 4, 464–472. [Google Scholar] [CrossRef]
- Liang, C.; Liu, Y.; Niu, A.; Liu, C.; Li, J.; Ning, D. Smartphone-app based point-of-care testing for myocardial infarction biomarker cTnI using an autonomous capillary microfluidic chip with self-aligned on-chip focusing (SOF) lenses. Lab. Chip. 2019, 19, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Stanford, J.B.; Warren, G.; Bond, S.; Bench-Capon, S.; Zinaman, M.J. Increased Likelihood of Pregnancy Using an App-Connected Ovulation Test System: A Randomized Controlled Trial. J. Womens Health 2020, 29, 84–90. [Google Scholar] [CrossRef]
- Ali, R.; Gürtin, Z.B.; Harpe, Z.C. Do fertility tracking applications offer women useful information about their fertile window? Reprod. Biomed. Online 2021, 42, 273–281. [Google Scholar] [CrossRef]
- Ji, Y.; Plourde, H.; Bouzo, V.; Kilgour, R.D.; Cohen, T.R. Validity and Usability of a Smartphone Image-Based Dietary Assessment App Compared to 3-Day Food Diaries in Assessing Dietary Intake Among Canadian Adults: Randomized Controlled Trial. JMIR Mhealth Uhealth 2020, 9, e16953. [Google Scholar] [CrossRef]
- Goodman, S.; Morrongiello, B.; Randall Simpson, J.; Meckling, K. Vitamin D intake among young Canadian adults: Validation of a mobile vitamin D calculator app. J. Nutr. Educ. Behav. 2015, 47, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Vemulapati, S.; Rey, E.; O’Dell, D.; Mehta, S.; Erickson, D. A Quantitative Point-of-Need Assay for the Assessment of Vitamin D(3) Deficiency. Sci. Rep. 2017, 26, 14142. [Google Scholar]
- Shim, H.W.; Shin, J.H.; Shin, S.C.; Lee, H.J.; So, K.S.; Lee, S.Y.; Jun, J.W.; Seo, J.K.; Lee, H.S.; Lee, S.Y.; et al. Analysis of Factors Affecting Neutralizing Antibody Production after COVID-19 Vaccination Using Newly Developed Rapid Point-of-Care Test. Diagnostics 2022, 9, 1924. [Google Scholar]
- Lee, S.; Kim, S.; Yoon, D.S.; Park, J.S.; Woo, H.; Lee, D.; Cho, S.Y.; Park, C.; Yoo, Y.K.; Lee, K.B.; et al. Sample-to-answer platform for the clinical evaluation of COVID-19 using a deep learning-assisted smartphone-based assay. Nat. Commun. 2023, 24, 2361. [Google Scholar]
- Lee, S.; O’Dell, D.; Hohenstein, J.; Colt, S.; Mehta, S.; Erickson, D. NutriPhone: A mobile platform for low-cost point-of-care quantification of vitamin B12 concentrations. Sci. Rep. 2016, 15, 28237. [Google Scholar]
- Albrecht, K.; Lotz, J.; Frommer, L.; Lackner, K.J.; Kahaly, G.J. A rapid point-of-care assay accurately measures vitamin D. J. Endocrinol. Invest. 2021, 44, 2485–2492. [Google Scholar] [PubMed]
- Liang, Z.Y.; Deng, Y.Q.; Tao, Z.Z. A quantum dot-based lateral flow immunoassay for the rapid, quantitative, and sensitive detection of specific IgE for mite allergens in sera from patients with allergic rhinitis. Anal. Bioanal. Chem. 2020, 412, 1785–1794. [Google Scholar]
- Pedreira-Rincón, J.; Rivas, L.; Comenge, J.; Skouridou, V.; Camprubí-Ferrer, D.; Muñoz, J.; O’Sullivan, C.K.; Chamorro-Garcia, A.; Parolo, C. A comprehensive review of competitive lateral flow assays over the past decade. Lab. Chip. 2025, 28, 2578–2608. [Google Scholar]
- Park, J. Smartphone based lateral flow immunoassay quantifications. J. Immunol. Methods 2024, 533, 113745. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.M.; Tomitaka, A. Machine Learning-Based Quantification of Lateral Flow Assay Using Smartphone-Captured Images. Biosensors 2025, 4, 19. [Google Scholar] [CrossRef]
- Papadopoulos, M.; Kokkinis, A.; Lamprou, E.; Kalligosfyri, P.M.; Koustoumpardis, P.N.; Kalogianni, D.P. Smartphone-Powered Automated Image Recognition Tool for Multianalyte Rapid Tests: Application to Infectious Diseases. Anal. Chem. 2025, 97, 13340–13349. [Google Scholar] [CrossRef]
- Brosamer, K.; Kourentzi, K.; Willson, R.C.; Vu, B.V. Glowstick-inspired smartphone-readable reporters for sensitive, multiplexed lateral flow immunoassays. Commun. Eng. 2023, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Du, K.; Lin, S.; Wang, Y. Deep learning on lateral flow immunoassay for the analysis of detection data. Front. Comput. Neurosci. 2023, 26, 1091180. [Google Scholar] [CrossRef] [PubMed]
| Experiment | Results | Substance | Lot 1 | Total | Accordance Rate | ||
|---|---|---|---|---|---|---|---|
| Repeatability | |||||||
| Within laboratory precision | No. of replicate/ App (Level/Result) | VITDSC-N | 25/25 (1/Deficiency) | 25/25 (1/Deficiency) | 100% | ||
| VITDSC-L | 25/25 (1/Deficiency) | 25/25 (1/Deficiency) | 100% | ||||
| VITDSC-M | 25/25 (2/Insufficient) | 25/25 (2/Insufficient) | 100% | ||||
| VITDSC-H | 25/25 (3/Sufficient) | 25/25 (3/Sufficient) | 100% | ||||
| TOTAL | 100/100 | 100/100 | 100% | ||||
| Reproducibility | |||||||
| Experiment | Results | Substance | Lot 1 | Lot 2 | Lot 3 | Total | Accordance Rate |
| Between lot precision | No. of replicate/ App (Level/Result) | VITDSC-N | 25/25 (1/Deficiency) | 25/25 (1/Deficiency) | 25/25 (1/Deficiency) | 75/75 (1/Deficiency) | 100% |
| VITDSC-L | 25/25 (1/Deficiency) | 25/25 (1/Deficiency) | 25/25 (1/Deficiency) | 75/75 (1/Deficiency) | 100% | ||
| VITDSC-M | 25/25 (2/Insufficient) | 25/25 (2/Insufficient) | 25/25 (2/Insufficient) | 75/75 (2/Insufficient) | 100% | ||
| VITDSC-H | 25/25 (3/Sufficient) | 25/25 (3/Sufficient) | 25/25 (3/Sufficient) | 75/75 (3/Sufficient) | 100% | ||
| TOTAL | 100/100 | 100/100 | 100/100 | 300/300 | 100% | ||
| Experiment | Results | Substance | Lot 1 | Total | Accordance Rate | ||
| Operator 1 | Operator 2 | Operator 3 | |||||
| Between-operator precision | No. of replicate/ App (Level/Result) | VITDSC-N | 25/25 (1/Deficiency) | 25/25 (1/Deficiency) | 25/25 (1/Deficiency) | 75/75 (1/Deficiency) | 100% |
| VITDSC-L | 25/25 (1/Deficiency) | 25/25 (1/Deficiency) | 25/25 (1/Deficiency) | 75/75 (1/Deficiency) | 100% | ||
| VITDSC-M | 25/25 (2/Insufficient) | 25/25 (2/Insufficient) | 25/25 (2/Insufficient) | 75/75 (2/Insufficient) | 100% | ||
| VITDSC-H | 25/25 (3/Sufficient) | 25/25 (3/Sufficient) | 25/25 (3/Sufficient) | 75/75 (3/Sufficient) | 100% | ||
| TOTAL | 100/100 | 100/100 | 100/100 | 300/300 | 100% | ||
| Experiment | Results | Substance | LOT1 | Total | Accordance Rate | ||
| Lab 1 | Lab 2 | Lab 3 | |||||
| Between laboratories precision | No. of replicate/ App (Level/Result) | VITDSC-N | 25/25 (1/Deficiency) | 25/25 (1/Deficiency) | 25/25 (1/Deficiency) | 75/75 (1/Deficiency) | 100% |
| VITDSC-L | 25/25 (1/Deficiency) | 25/25 (1/Deficiency) | 25/25 (1/Deficiency) | 75/75 (1/Deficiency) | 100% | ||
| VITDSC-M | 25/25 (2/Insufficient) | 25/25 (2/Insufficient) | 25/25 (2/Insufficient) | 75/75 (2/Insufficient) | 100% | ||
| VITDSC-H | 25/25 (3/Sufficient) | 25/25 (3/Sufficient) | 25/25 (3/Sufficient) | 75/75 (3/Sufficient) | 100% | ||
| TOTAL | 100/100 | 100/100 | 100/100 | 300/300 | 100% | ||
| Concentration(ng/mL) | App Level | No of Samples | Sex | |
|---|---|---|---|---|
| Female | Male | |||
| <10 | 1 | 2 | 2 | 0 |
| 10.0–19.9 | 1 | 13 | 7 | 6 |
| 20.0–29.9 | 2 | 39 | 29 | 10 |
| 30.0–40.9 | 3 | 9 | 5 | 4 |
| 41.0–60.9 | 3 | 24 | 23 | 1 |
| 61.0–79.9 | 3 | 11 | 9 | 2 |
| >80.0 | 3 | 2 | 2 | 0 |
| Total | 100 | 77 | 23 | |
| AOS | Atellica IM 1600 Analyzer (Atellica IM Vitamin D Total (VitD) | Total | |||
| Vita-D Rapid Kit | Result | 0~<20 ng/mL (Level 1) | 20~30 ng/mL (Level 2) | >30~100 ng/mL (Level 3) | |
| 0~<20 ng/mL (Level 1) | 14 | 1 | 0 | 15 | |
| 20~30 ng/mL (Level 2) | 1 | 38 | 1 | 40 | |
| >30~100 ng/mL (Level 3) | 0 | 0 | 45 | 45 | |
| Total | 15 | 39 | 46 | 100 | |
| Agreement rate (95% CI, %) | 93.3% (70.2–98.8%) | 97.4% (86.8–99.6%) | 97.8% (88.7–99.6%) | 97.0% (93.0–99.1%) | |
| iOS | Atellica IM 1600 Analyzer (Atellica IM Vitamin D Total (VitD) | Total | |||
| Vita-D Rapid Kit | Result | 0~<20 ng/mL (Level 1) | 20~30 ng/mL (Level 2) | >30~100 ng/mL (Level 3) | |
| 0~<20 ng/mL (Level 1) | 14 | 1 | 0 | 15 | |
| 20~30 ng/mL (Level 2) | 1 | 38 | 1 | 40 | |
| >30~100 ng/mL (Level 3) | 0 | 0 | 45 | 45 | |
| Total | 15 | 39 | 46 | 100 | |
| Agreement rate (95% CI, %) | 93.3% (70.2–98.8%) | 97.4% (86.8–99.6%) | 97.8% (88.7–99.6%) | 97.0% (93.0–99.1%) | |
| Concentration (ng/mL) | App Level | No of Samples | Sex | |
|---|---|---|---|---|
| Female | Male | |||
| <10 | 1 | 1 | 1 | 0 |
| 10.0–19.9 | 1 | 11 | 9 | 2 |
| 20.0–30.0 | 2 | 7 | 4 | 3 |
| 30.1–39.9 | 3 | 2 | 2 | 0 |
| >40.0 | 3 | 1 | 1 | 0 |
| Total | 22 | 17 | 5 | |
| AOS | Atellica IM 1600 Analyzer (Atellica IM Vitamin D Total (VitD)/(Serum) | Total | |||
| Vita-D Rapid Kit / (Capillary blood) | Result | 0~<20 ng/mL (Level 1) | 20~30 ng/mL (Level 2) | >30~100 ng/mL (Level 3) | |
| 0~<20 ng/mL (Level 1) | 12 | 1 | 0 | 13 | |
| 20~30 ng/mL (Level 2) | 0 | 6 | 0 | 6 | |
| >30~100 ng/mL (Level 3) | 0 | 0 | 3 | 3 | |
| Total | 12 | 7 | 3 | 22 | |
| Agreement rate (95% CI, %) | 100% (75.8–100%) | 86% (48.7–97.4%) | 100% (43.9–100%) | 95.5% (78.2–99.1%) | |
| iOS | Atellica IM 1600 Analyzer (Atellica IM Vitamin D Total (VitD)/(Serum) | Total | |||
| Vita-D Rapid Kit / (Capillary blood) | Result | 0~<20 ng/mL (Level 1) | 20~30 ng/mL (Level 2) | >30~100 ng/mL (Level 3) | |
| 0~<20 ng/mL (Level 1) | 12 | 1 | 0 | 13 | |
| 20~30 ng/mL (Level 2) | 0 | 6 | 0 | 6 | |
| >30~100 ng/mL (Level 3) | 0 | 0 | 3 | 3 | |
| Total | 12 | 7 | 3 | 22 | |
| Agreement rate (95% CI, %) | 100% (75.8–100%) | 86% (48.7–97.4%) | 100% (43.9–100%) | 95.5% (78.2–99.1%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Kim, S.H.; Kim, M.; Park, N.; Gu, J.; Kim, S.J.; Lee, S.Y.; Seo, J. Development and Evaluation of a Smartphone App-Based Rapid 25-Hydroxy Vitamin D Test. Diagnostics 2025, 15, 2916. https://doi.org/10.3390/diagnostics15222916
Han S, Kim SH, Kim M, Park N, Gu J, Kim SJ, Lee SY, Seo J. Development and Evaluation of a Smartphone App-Based Rapid 25-Hydroxy Vitamin D Test. Diagnostics. 2025; 15(22):2916. https://doi.org/10.3390/diagnostics15222916
Chicago/Turabian StyleHan, SoYeong, Seung Hyun Kim, MyungJin Kim, NaMi Park, Junnan Gu, Sun Jong Kim, Suk Yong Lee, and Jeongku Seo. 2025. "Development and Evaluation of a Smartphone App-Based Rapid 25-Hydroxy Vitamin D Test" Diagnostics 15, no. 22: 2916. https://doi.org/10.3390/diagnostics15222916
APA StyleHan, S., Kim, S. H., Kim, M., Park, N., Gu, J., Kim, S. J., Lee, S. Y., & Seo, J. (2025). Development and Evaluation of a Smartphone App-Based Rapid 25-Hydroxy Vitamin D Test. Diagnostics, 15(22), 2916. https://doi.org/10.3390/diagnostics15222916







