Association Between Osseous Shoulder Morphology and Pathoanatomical Characteristics of Calcific Deposits in Rotator Cuff Calcific Tendinitis
Abstract
1. Introduction
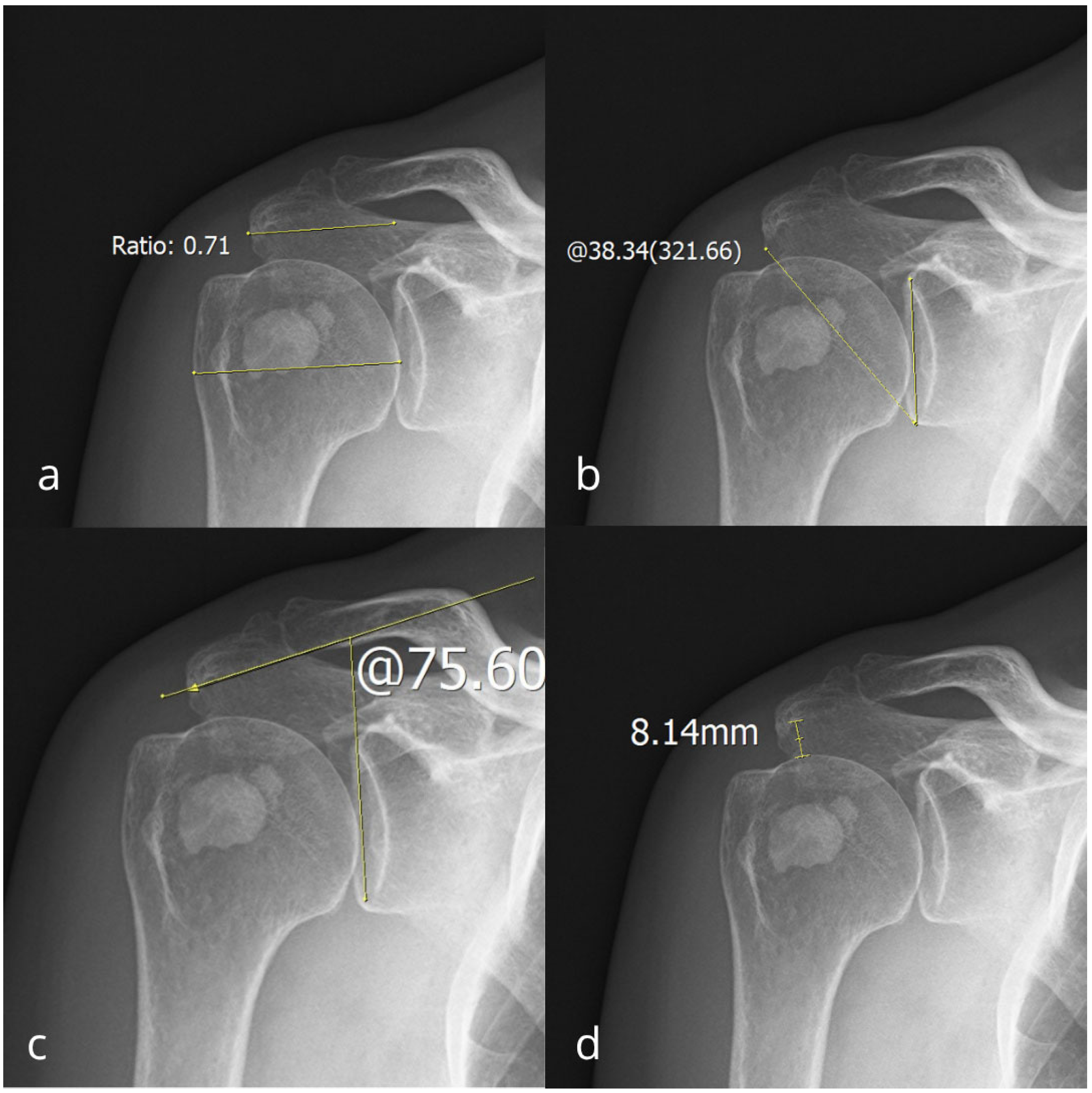
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clavert, P.; Sirveaux, F. Shoulder calcifying tendinitis. Rev. Chir. Orthop. Reparatrice Appar. Mot. 2008, 94, S336–S355. [Google Scholar] [CrossRef] [PubMed]
- Bechay, J.; Lawrence, C.; Namdari, S. Calcific tendinopathy of the rotator cuff: A review of operative versus nonoperative management. Phys. Sportsmed. 2020, 48, 241–246. [Google Scholar] [CrossRef]
- Sougué, C.; Darrieutort-Laffite, C.; Maugars, Y.; Le Goff, B. Location of calcifications of rotator cuff on ultrasound in 74 patients: Near the junction between the supraspinatus and infraspinatus tendons in 96% of the cases. Jt. Bone Spine 2021, 88, 105107. [Google Scholar] [CrossRef] [PubMed]
- Uhthoff, H.K.; Sarkar, K. Calcifying tendinitis. Baillieres Clin. Rheumatol. 1989, 3, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Lanza, E.; Banfi, G.; Serafini, G.; Lacelli, F.; Orlandi, D.; Bandirali, M.; Sardanelli, F.; Sconfienza, L.M. Ultrasound-guided percutaneous irrigation in rotator cuff calcific tendinopathy: What is the evidence? A systematic review with proposals for future reporting. Eur. Radiol. 2015, 25, 2176–2183. [Google Scholar] [CrossRef]
- Messina, C.; Sconfienza, L.M. Ultrasound-guided percutaneous irrigation of calcific tendinopathy. Semin. Musculoskelet. Radiol. 2016, 20, 409–413. [Google Scholar] [CrossRef]
- Louwerens, J.K.G.; Sierevelt, I.N.; van Hove, R.P.; van den Bekerom, M.P.J.; van Noort, A. Prevalence of calcific deposits within the rotator cuff tendons in adults with and without subacromial pain syndrome: Clinical and radiologic analysis of 1219 patients. J. Shoulder Elbow. Surg. 2015, 24, 1588–1593. [Google Scholar] [CrossRef]
- Oliva, F.; Via, A.G.; Maffulli, N. Calcific tendinopathy of the rotator cuff tendons. Sports Med. Arthrosc. Rev. 2011, 19, 237–243. [Google Scholar] [CrossRef]
- Sansone, V.; Maiorano, E.; Galluzzo, A.; Pascale, V. Calcific tendinopathy of the shoulder: Clinical perspectives on the mechanisms, pathogenesis, and treatment. Orthop. Res. Rev. 2018, 10, 63–72. [Google Scholar] [CrossRef]
- Sansone, V.C.; Meroni, R.; Boria, P.; Pisani, S.; Maiorano, E. Are occupational repetitive movements of the upper arm associated with rotator cuff calcific tendinopathies? Rheumatol. Int. 2015, 35, 273–280. [Google Scholar] [CrossRef]
- Codman, E.A. The Shoulder: Rupture of the Supraspinatus Tendon and Other Lesions in or About the Subacromial Bursa; Thomas Todd Co.: Boston, MA, USA, 1934. [Google Scholar]
- Boyle, S.; Smith, G.C.S. Calcific tendinitis. In Textbook of Shoulder Surgery; Trail, I.A., Rangan, A., Nixon, M., Eds.; Springer: Berlin, Germany, 2019; pp. 145–155. [Google Scholar]
- Uhthoff, H.K. Calcifying tendinitis, an active cell-mediated calcification. Virchows Arch. 1975, 366, 51–58. [Google Scholar] [CrossRef]
- Uhthoff, H.K.; Loehr, J.W. Calcific tendinopathy of the rotator cuff: Pathogenesis, diagnosis, and management. J. Am. Acad. Orthop. Surg. 1997, 5, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Oliva, F.; Via, A.G.; Maffulli, N. Physiopathology of intratendinous calcific deposition. BMC Med. 2012, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Catapano, M.; Robinson, D.M.; Schowalter, S.; McInnis, K.C. Clinical evaluation and management of calcific tendinopathy: An evidence-based review. J. Osteopath. Med. 2022, 122, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.H. Mechanobiological response of tendon stem cells: Implications of tendon homeostasis and pathogenesis of tendinopathy. J. Orthop. Res. 2010, 28, 639–643. [Google Scholar] [CrossRef]
- Balke, M.; Schmidt, C.; Dedy, N.; Banerjee, M.; Bouillon, B.; Liem, D. Correlation of acromial morphology with impingement syndrome and rotator cuff tears. Acta Orthop. 2013, 84, 178–183. [Google Scholar] [CrossRef]
- Bigliani, L.U.; Ticker, J.B.; Flatow, E.L.; Soslowsky, L.J.; Mow, V.C. The relationship of acromial architecture to rotator cuff disease. Clin. Sports Med. 1991, 10, 823–838. [Google Scholar] [CrossRef]
- Gill, T.J.; McIrvin, E.; Kocher, M.S.; Homa, K.; Mair, S.D.; Hawkins, R.J. The relative importance of acromial morphology and age with respect to rotator cuff pathology. J. Shoulder Elbow Surg. 2002, 11, 327–330. [Google Scholar] [CrossRef]
- Ma, Q.; Sun, C.; Du, R.; Liu, P.; Wu, S.; Zhang, W.; Fu, L.; Cai, X. Morphological characteristics of acromion and acromioclavicular joint in patients with shoulder impingement syndrome and related recommendations: A three-dimensional analysis based on multiplanar reconstruction of computed tomography scans. Orthop. Surg. 2021, 13, 1309–1318. [Google Scholar] [CrossRef]
- Nyffeler, R.W.; Werner, C.M.; Sukthankar, A.; Schmid, M.R.; Gerber, C. Association of a large lateral extension of the acromion with rotator cuff tears. J. Bone Jt. Surg. 2006, 88, 800–805. [Google Scholar] [CrossRef]
- Gärtner, J.; Heyer, A. Calcific tendinitis of the shoulder. Der Orthop. 1995, 24, 284–302. [Google Scholar]
- Bosworth, B. Calcium deposits in the shoulder and subacromial bursitis: A survey of 12,122 shoulders. JAMA 1941, 116, 2477–2482. [Google Scholar] [CrossRef]
- Suter, T.; Gerber Popp, A.; Zhang, Y.; Zhang, C.; Gerber, C.; Jost, B. The influence of radiographic projection on the critical shoulder angle: A comparative study of radiographs and 3D-CT scans. J. Shoulder Elbow Surg. 2015, 24, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Spiegl, U.J.; Horan, M.P.; Smith, S.W.; Millett, P.J. The critical shoulder angle is associated with rotator cuff tears and shoulder osteoarthritis and is affected by radiographic projection. Clin. Orthop. Relat. Res. 2016, 474, 2452–2461. [Google Scholar] [CrossRef]
- Nutton, R.W.; Stothard, J. Acute calcific supraspinatus tendinitis in a three-year-old child. J. Bone Jt. Surg. Br. 1987, 69, 148. [Google Scholar] [CrossRef]
- Choi, E.S.; Park, K.J.; Kim, Y.M.; Kim, D.S.; Shon, H.; Park, J.K. Calcific tendinitis of the supraspinatus tendon in a 7-year-old girl—A case report. J. Kor. Orthop. Assoc. 2007, 42, 400–403. [Google Scholar] [CrossRef]
- Fong, C.M. Calcific tendinitis of the supraspinatus tendon in a 7-year-old boy: Diagnostic challenges. Hong Kong Med. J. 2011, 17, 414–416. [Google Scholar]
- Wako, M.; Ichikawa, J.; Koyama, K.; Takayama, Y.; Haro, H. Calcific tendinitis of the supraspinatus tendon in an infant. Case Rep. Orthop. 2020, 2020, 9842489. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, I.W.; Lee, S.; Shin, S.J. Diagnosis and treatment of calcific tendinitis of the shoulder. Clin. Shoulder Elbow. 2020, 23, 210. [Google Scholar] [CrossRef]
- Titchener, A.G.; White, J.J.; Hinchliffe, S.R.; Tambe, A.A.; Hubbard, R.B.; Clark, D.I. Comorbidities in rotator cuff disease: A case-control study. J. Shoulder Elbow Surg. 2014, 23, 1282–1288. [Google Scholar] [CrossRef]
- De Carli, A.; Pulcinelli, F.; Delle Rose, G.; Pitino, D.; Ferretti, A. Calcific tendinitis of the shoulder. Joints 2014, 2, 130–136. [Google Scholar] [CrossRef] [PubMed]
- de Witte, P.B.; van Adrichem, R.A.; Selten, J.W.; Nagels, J.; Reijnierse, M.; Nelissen, R.G. Radiological and clinical predictors of long-term outcome in rotator cuff calcific tendinitis. Eur. Radiol. 2016, 26, 3401–3411. [Google Scholar] [CrossRef] [PubMed]
- Amroodi, M.N.; Kordkandi, S.A.; Moghtadaei, M.; Farahini, H.; Amiri, S.; Hajializade, M. A study of characteristic features and diagnostic roles of X-ray and MRI in calcifying tendinitis of the shoulder. Med. J. Islam. Repub. Iran. 2022, 36, 79. [Google Scholar] [CrossRef]
- Le Goff, B.; Berthelot, J.M.; Guillot, P.; Glémarec, J.; Maugars, Y. Assessment of calcific tendonitis of rotator cuff by ultrasonography: Comparison between symptomatic and asymptomatic shoulders. Jt. Bone Spine 2010, 77, 258–263. [Google Scholar] [CrossRef]
- Compagnoni, R.; Menon, A.; Radaelli, S.; Lanzani, F.; Gallazzi, M.B.; Tassi, A.; Randelli, P.S. Long-term evolution of calcific tendinitis of the rotator cuff: Clinical and radiological evaluation 10 years after diagnosis. J. Orthop. Traumatol. 2021, 22, 42. [Google Scholar] [CrossRef]
- Del Castillo-González, F.; Ramos-Álvarez, J.J.; Rodríguez-Fabián, G.; González-Pérez, J.; Calderón-Montero, J. Treatment of the calcific tendinopathy of the rotator cuff by ultrasound-guided percutaneous needle lavage. Two years prospective study. Muscles Ligaments Tendons J. 2015, 4, 407–412. [Google Scholar] [CrossRef]
- Hurt, G.; Baker, C.L., Jr. Calcific tendinitis of the shoulder. Orthop. Clin. N. Am. 2003, 34, 567–575. [Google Scholar] [CrossRef]
- Birsel, O.; Çalışkan, E.; Eren, İ.; Yürük, B.; Özben, H.; Demirhan, M. The impact of acromial morphology on the localization and size of calcific tendonitis in the rotator cuff. Acta Orthop. Traumatol. Turc. 2024, 58, 263–268. [Google Scholar] [CrossRef]
- Gerber, C.; Snedeker, J.G.; Baumgartner, D.; Viehöfer, A.F. Supraspinatus tendon load during abduction is dependent on the size of the critical shoulder angle: A biomechanical analysis. J. Orthop. Res. 2014, 32, 952–957. [Google Scholar] [CrossRef]
- Moor, B.K.; Bouaicha, S.; Rothenfluh, D.A.; Sukthankar, A.; Gerber, C. Is there an association between the individual anatomy of the scapula and the development of rotator cuff tears? J. Bone Jt. Surg. Br. 2013, 95, 935–941. [Google Scholar] [CrossRef]
- Ludewig, P.M.; Braman, J.P. Shoulder impingement: Biomechanical considerations in rehabilitation. Man. Ther. 2011, 16, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Prietzel, A.; Languth, T.; Bülow, R.; Ittermann, T.; Laqua, R.; Haralambiev, L.; Wassilew, G.I.; Ekkernkamp, A.; Bakir, M.S. Establishing Normative Values for Acromion Anatomy: A Comprehensive MRI-Based Study in a Healthy Population of 996 Participants. Diagnostics. 2024, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Balke, M.; Banerjee, M.; Vogler, T.; Akoto, R.; Bouillon, B.; Liem, D. Acromial morphology in patients with calcific tendinitis of the shoulder. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Kircher, J.; Morhard, M.; Patzer, T.; Magosch, P.; Lichtenberg, S.; Habermeyer, P. Do anatomical variants of the acromion shape in the frontal plane influence pain and function in calcifying tendinitis of the shoulder? Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 368–372. [Google Scholar] [CrossRef]
- Heuberer, P.R.; Plachel, F.; Willinger, L.; Moroder, P.; Laky, B.; Pauzenberger, L.; Lomoschitz, F.; Anderl, W. Critical shoulder angle combined with age predicts five shoulder pathologies: A retrospective analysis of 1000 cases. BMC Musculoskelet. Disord. 2017, 18, 259–268. [Google Scholar] [CrossRef]
- Hsu, T.-H.; Lin, C.-L.; Wu, C.-W.; Chen, Y.-W.; Vitoonpong, T.; Lin, L.-C.; Huang, S.-W. Accuracy of Critical Shoulder Angle and Acromial Index for Predicting Supraspinatus Tendinopathy. Diagnostics 2022, 12, 283. [Google Scholar] [CrossRef]
- Yaka, H.; Özer, M.; Kanatli, U. A New Predictive Parameter for Rotator Cuff Tears: Acromial Incidence Angle. Orthop. J. Sports Med. 2025, 13, 4. [Google Scholar] [CrossRef]
- Moor, B.K.; Röthlisberger, M.; Müller, D.; Zumstein, M.A.; Bouaicha, S.; Ehlinger, M.; Gerber, C. Age, trauma, and the critical shoulder angle accurately predict supraspinatus tendon tears. Orthop. Traumatol. Surg. Res. 2014, 100, 489–494. [Google Scholar] [CrossRef]
- Ricci, V.; Mezian, K.; Chang, K.-V.; Özçakar, L. Clinical/Sonographic Assessment and Management of Calcific Tendinopathy of the Shoulder: A Narrative Review. Diagnostics. 2022, 12, 3097. [Google Scholar] [CrossRef]
- Brolin, T.J.; Updegrove, G.F.; Horneff, J.G. Classifications in Brief: Hamada Classification of Massive Rotator Cuff Tears. Clin. Orthop. Relat. Res. 2017, 475, 2819–2823. [Google Scholar] [CrossRef]
- Venkat, V.; Sakalecha, A.K.; Kale, R.M.; Reddy, H.; Sakalecha, A.K. Evaluation of Acromiohumeral Interval on Chest Radiographs in Predicting Rotator Cuff Pathology: A Cross-Sectional Study. Cureus 2025, 17, e91317. [Google Scholar] [CrossRef]


| 95% Confidence Interval | Shapiro–Wilk | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Lower | Upper | Median | SD | Min | Max | W | p | |
| CSA | 35.11 | 34.80 | 35.41 | 34.80 | 4.71 | 23.69 | 48.65 | 0.91 | <0.001 |
| LAA | 92.29 | 91.68 | 92.90 | 93.24 | 9.31 | 66.25 | 118.52 | 0.99 | <0.001 |
| AI | 0.72 | 0.71 | 0.72 | 0.72 | 0.08 | 0.48 | 1.20 | 0.99 | <0.001 |
| AHI | 8.56 | 8.41 | 8.71 | 8.00 | 2.33 | 2.00 | 18.00 | 0.98 | <0.001 |
| Deposit Size | CSA | LAA | AI | AHI | Bosworth | Gartner | |
|---|---|---|---|---|---|---|---|
| Deposit size | — | ||||||
| CSA | −0.08 * | — | |||||
| CI lower | −0.14 | ||||||
| upper | −0.02 | ||||||
| LAA | 0.01 | −0.32 *** | — | ||||
| CI lower | −0.06 | −0.38 | |||||
| upper | 0.08 | −0.28 | |||||
| AI | −0.05 | 0.55 *** | 0.12 *** | — | |||
| CI lower | −0.12 | 0.50 | 0.06 | ||||
| upper | 0.02 | 0.59 | 0.18 | ||||
| AHI | 0.12 *** | −0.50 *** | 0.20 *** | 0.11 *** | — | ||
| CI lower | 0.06 | −0.55 | 0.14 | 0.05 | |||
| upper | 0.18 | −0.45 | 0.26 | 0.17 | |||
| Bosworth | 0.90 *** | −0.08 * | 0.01 | −0.08 * | 0.12 *** | — | |
| CI lower | 0.89 | −0.14 | −0.06 | −0.14 | 0.08 | ||
| upper | 0.91 | −0.02 | 0.08 | −0.02 | 0.18 | ||
| Gartner | 0.10 * | 0.00 | 0.05 | 0.09 ** | −0.00 | 0.09 ** | — |
| CI lower | 0.04 | −0.07 | −0.02 | 0.03 | −0.07 | 0.03 | |
| upper | 0.16 | 0.07 | 0.12 | 0.15 | 0.07 | 0.15 |
| χ2 | df | p | ε2 | |
|---|---|---|---|---|
| CSA | 0.86 | 2 | 0.652 | 9.49 × 10−4 |
| LAA | 8.15 | 2 | 0.017 | 0.00906 |
| AI | 7.68 | 2 | 0.021 | 0.00851 |
| AHI | 3.90 | 2 | 0.142 | 0.00433 |
| Gartner | CSA | LAA | AI | AHI | |||||
|---|---|---|---|---|---|---|---|---|---|
| W | p | W | p | W | p | W | p | ||
| 1 | 2 | −1.10 | 0.752 | 4.02 | 0.012 | 0.88 | 0.807 | 2.36 | 0.218 |
| 1 | 3 | 0.12 | 0.996 | 1.94 | 0.354 | 3.71 | 0.024 | −0.20 | 0.990 |
| 2 | 3 | 1.20 | 0.672 | −1.96 | 0.347 | 3.04 | 0.081 | −2.40 | 0.206 |
| χ2 | df | p | ε2 | |
|---|---|---|---|---|
| CSA | 5.62 | 2 | 0.060 | 0.00624 |
| LAA | 0.44 | 2 | 0.801 | 4.93 × 10−4 |
| AI | 8.77 | 2 | 0.012 | 0.00972 |
| AHI | 12.54 | 2 | 0.002 | 0.01391 |
| Bosworth | CSA | LAA | AI | AHI | |||||
|---|---|---|---|---|---|---|---|---|---|
| W | p | W | p | W | p | W | p | ||
| 1 | 2 | −1.24 | 0.655 | −0.43 | 0.950 | 0.60 | 0.905 | 2.36 | 0.217 |
| 1 | 3 | −3.05 | 0.079 | 0.35 | 0.967 | −2.62 | 0.153 | 4.58 | 0.003 |
| 2 | 3 | −2.57 | 0.164 | 0.91 | 0.795 | −4.06 | 0.011 | 3.59 | 0.030 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matković, A.; Grgić, M.; Trkulja, I.; Ivković, M.; Ferenc, T.; Jurjević, N.; Šebečić, B.; Vidjak, V. Association Between Osseous Shoulder Morphology and Pathoanatomical Characteristics of Calcific Deposits in Rotator Cuff Calcific Tendinitis. Diagnostics 2025, 15, 2908. https://doi.org/10.3390/diagnostics15222908
Matković A, Grgić M, Trkulja I, Ivković M, Ferenc T, Jurjević N, Šebečić B, Vidjak V. Association Between Osseous Shoulder Morphology and Pathoanatomical Characteristics of Calcific Deposits in Rotator Cuff Calcific Tendinitis. Diagnostics. 2025; 15(22):2908. https://doi.org/10.3390/diagnostics15222908
Chicago/Turabian StyleMatković, Andro, Mia Grgić, Ines Trkulja, Marija Ivković, Thomas Ferenc, Nikolina Jurjević, Božidar Šebečić, and Vinko Vidjak. 2025. "Association Between Osseous Shoulder Morphology and Pathoanatomical Characteristics of Calcific Deposits in Rotator Cuff Calcific Tendinitis" Diagnostics 15, no. 22: 2908. https://doi.org/10.3390/diagnostics15222908
APA StyleMatković, A., Grgić, M., Trkulja, I., Ivković, M., Ferenc, T., Jurjević, N., Šebečić, B., & Vidjak, V. (2025). Association Between Osseous Shoulder Morphology and Pathoanatomical Characteristics of Calcific Deposits in Rotator Cuff Calcific Tendinitis. Diagnostics, 15(22), 2908. https://doi.org/10.3390/diagnostics15222908






