Associations Between Th17 Cell Markers (IL-23R, CCR6, and IL-17) and Clinical Profiles in Sjögren’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject Recruitment
2.2. Antibody Quantification
2.3. Turbidimetry Assays
2.4. Blood Lipid Profile and ESR
2.5. Flow Cytometry
2.6. Multiplex Assay
2.7. Statistical Analysis
3. Results
3.1. Clinical and Demographic Characteristics
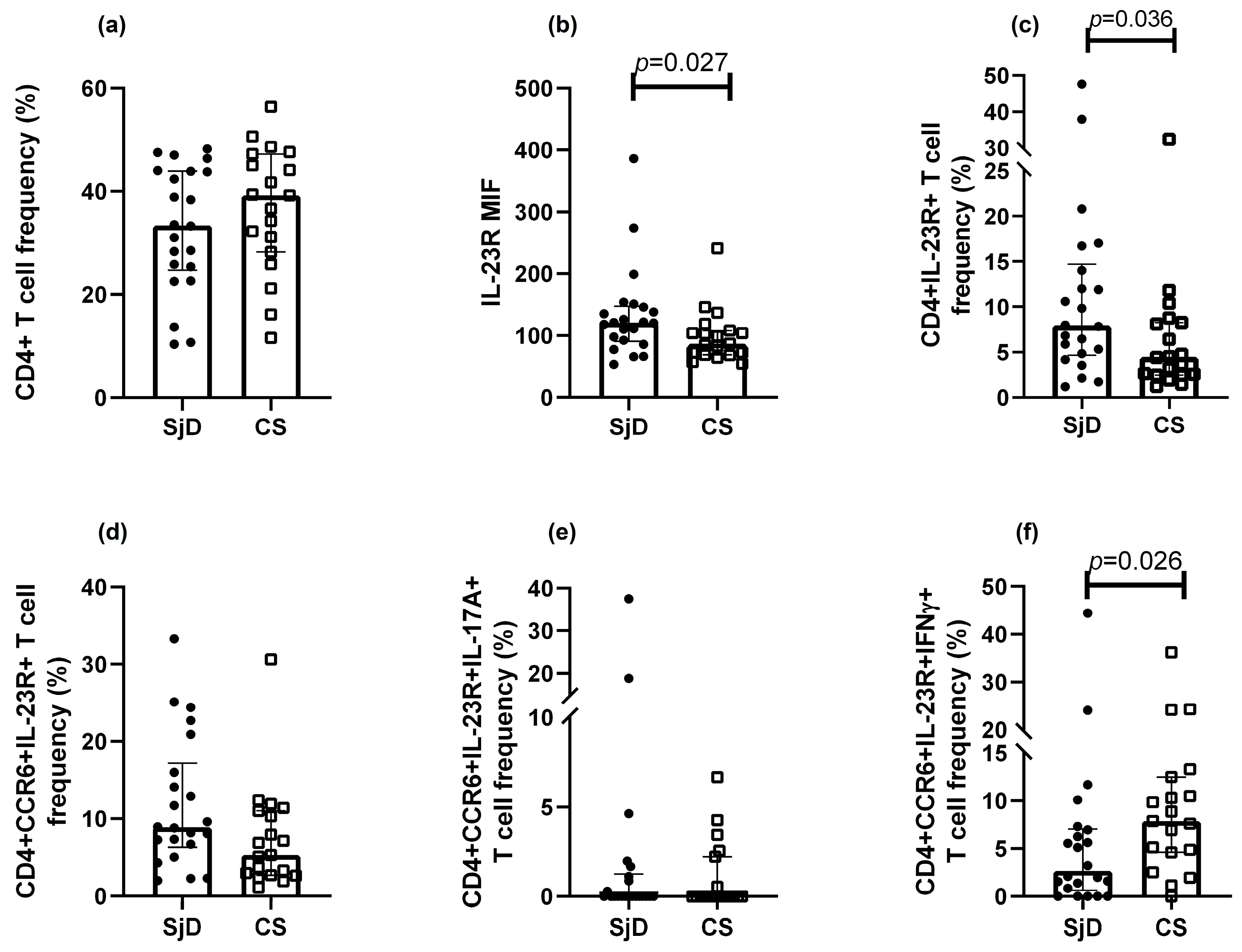
3.2. Immunophenotyping of Th17 Cells
3.3. Th17 Population Frequencies
3.4. Cytokine Levels
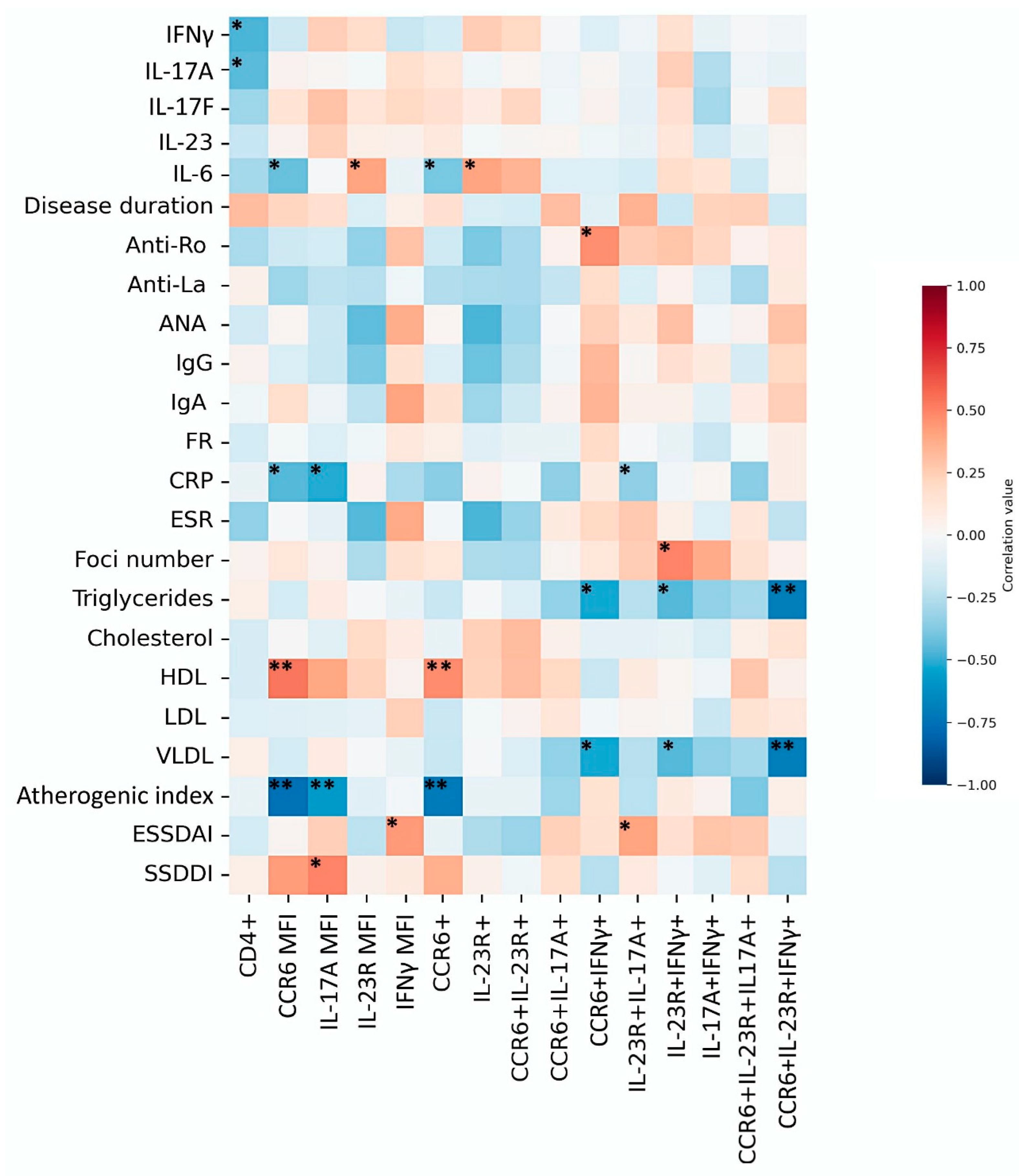
3.5. Comparison Between Th17 Populations and Clinical Parameters
3.6. Clinical Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANA | Antinuclear antibodies |
| CRP | C-reactive protein |
| CS | Control subjects |
| dL | Deciliter |
| ESR | Erythrocyte sedimentation rate |
| ESSDAI | EULAR Sjögren’s Syndrome Disease Activity Index |
| FAS | Fatty acid synthesis |
| GC | Germinal center |
| h | Hour |
| HDL | High-density lipoprotein |
| Ig | Immunoglobulin |
| IL-23R | Interleukin-23 receptor |
| L | Liter |
| LD | Limit of detection |
| LDL | Low-density lipoprotein |
| mg | Milligram |
| MIF | Median intensity fluorescence |
| mL | Milliliter |
| mm | Millimeter |
| PBMCs | Peripheral blood mononuclear cells |
| RF | Rheumatoid factor |
| RT | Room temperature |
| SGE | Salivary gland epithelium |
| SjD | Sjögren’s disease |
| SSDDI | Sjögren’s Syndrome Disease Damage Index |
| Th | T helper cell |
| U | Unit |
| VLDL | Very low-density lipoprotein |
References
- Ramos-Casals, M.; Baer, A.N.; del Pilar Brito-Zerón, M.; Hammitt, K.M.; Bouillot, C.; Retamozo, S.; Mackey, A.; Yarowsky, D.; Turner, B.; Blanck, J.; et al. 2023 International Rome consensus for the nomenclature of Sjögren disease. Nat. Rev. Rheumatol. 2023, 21, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Brito-Zerón, P.; Retamozo, S.; Ramos-Casals, M. Sjögren syndrome. Med. Clín. 2023, 160, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Ma, J.; Cui, J.; Gu, Y.; Shan, Y. Subpopulation dynamics of T and B lymphocytes in Sjögren’s syndrome: Implications for disease activity and treatment. Front. Immunol. 2024, 15, 1468469. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar] [CrossRef]
- Verstappen, G.M.; Corneth, O.B.J.; Bootsma, H.; Kroese, F.G.M. Th17 cells in primary Sjögren’s syndrome: Pathogenicity and plasticity. J. Autoimmun. 2018, 87, 16–25. [Google Scholar] [CrossRef]
- Verstappen, G.M.; Meiners, P.M.; Corneth, O.B.J.; Visser, A.; Arends, S.; Abdulahad, W.H.; Hendriks, R.W.; Vissink, A.; Kroese, F.G.M.; Bootsma, H. Attenuation of Follicular Helper T Cell-Dependent B Cell Hyperactivity by Abatacept Treatment in Primary Sjögren’s Syndrome: Abatacept in Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2017, 69, 1850–1861. [Google Scholar] [CrossRef]
- Blokland, S.L.M.; Hillen, M.R.; Kruize, A.A.; Meller, S.; Homey, B.; Smithson, G.M.; Radstake, T.R.D.J.; van Roon, J.A.G. Increased CCL25 and T Helper Cells Expressing CCR9 in the Salivary Glands of Patients with Primary Sjögren’s Syndrome: Potential New Axis in Lymphoid Neogenesis. Arthritis Rheumatol. 2017, 6, 2038–2051. [Google Scholar] [CrossRef]
- Fei, Y.; Zhang, W.; Lin, D.; Wu, C.; Li, M.; Zhao, Y.; Zeng, X.; Zhang, F. Clinical parameter and Th17 related to lymphocytes infiltrating degree of labial salivary gland in primary Sjögren’s syndrome. Clin. Rheumatol. 2014, 33, 523–529. [Google Scholar] [CrossRef]
- Schinocca, C.; Rizzo, C.; Fasano, S.; Grasso, G.; La Barbera, L.; Ciccia, F.; Guggino, G. Role of the IL-23/IL-17 Pathway in Rheumatic Diseases: An Overview. Front. Immunol. 2021, 12, 637829. [Google Scholar] [CrossRef]
- Nalbant, A.; Eskier, D. Genes associated with T helper 17 cell differentiation and function. Front. Biosci. Elite 2016, 8, 427–435. [Google Scholar] [CrossRef]
- Aqrawi, L.A.; Ivanchenko, M.; Björk, A.; Sepúlveda, J.I.R.; Imgenberg-Kreuz, J.; Kvarnström, M.; Haselmayer, P.; Jensen, J.L.; Nordmark, G.; Chemin, K. Diminished CXCR5 expression in peripheral blood of patients with Sjögren’s syndrome may relate to both genotype and salivary gland homing: CXCR5 expression in SS. Clin. Exp. Immunol. 2018, 192, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, J.; Zhu, L.; Yang, Y.; Jin, Y.; Jia, R.; Liu, X.; Liu, Y.; Sun, X.; Li, Z. The Clinical Relevance of IL-17-Producing CD4+CD161+ Cell and Its Subpopulations in Primary Sjögren’s Syndrome. J. Immunol. Res. 2015, 2015, 307453. [Google Scholar] [CrossRef] [PubMed]
- Alunno, A.; Carubbi, F.; Bistoni, O.; Caterbi, S.; Bartoloni, E.; Di Benedetto, P.; Cipriani, P.; Giacomelli, R.; Gerli, R. Interleukin (IL)-17-producing pathogenic T lymphocytes co-express CD20 and are depleted by rituximab in primary Sjögren’s syndrome: A pilot study. Clin. Exp. Immunol. 2016, 184, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.S.; Kim, S.H.; Bunin, V.; Shin, M.S.; Lee, N.; Parke, A.; Kang, I. Altered Th17 and Th1 cells responses in patients with primary Sjogren syndrome (59.10). J. Immunol. 2012, 188 (Suppl. S1), 4. [Google Scholar] [CrossRef]
- Kleiner, J.C.; Krebs, C.F. Persistence, Pathogenicity and Plasticity: The Role of IL-23 in Th17 Fate. J. Cell. Immunol. 2022, 4, 121–130. [Google Scholar]
- Pawlak, M.; DeTomaso, D.; Schnell, A.; Zu Horste, G.M.; Lee, Y.; Nyman, J.; Dionne, D.; Regan, B.M.; Singh, V.; Delorey, T.; et al. Induction of a colitogenic phenotype in Th1-like cells depends on interleukin-23 receptor signaling. Immunity 2022, 55, 1663–1679.e6. [Google Scholar] [CrossRef]
- Paulissen, S.M.J.; van Hamburg, J.P.; Dankers, W.; Lubberts, E. The role and modulation of CCR6+ Th17 cell populations in rheumatoid arthritis. Cytokine 2015, 74, 43–53. [Google Scholar] [CrossRef]
- Katsifis, G.E.; Rekka, S.; Moutsopoulos, N.M.; Pillemer, S.; Wahl, S.M. Systemic and Local Interleukin-17 and Linked Cytokines Associated with Sjögren’s Syndrome Immunopathogenesis. Am. J. Pathol. 2009, 175, 1167–1177. [Google Scholar] [CrossRef]
- Mieliauskaite, D.; Dumalakiene, I.; Rugiene, R.; Mackiewicz, Z. Expression of IL-17, IL-23 and Their Receptors in Minor Salivary Glands of Patients with Primary Sjögren’s Syndrome. Clin. Dev. Immunol. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Nguyen, C.Q.; Hu, M.H.; Li, Y.; Stewart, C.; Peck, A.B. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjögren’s syndrome: Findings in humans and mice. Arthritis Rheum. 2008, 58, 734–743. [Google Scholar] [CrossRef]
- Alunno, A.; Caterbi, S.; Bistoni, O.; Bartoloni, E.; Santoboni, G.; Mirabelli, G.; Cannarile, F.; Valentini, V.; Terenzi, R.; Gerli, R. A3.4 CD3+CD4–CD8– Double Negative Th17 Cells: New Insights in the Pathogenesis of Primary Sjögren’s Syndrome. Ann. Rheum. Dis. 2013, 72, A14–A15. [Google Scholar] [CrossRef]
- Nanke, Y.; Kobashigawa, T.; Yago, T.; Kawamoto, M.; Yamanaka, H.; Kotake, S. Detection of IFN-γ+IL-17+ cells in salivary glands of patients with Sjögren’s syndrome and Mikulicz’s disease: Potential role of Th17•Th1 in the pathogenesis of autoimmune diseases. Nihon Rinsho Meneki Gakkai Kaishi. 2016, 39, 473–477. [Google Scholar] [CrossRef]
- Dohlman, T.H.; Chauhan, S.K.; Kodati, S.; Hua, J.; Chen, Y.; Omoto, M.; Sadrai, Z.; Dana, R. The CCR6/CCL20 Axis Mediates Th17 Cell Migration to the Ocular Surface in Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4081. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, C.S.; Hwang, C.S.; Pitcher, J.D.; Pangelinan, S.B.; Rahimy, E.; Chen, W.; Yoon, K.-C.; Farley, W.J.; Niederkorn, J.Y.; Stern, M.E.; et al. Age-related T-cell cytokine profile parallels corneal disease severity in Sjogren’s syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology 2010, 49, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, M.; Tsuboi, H.; Matsuo, N.; Asashima, H.; Hirota, T.; Kondo, Y.; Iwakura, Y.; Takahashi, S.; Matsumoto, I.; Sumida, T. A Crucial Role of RORγt in the Development of Spontaneous Sialadenitis-like Sjögren’s Syndrome. J. Immunol. 2015, 194, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wan, Y. Molecular control of pathogenic Th17 cells in autoimmune diseases. Int. Immunopharmacol. 2020, 80, 106187. [Google Scholar] [CrossRef]
- López-Villalobos, E.F.; Muñoz-Valle, J.F.; Palafox-Sánchez, C.A.; García-Arellano, S.; Martínez-Fernández, D.E.; Orozco-Barocio, G.; García-Espinoza, J.A.; Oregon-Romero, E. Cytokine profiles and clinical characteristics in primary Sjögren’s syndrome patient groups. Clin. Lab. Anal. 2021, 35, e23629. [Google Scholar] [CrossRef]
- Carrillo-Ballesteros, F.J.; Palafox-Sánchez, C.A.; Franco-Topete, R.A.; Muñoz-Valle, J.F.; Orozco-Barocio, G.; Martínez-Bonilla, G.E.; Gómez-López, C.E.; Marín-Rosales, M.; López-Villalobos, E.F.; Luquin, S.; et al. Expression of BAFF and BAFF receptors in primary Sjögren’s syndrome patients with ectopic germinal center-like structures. Clin. Exp. Med. 2020, 20, 615–626. [Google Scholar] [CrossRef]
- Mitsdoerffer, M.; Lee, Y.; Jäger, A.; Kim, H.J.; Korn, T.; Kolls, J.K.; Cantor, H.; Bettelli, E.; Kuchroo, V.K. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl. Acad. Sci. USA 2010, 107, 14292–14297. [Google Scholar] [CrossRef]
- Bystrom, J.; Taher, T.E.; Henson, S.M.; Gould, D.J.; Mageed, R.A. Metabolic requirements of Th17 cells and of B cells: Regulation and defects in health and in inflammatory diseases. Front Immunol. 2022, 13, 990794. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, T.; Zhang, P.; Zhou, S.; Chen, J.; Jiang, W.; Yu, S.; Xie, W.; Wang, J.; Xu, Z.; et al. The relationship between serum levels of cholesterol and disease activity in Sjogren’s syndrome: A retrospective study. Int. J. Clin. Exp. Med. 2019, 11, 13037. [Google Scholar]
- Qin, Y.; Gao, C.; Luo, J. Metabolism Characteristics of Th17 and Regulatory T Cells in Autoimmune Diseases. Front. Immunol. 2022, 13, 828191. [Google Scholar] [CrossRef]
- Feingold, K.R. Introduction to Lipids and Lipoproteins. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: http://www.ncbi.nlm.nih.gov/books/NBK305896 (accessed on 12 January 2025).
- Chen, Y.; Luo, X.; Deng, C.; Zhao, L.; Gao, H.; Zhou, J.; Peng, L.; Yang, H.; Li, M.; Zhang, W.; et al. Immunometabolic alteration of CD4+ T cells in the pathogenesis of primary Sjögren’s syndrome. Clin. Exp. Med. 2024, 24, 163. [Google Scholar] [CrossRef] [PubMed]
- Gruaz, L.; Delucinge-Vivier, C.; Descombes, P.; Dayer, J.M.; Burger, D. Blockade of T Cell Contact-Activation of Human Monocytes by High-Density Lipoproteins Reveals a New Pattern of Cytokine and Inflammatory Genes. PLoS ONE 2010, 5, e9418. [Google Scholar] [CrossRef]
- Tiniakou, I.; Drakos, E.; Sinatkas, V.; Van Eck, M.; Zannis, V.I.; Boumpas, D.; Verginis, P.; Kardassis, D. High-Density Lipoprotein Attenuates Th1 and Th17 Autoimmune Responses by Modulating Dendritic Cell Maturation and Function. J. Immunol. 2015, 194, 4676–4687. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.; Smith, A.J.; Blaszczak, A.; Shantaram, D.; Bergin, S.M.; Jalilvand, A.; Wright, V.; Wyne, K.L.; Dewal, R.S.; Baer, L.A.; et al. Interferon gamma mediates the reduction of adipose tissue regulatory T cells in human obesity. Nat. Commun. 2022, 13, 5606. [Google Scholar] [CrossRef]


| Variables | SjD Patients (n = 22) | Control Subjects (n = 19) |
|---|---|---|
| Demographics | ||
| Gender; Male/Female | 1/21 | 1/18 |
| Age, years; median (p25–p75) | 56 (49.5–66.2) | 55 (52–63) |
| Disease duration, years; median (p25–p75) | 3.0 (2.0–6.5) | - |
| Inflammatory markers | ||
| ESR, mm/H; median (p25–p75) | 33.0 (21.2–42.7) | 24.0 (13.0–33.0) |
| CRP, mg/L; median (p25–p75) | 11.7 (8.9–16.1) | 8.5 (2.4–12.8) |
| Autoantibodies | ||
| RF, U/mL; median (p25–p75) | 35.7 (8.3–89.3) | 1.32 (0.0–4.77) |
| Anti-Ro, U/mL; median (p25–p75); % | 2.2 (0.7–481.3); 41.7% | 0.37 (0.15–0.91) |
| Anti-La, U/mL; median (p25–p75); % | 2.9 (0.04–96.8); 29.2% | 0.40 (0.82–0.94) |
| ANA, titer; median (range) | 1:320 (1:160–1:1280) | - |
| Clinical parameters | ||
| IgG, mg/dL; median (p25–p75) | 1563.1 (1351.2–2358.6) | 1338.4 (1060.3–1557.1) |
| IgA, mg/dL; median (p25–p75) | 325.0 (261.7–449.5) | 279.0 (250.0–321.0) |
| Total cholesterol, mg/dL; median (p25–p75) | 211.5 (173.3–233.3) | 195.7 (180.1–233.0) |
| HDL, mg/dL; median (p25–p75) | 48.4 (31.1–60.3) | 48.4 (42.8–58.8) |
| LDL, mg/dL; median (p25–p75) | 67.8 (47.29–80.5) | 62.6 (50.1–78.7) |
| VLDL, mg/dL; median (p25–p75) | 22.0 (19.4–37.4) | 27.62 (19.2–35.0) |
| Triglycerides, mg/dL; median (p25–p75) | 110.4 (97.1–187.2) | 143.1 (111.5–182.2) |
| Atherogenic index; median (p25–p75) | 4.3 (3.6–5.3) | 4.3 (3.5–5.1) |
| Foci number ≥ 1 focus/4 mm2; median (p25–p75) | 2.0 (1.0–3.0) | - |
| SSDDI, score; median (p25–p75) | 2.0 (1.0–3.0) | - |
| ESSDAI, score; median (p25–p75) | 4.0 (2.0–5.7) | - |
| Treatment | ||
| Prednisone, % | 16.7 | - |
| Hydroxychloroquine, % | 83.3 | - |
| Methotrexate, % | 20.8 | - |
| Azathioprine, % | 37.5 | - |
| Mycophenolate mofetil, % | 8.3 | - |
| Leflunomide, % | 4.2 | - |
| Rituximab, % | 8.3 | - |
| Abatacept, % | 4.2 | - |
| Cyclophosphamide, % | 4.2 | - |
| Anti-Ro Positivity | |||
|---|---|---|---|
| Anti-Ro positive group (n = 9) | Anti-Ro negative group (n = 13) | p-value | |
| IL-23R, MIF; median (p25–p75) | 111.0 (7.75–121.5) | 138.0 (94.95–176.50) | 0.051 |
| IL-23R+ Th cells %, median (p25–p75) | 6.44 (2.94–8.90) | 12.0 (5.59–18.90) | 0.043 * |
| CCR6+IFN-γ+ Th cells %; median (p25–p75) | 6.090 (2.13–7.58) | 1.62 (1.06–3.40) | 0.082 |
| Anti-La positivity | |||
| Anti-La positive group (n = 6) | Anti-La negative group (n = 15) | p-value | |
| Cholesterol, mg/dL; median (p25–p75) | 169.07 (136.28–212.38) | 221.0 (204.81–236.83) | 0.036 * |
| LDL, mg/dL; median (p25–p75) | 54.77 (41.34–63.38) | 73.45 (58.03–92.30) | 0.036 * |
| Hypergammaglobulinemia positivity | |||
| High IgG levels group (n = 11) | Normal IgG levels group (n = 9) | p-value | |
| CCR6+IFN-γ+ Th cells %; median (p25–p75) | 4.02 (2.64–6.76) | 1.60 (1.06–6.12) | 0.057 |
| Triglycerides, mg/dL; median (p25–p75) | 103.37 (87.11–118.18) | 155.39 (109.97–216.79) | 0.036 * |
| VLDL, mg/dL; median (p25–p75) | 20.67 (17.42–23.64) | 31.08 (21.99–43.36) | 0.036 * |
| Rheumatoid factor positivity | |||
| RF positive group (n = 11) | RF negative group (n = 10) | p-value | |
| Cholesterol, mg/dL; median (p25–p75) | 204.81 (137.95–223.27) | 224.38 (209.80–242.18) | 0.043 |
| LDL; median (p25–p75) | 50.14 (39.54–70.77) | 75.82 (66.64–84.94) | 0.029 * |
| Hypertriglyceridemia | |||
| High Triglyceride levels group (n = 6) | Normal Triglyceride levels group (n = 15) | p-value | |
| CCR6+IL-23R+IFN-γ+ Th cells %; median (p25–p75) | 0.42 (0.0–2.58) | 5.13 (1.61–7.32) | 0.029 * |
| Abnormal HDL levels | |||
| Low HDL levels group (n = 6) | Normal HDL levels group (n = 15) | p-value | |
| CCR6, MIF; median (p25–p75) | 99.10 (92.17–176.50) | 286.00 (189.0–363.0) | 0.003 * |
| IL-17A, MIF; median (p25–p75) | 78.50 (54.55–103.42) | 105.00 (94.3–118.0) | 0.029 * |
| CCR6+ Th cells %; median (p25–p75) | 6.51 (5.55–14.57) | 28.40 (16.9–40.10) | 0.018 * |
| IL-17A+ Th cells %; median (p25–p75) | 0.00 (0.00–0.03) | 0.05 (0.00–0.27) | 0.018 * |
| IFN-γ+ Th cells %; median (p25–p75) | 1.48 (0.82–2.29) | 2.22 (1.19–5.45) | 0.080 |
| CCR6+IL-17A+ Th cells %; median (p25–p75) | 0.00 (0.00–0.01) | 0.14 (0.00–0.42) | 0.036 * |
| CCR6+IL-23R+IL-17A+ Th cells %; median (p25–p75) | 0.00 (0.00–0.00) | 0.28 (0.00–1.98) | 0.066 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Villalobos, E.F.; Garcia-Espinoza, J.A.; García-Chagollán, M.; Uribe-Martínez, J.F.; Cerpa-Cruz, S.; Muñoz-Valle, J.F.; Palafox-Sánchez, C.A.; Oregon-Romero, E. Associations Between Th17 Cell Markers (IL-23R, CCR6, and IL-17) and Clinical Profiles in Sjögren’s Disease. Diagnostics 2025, 15, 2909. https://doi.org/10.3390/diagnostics15222909
López-Villalobos EF, Garcia-Espinoza JA, García-Chagollán M, Uribe-Martínez JF, Cerpa-Cruz S, Muñoz-Valle JF, Palafox-Sánchez CA, Oregon-Romero E. Associations Between Th17 Cell Markers (IL-23R, CCR6, and IL-17) and Clinical Profiles in Sjögren’s Disease. Diagnostics. 2025; 15(22):2909. https://doi.org/10.3390/diagnostics15222909
Chicago/Turabian StyleLópez-Villalobos, Erika Fabiola, Jose Antonio Garcia-Espinoza, Mariel García-Chagollán, Jefte Felipe Uribe-Martínez, Sergio Cerpa-Cruz, José Francisco Muñoz-Valle, Claudia Azucena Palafox-Sánchez, and Edith Oregon-Romero. 2025. "Associations Between Th17 Cell Markers (IL-23R, CCR6, and IL-17) and Clinical Profiles in Sjögren’s Disease" Diagnostics 15, no. 22: 2909. https://doi.org/10.3390/diagnostics15222909
APA StyleLópez-Villalobos, E. F., Garcia-Espinoza, J. A., García-Chagollán, M., Uribe-Martínez, J. F., Cerpa-Cruz, S., Muñoz-Valle, J. F., Palafox-Sánchez, C. A., & Oregon-Romero, E. (2025). Associations Between Th17 Cell Markers (IL-23R, CCR6, and IL-17) and Clinical Profiles in Sjögren’s Disease. Diagnostics, 15(22), 2909. https://doi.org/10.3390/diagnostics15222909







