Autoimmune Metaplastic Atrophic Gastritis Reporting: Are Pathologists and Endoscopists on the Same Page?
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Biopsy Review
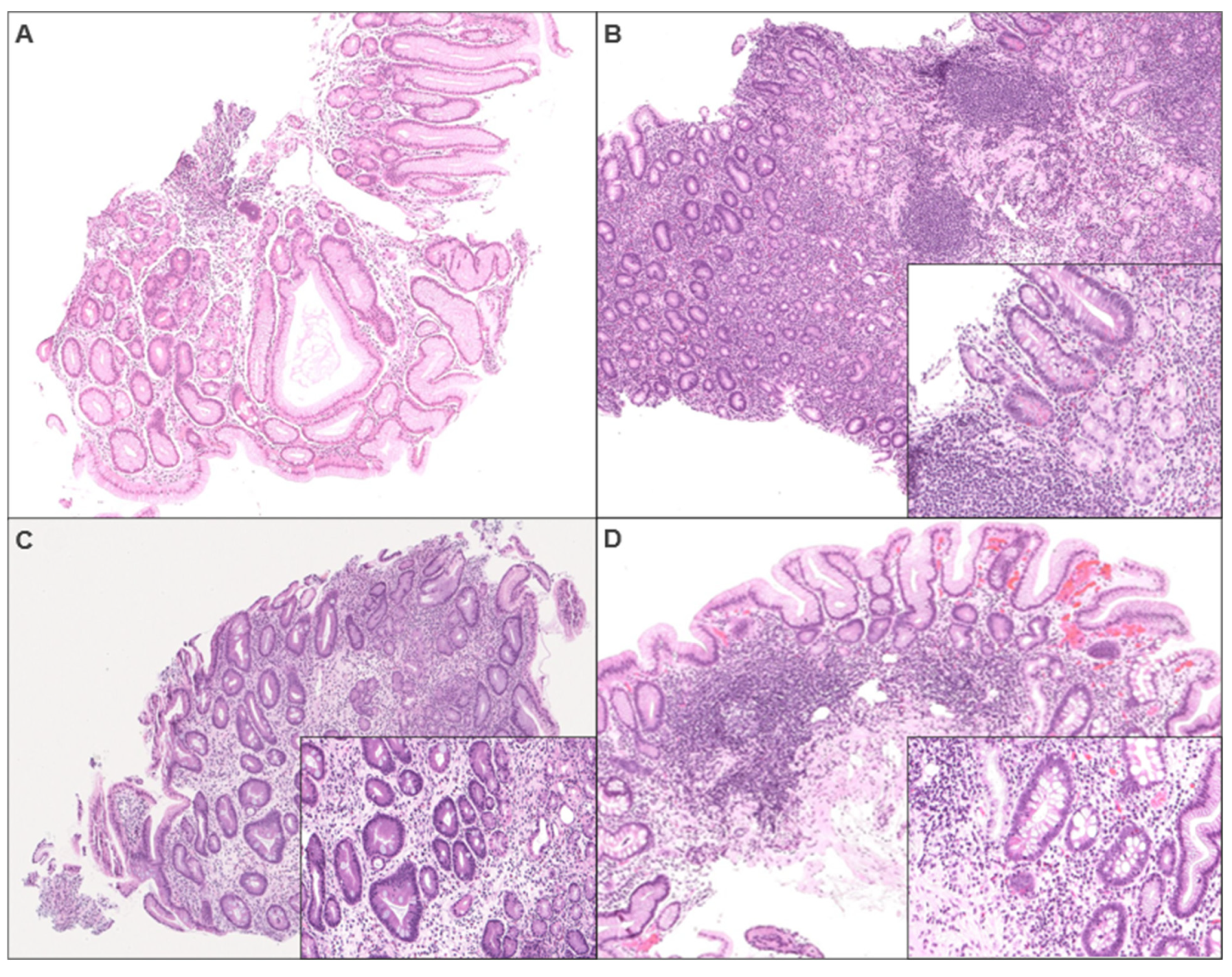
2.3. Histology Comment Score
2.4. Endoscopy Report Score
2.5. Chart Review
2.6. Statistical Analyses
3. Results
3.1. Cases
3.2. Indication for Endoscopy and Tissue Sampling
3.3. Biopsy Review
3.4. Histology Comment Scores and Their Correlation with Histology
3.5. Endoscopy Report Scores and Their Correlation with Histology
3.6. Histology Comment Score and Endoscopy Report Score vs. Tissue Sampling and Gender
3.7. Follow-Up
3.7.1. Follow-Up Laboratory Testing
3.7.2. Follow-Up Biopsy vs. Histology and Histology Comment Score
3.7.3. Follow-Up Biopsy vs. Endoscopy Report Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMAG | Autoimmune metaplastic atrophic gastritis |
| NET | Neuroendocrine tumor |
| ECL | Enterochromaffin-like |
| HCl | Hydrochloric acid |
| OLGA | Operative Link for Gastritis Assessment |
| US | United States |
| H&E | Hematoxylin and eosin |
| GI | Gastrointestinal |
| ANA | Antinuclear antibody |
| EGD | Esophagogastroduodenoscopy |
| ES1 | Initial endoscopy report score based on report terminology |
| ES2 | Subsequent endoscopy report score based on endoscopy images |
| AGA | American Gastroenterological Association |
| ENETS | European Neuroendocrine Tumor Society |
References
- Lenti, M.V.; Rugge, M.; Lahner, E.; Miceli, E.; Toh, B.H.; Genta, R.M.; De Block, C.; Hershko, C.; Di Sabatino, A. Autoimmune gastritis. Nat. Rev. Dis. Primers 2020, 6, 56. [Google Scholar] [CrossRef]
- Massironi, S.; Zilli, A.; Elvevi, A.; Invernizzi, P. The changing face of chronic autoimmune atrophic gastritis: An updated comprehensive perspective. Autoimmun. Rev. 2019, 18, 215–222. [Google Scholar] [CrossRef]
- Irvine, W.J.; Cullen, D.R.; Mawhinney, H. Natural history of autoimmune achlorhydric atrophic gastritis A 1–15-year follow-up study. Lancet 1974, 304, 482–485. [Google Scholar] [CrossRef]
- Miceli, E.; Lenti, M.V.; Padula, D.; Luinetti, O.; Vattiato, C.; Monti, C.M.; Di Stefano, M.; Corazza, G.R. Common features of patients with autoimmune atrophic gastritis. Clin. Gastroenterol. Hepatol. 2012, 10, 812–814. [Google Scholar] [CrossRef]
- Dilaghi, E.; Dottori, L.; Pivetta, G.; Dalla Bella, M.; Esposito, G.; Ligato, I.; Pilozzi, E.; Annibale, B.; Lahner, E. Incidence and predictors of gastric neoplastic lesions in corpus-restricted atrophic gastritis: A single-center cohort study. Am. J. Gastroenterol. 2023, 118, 2157–2165. [Google Scholar] [CrossRef]
- Mahmud, N.; Stashek, K.; Katona, B.W.; Tondon, R.; Shroff, S.G.; Roses, R.; Furth, E.E.; Metz, D.C. The incidence of neoplasia in patients with autoimmune metaplastic atrophic gastritis: A renewed call for surveillance. Ann. Gastroenterol. 2018, 32, 67. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Kim, N. Review of atrophic gastritis and intestinal metaplasia as a premalignant lesion of gastric cancer. J. Cancer Prev. 2015, 20, 25. [Google Scholar] [CrossRef]
- Eshmuratov, A.; Nah, J.C.; Kim, N.; Lee, H.S.; Lee, H.E.; Lee, B.H.; Uhm, M.S.; Park, Y.S.; Lee, D.H.; Jung, H.C.; et al. The correlation of endoscopic and histological diagnosis of gastric atrophy. Dig. Dis. Sci. 2010, 55, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and grading of gastritis: The updated Sydney system. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- Rugge, M.; Correa, P.; Di Mario, F.; El-Omar, E.; Fiocca, R.; Geboes, K.; Genta, R.M.; Graham, D.Y.; Hattori, T.; Malfertheiner, P.; et al. OLGA staging for gastritis: A tutorial. Dig. Liver Dis. 2008, 40, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Lash, J.G.; Genta, R.M. Adherence to the Sydney System guidelines increases the detection of H elicobacter gastritis and intestinal metaplasia in 400 738 sets of gastric biopsies. Aliment. Pharmacol. Ther. 2013, 38, 424–431. [Google Scholar] [CrossRef]
- Shah, S.C.; Piazuelo, M.B.; Kuipers, E.J.; Li, D. AGA clinical practice update on the diagnosis and management of atrophic gastritis: Expert review. Gastroenterology 2021, 161, 1325–1332. [Google Scholar] [CrossRef]
- Pittman, M.E.; Voltaggio, L.; Bhaijee, F.; Robertson, S.A.; Montgomery, E.A. Autoimmune metaplastic atrophic gastritis: Recognizing precursor lesions for appropriate patient evaluation. Am. J. Surg. Pathol. 2015, 39, 1611–1620. [Google Scholar] [CrossRef]
- Cockburn, A.N.; Morgan, C.J.; Genta, R.M. Neuroendocrine proliferations of the stomach: A pragmatic approach for the perplexed pathologist. Adv. Anat. Pathol. 2013, 20, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.A.; Chandrasekhara, V.; Chathadi, K.V.; Decker, G.A.; Early, D.S.; Fisher, D.A.; Foley, K.; Hwang, J.H.; Jue, T.L.; Lightdale, J.R.; et al. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest. Endosc. 2015, 82, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Coati, I.; Fassan, M.; Farinati, F.; Graham, D.Y.; Genta, R.M.; Rugge, M. Autoimmune gastritis: Pathologist’s viewpoint. World J. Gastroenterol. 2015, 21, 12179. [Google Scholar] [CrossRef] [PubMed]
- Basuroy, R.; Srirajaskanthan, R.; Prachalias, A.; Quaglia, A.; Ramage, J.K. The investigation and management of gastric neuroendocrine tumours. Aliment. Pharmacol. Ther. 2014, 39, 1071–1084. [Google Scholar] [CrossRef]
- Panzuto, F.; Ramage, J.; Pritchard, D.M.; van Velthuysen, M.L.; Schrader, J.; Begum, N.; Sundin, A.; Falconi, M.; O’Toole, D. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for gastroduodenal neuroendocrine tumours (NETs) G1–G3. J. Neuroendocrinol. 2023, 35, e13306. [Google Scholar] [CrossRef]
- Torbenson, M.; Abraham, S.C.; Boitnott, J.; Yardley, J.H.; Wu, T.T. Autoimmune gastritis: Distinct histological and immunohistochemical findings before complete loss of oxyntic glands. Mod. Pathol. 2002, 15, 102–109. [Google Scholar] [CrossRef] [PubMed]
- McColl, K.E.; El-Omar, E.; Gillen, D. Helicobacter pylori gastritis and gastric physiology. Gastroenterol. Clin. N. Am. 2000, 29, 687–703. [Google Scholar] [CrossRef]
- Taha, A.S.; Huxham, I.M.; Motley, P.; Morton, R.; Beattie, A.D. Reduced gastric mucosal vascularity in patients with chronic gastritis. Eur. J. Gastroenterol. Hepatol. 1998, 10, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, R.; Shao, L.; Zhang, Q.; Xu, R.; Zhang, S. Gastric lesions in patients with autoimmune metaplastic atrophic gastritis: A retrospective study in a single center. Scand. J. Gastroenterol. 2022, 57, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P. Vitamin B12 deficiency. N. Engl. J. Med. 2013, 368, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Chlumska, A.; Boudova, L.; Benes, Z.; Zamecnik, M. Autoimmune gastritis. A clinicopathologic study of 25 cases. Cesk Patol 2005, 41, 137–142. [Google Scholar]
- Poveda, J.C.; Chahar, S.; Garcia-Buitrago, M.T.; Montgomery, E.A.; McDonald, O.G. The Morphologic Spectrum of Gastric Type 1 Enterochromaffin–Like Cell Neuroendocrine Tumors. Mod. Pathol. 2023, 36, 100098. [Google Scholar] [CrossRef]

| Histology Comment Score | |
|---|---|
| 1 | Alternative differential diagnosis including early AMAG |
| 2 | Possible early AMAG |
| 3 | Can be seen in AMAG/atrophic gastritis, NOS |
| 4 | Suggestive of AMAG/features resembling AMAG |
| 5 | Consistent with/suspicious for AMAG |
| Endoscopy Report Score | |
|---|---|
| 1 | Normal gastric mucosa |
| 2 | Gastric erythema |
| 3 | Inconclusive gastritis (papules/nodules/pseudopolyps/mildly edematous mucosa) |
| 4 | Features of atrophy reported (mosaic pattern, nodular appearance, loss of gastric folds) |
| 5 | Atrophy present (visible blood vessels in corpus) |
| Demographics | |
| Age (mean, median, range) | 64.6, 65, (30–86) |
| Sex (n, %) | |
| Male | 25 (38) |
| Female | 40 (62) |
| Clinical Findings | |
| Other autoimmune conditions (n, %) | 15 (23) (NA = 4) a |
| Hypothyroidism | 7 (11) |
| Hashimoto’s thyroiditis | 1 (2) |
| Graves’ disease | 1 (2) |
| Type 1 diabetes | 2 (3) |
| Other | 4 (7) |
| Indications for endoscopic evaluation (n, %) | |
| Anemia/GI bleeding | 19 (29) |
| Dysphagia/pain | 27 (42) |
| Follow-up for gastric pathology/neoplasm | 18 (28) |
| Follow-up for esophageal pathology | 8 (12) |
| Other GI related symptoms | 8 (12) |
| Others | 5 (8) |
| Biopsy sites (n, %) | |
| Antrum | 31 (48) |
| Corpus | 30 (46) |
| Cardia | 4 (6) |
| Greater curvature | 0 (0) |
| Lesser curvature | 1 (2) |
| Random | 21 (32) |
| Targeted biopsy | 32 (49) |
| w/ flat mucosa evaluation | 21 (32) |
| w/o flat mucosa evaluation | 11 (17) |
| Biopsy Review | |
| Biopsy sites showing features of AMAG or NET (n, %) | |
| Antrum | 2 (3) |
| Corpus | 46 (71) |
| Cardia | 5 (8) |
| Greater curvature | 2 (3) |
| Lesser curvature | 1 (2) |
| Random | 19 (29) |
| Targeted biopsy | 29 (45) |
| Targeted lesions | |
| Polyp/nodule/mass b | 20 (69) |
| Ulcer/erosion b | 6 (21) |
| Scar b | 2 (7) |
| Others b | 3 (10) |
| Targeted biopsy shows NET b | 10 (34) |
| Atrophy grade (mean, median, range) | 2.8, 3, (1–3) |
| Synaptophysin and/or chromogranin IHC (n, %) | 61 (94) |
| ECL hyperplasia pattern | |
| Negative | 1 (2) |
| Linear | 5 (8) |
| Linear and micronodular | 47 (72) |
| NET | 12 (18) |
| Helicobacter pylori IHC (n, %) | 59 (91) |
| Positive b | 3 (5) |
| Negative b | 56 (95) |
| Histology Comment Score | |
| Histology comment score (mean, median, range) | 4.2, 5, (1–5) (NA = 2) |
| 1 | 3 (5%) |
| 2 | 3 (5%) |
| 3 | 11 (17%) |
| 4 | 7 (11%) |
| 5 | 39 (57%) |
| Endoscopy Report Score | |
| Endoscopy report score #1 (mean, median, range) | 2.9, 3, (1–5) (NA = 2) |
| 1 | 9 (14%) |
| 2 | 14 (22%) |
| 3 | 24 (37%) |
| 4 | 7 (11%) |
| 5 | 9 (14%) |
| Endoscopy report score #2 (mean, median, range) | 3.1, 3, (1–5) (NA = 2) |
| 1 | 5 (8%) |
| 2 | 16 (25%) |
| 3 | 20 (31%) |
| 4 | 12 (18%) |
| 5 | 10 (15%) |
| Follow-Up | |
| Follow-up available (n, %) | 26 (40) |
| Follow-up duration (months; mean, median, range) | 29.4, 26.3, (2–84) |
| Follow-up biopsy (n, %) | |
| Performed | 24 (37) |
| Not performed | 41 (63) |
| Number of follow-up biopsy (mean, median, range) | 2.2, 2, (1–8) |
| Follow-up laboratory testing (n, %) | |
| Performed | 57 (88) |
| Not performed | 8 (12) |
| Number of follow-up laboratory testing (mean, median, range) | 2.4, 2, (0–5) |
| Endoscopy report score #1 | - | p < 0.01 * rs = 0.78 | p = 0.06 rs = 0.30 | p = 0.72 rs = 0.09 | p = 0.75 rs = 0.04 |
| Endoscopy report score #2 | p < 0.01 * rs = 0.78 | - | p = 0.52 rs = 0.14 | p = 0.54 rs = 0.13 | p = 0.75 rs = 0.05 |
| ECL hyperplasia | p = 0.06 rs = 0.30 | p = 0.52 rs = 0.14 | - | p = 0.75 rs = 0.05 | p = 0.04 * rs = 0.32 |
| Histology comment score | p = 0.72 rs = 0.09 | p = 0.54 rs = 0.13 | p = 0.75 rs = 0.05 | - | p = 0.52 rs = 0.15 |
| Atrophy grade | p = 0.75 rs = 0.04 | p = 0.75 rs = 0.05 | p = 0.04 * rs = 0.32 | p = 0.52 rs = 0.15 | - |
| Endoscopy report score #1 | Endoscopy report score #2 | ECL cell hyperplasia | Histology comment score | Atrophy grade |
| Laboratory Test | Number (%) of Patients with Performed Test |
|---|---|
| H. pylori serology | 2 (3.1) |
| Serum chloride | 48 (73.8) |
| Anti-parietal cell antibody | 12 (18.5) |
| Vitamin B12 | 29 (44.6) |
| Hemoglobin | 54 (83.1) |
| Serum gastrin | 13 (20.0) |
| Serum pepsinogen I/II | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vienneau, N.; Lee, H.; Zhang, X.; Park, E.; Cleary, M.; Zhou, J.; Tarar, S.; Liu, M.; Tadros, M. Autoimmune Metaplastic Atrophic Gastritis Reporting: Are Pathologists and Endoscopists on the Same Page? Diagnostics 2025, 15, 2906. https://doi.org/10.3390/diagnostics15222906
Vienneau N, Lee H, Zhang X, Park E, Cleary M, Zhou J, Tarar S, Liu M, Tadros M. Autoimmune Metaplastic Atrophic Gastritis Reporting: Are Pathologists and Endoscopists on the Same Page? Diagnostics. 2025; 15(22):2906. https://doi.org/10.3390/diagnostics15222906
Chicago/Turabian StyleVienneau, Nicole, Hwajeong Lee, Xulang Zhang, Eundong Park, Madeline Cleary, Jing Zhou, Shunsa Tarar, Meng Liu, and Micheal Tadros. 2025. "Autoimmune Metaplastic Atrophic Gastritis Reporting: Are Pathologists and Endoscopists on the Same Page?" Diagnostics 15, no. 22: 2906. https://doi.org/10.3390/diagnostics15222906
APA StyleVienneau, N., Lee, H., Zhang, X., Park, E., Cleary, M., Zhou, J., Tarar, S., Liu, M., & Tadros, M. (2025). Autoimmune Metaplastic Atrophic Gastritis Reporting: Are Pathologists and Endoscopists on the Same Page? Diagnostics, 15(22), 2906. https://doi.org/10.3390/diagnostics15222906






