Quantitative Bone SPECT/CT in Diabetic Foot Osteomyelitis: Diagnostic Performance Within-Patient Lesion–Contralateral Separation and Associations with Inflammatory Burden
Abstract
1. Introduction
2. Materials and Methods
2.1. SPECT/CT Acquisition and SUV Quantification
2.2. Image Reconstruction and Corrections
2.3. SUV Calculation
2.4. VOI Definition and Metrics
- SUVmax—the maximum voxel within the VOI;
- SUVmean—the mean of all voxels within the VOI.
2.5. Laboratory Analysis
2.6. Statistical Analysis
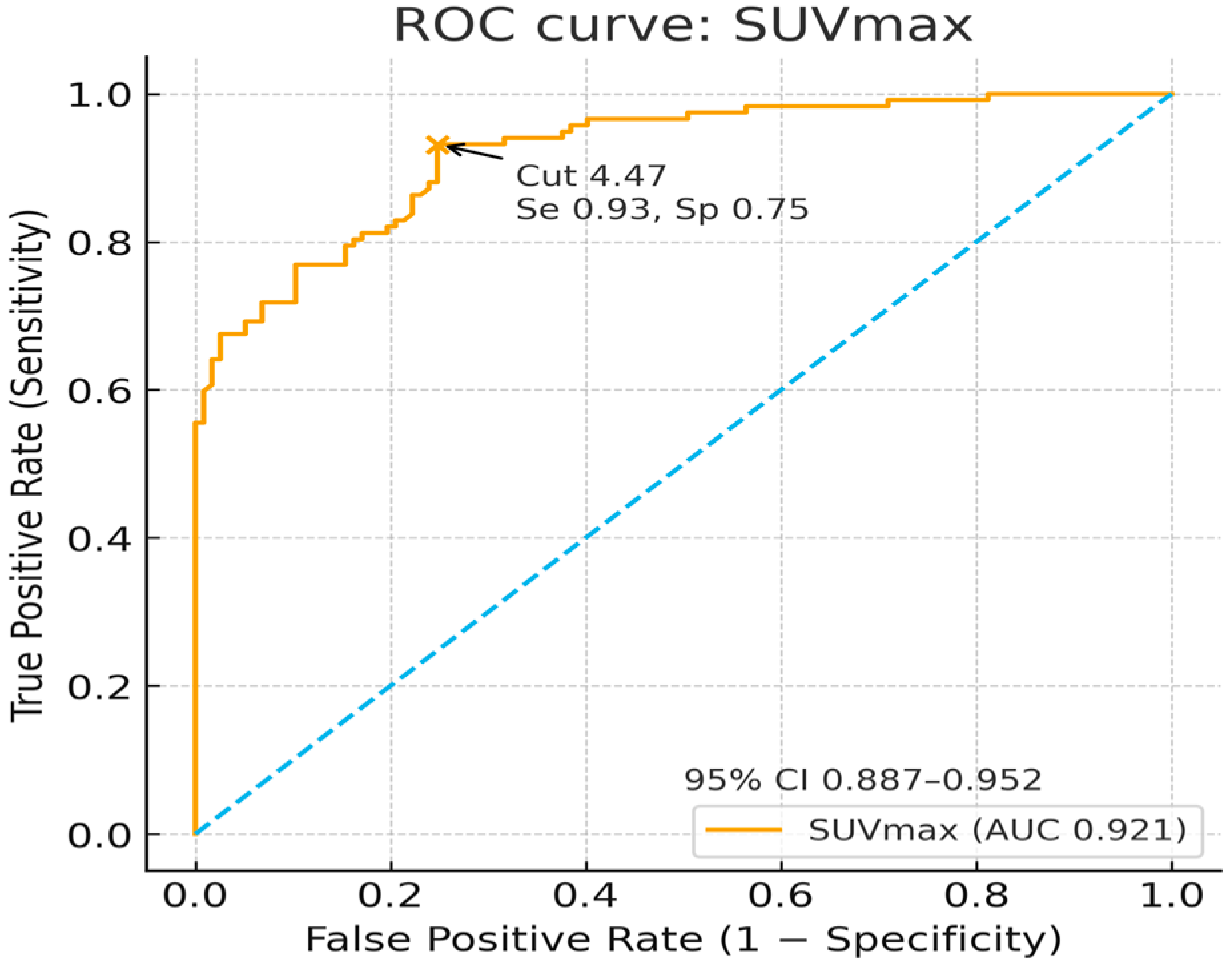
3. Results
4. Discussion
5. Limitations
6. Future Directions
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Lim, J.Z.; Ng, N.S.; Thomas, C. Prevention and treatment of diabetic foot ulcers. J. R. Soc. Med. 2017, 110, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Blonde, L.; Umpierrez, G.E.; Reddy, S.S.; McGill, J.B.; Berga, S.L.; Bush, M.; Chandrasekaran, S.; DeFronzo, R.A.; Einhorn, D.; Galindo, R.J.; et al. American Association of Clinical Endocrinology clinical practice guideline: Developing a diabetes mellitus comprehensive care plan—2022 update. Endocr. Pract. 2022, 28, 923–1049. [Google Scholar] [CrossRef] [PubMed]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Elmotaleb Hussein, M.A.; Nabil Hanna, I.; Japer Nashwan, A.J.; Saleh, M.; Abdel Wahed, W.Y.; Mohamed Mansour, A.M.; Ezz Al Arab, M.R.; Fawzy, N.; Sakr, Y.; et al. The potential impact and diagnostic value of inflammatory markers on diabetic foot progression in type II diabetes mellitus: A case-control study. Med. Clin. 2024, 162, e33–e39. [Google Scholar] [CrossRef]
- Eren, M.A.; Güneş, A.E.; Ceylan, M.R.; İncebıyık, H.; Aydın, M.S.; Dusak, A.; Sabuncu, S. Pilot study of the diagnostic value of CRP: Albumin ratio for osteomyelitis in patients with diabetic foot ulcers. J. Wound Care 2022, 31 (Suppl. 3), S25–S28. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, X.; He, M.; Wang, W.; Zhao, Y. Higher C-reactive protein/albumin ratio is a potential marker for predicting amputation in patients with diabetic foot infection. Arch. Endocrinol. Metab. 2025, 69, e240397. [Google Scholar] [CrossRef]
- Lee, Y.J.; Sadigh, S.; Mankad, K.; Kapse, N.; Rajeswaran, G. The imaging of osteomyelitis. Quant. Imaging Med. Surg. 2016, 6, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Gholamrezanezhad, A.; Basques, K.; Batouli, A.; Matcuk, G.; Alavi, A.; Jadvar, H. Clinical nononcologic applications of PET/CT and PET/MRI in musculoskeletal, orthopedic, and rheumatologic imaging. AJR Am. J. Roentgenol. 2018, 210, W245–W263. [Google Scholar] [CrossRef]
- Adams, C.; Banks, K.P. Bone scan. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Johnson, J.E.; Kennedy, E.J.; Shereff, M.J.; Patel, N.C.; Collier, B.D. Prospective study of bone, indium-111-labeled white blood cell, and gallium-67 scanning for the evaluation of osteomyelitis in the diabetic foot. Foot Ankle Int. 1996, 17, 10–16. [Google Scholar] [CrossRef]
- Unal, S.N.; Birinci, H.; Baktiroğlu, S.; Cantez, S. Comparison of Tc-99m methylene diphosphonate, Tc-99m human immune globulin, and Tc-99m-labeled white blood cell scintigraphy in the diabetic foot. Clin. Nucl. Med. 2001, 26, 1016–1021. [Google Scholar] [CrossRef]
- Schauwecker, D.S.; Park, H.M.; Burt, R.W.; Mock, B.H.; Wellman, H.N. Combined bone scintigraphy and indium-111 leukocyte scans in neuropathic foot disease. J. Nucl. Med. 1988, 29, 1651–1655. [Google Scholar]
- Park, S.B.; Lim, C.H.; Chun, D.I.; Kim, Y.J.; Kim, T.H.; Park, J.M. The usefulness of quantitative 99mTc-HMPAO WBC SPECT/CT for predicting lower extremity amputation in diabetic foot infection. Sci. Rep. 2024, 14, 9260. [Google Scholar] [CrossRef]
- Donohoe, K.J.; Brown, M.L.; Collier, B.D.; Carretta, R.F.; Henkin, R.E.; O’Mara, R.E. Society of Nuclear Medicine Procedure Guideline for Bone Scintigraphy; Version 3.0; Society of Nuclear Medicine: Reston, VA, USA, 2003. [Google Scholar]
- El Sheikh, W.M.; Alahmar, I.E.; Salem, G.M.; El-Sheikh, M.A. Tumor necrosis factor alpha in peripheral neuropathy in type 2 diabetes mellitus. Egypt. J. Neurol. Psychiatry Neurosurg. 2019, 55, 37. [Google Scholar] [CrossRef]
- Lavery, L.A.; Peters, E.J.G.; Armstrong, D.G.; Wendel, C.S.; Murdoch, D.P.; Lipsky, B.A. Risk factors for developing osteomyelitis in patients with diabetic foot wounds. Diabetes Res. Clin. Pract. 2009, 83, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Mutluoglu, M.; Sivrioglu, A.K.; Eroglu, M.; Uzun, G.; Turhan, V.; Ay, H.; Lipsky, B.A. The implications of the presence of osteomyelitis on outcomes of infected diabetic foot wounds. Scand. J. Infect. Dis. 2013, 45, 497–503. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Itani, K.; Norden, C.; Uzun, G.; Turhan, V.; Ay, H.; Lipsky, B.A. Treating foot infections in diabetic patients: A randomized, multicenter, open-label trial of linezolid versus ampicillin-sulbactam/amoxicillin-clavulanate. Clin. Infect. Dis. 2004, 38, 17–24. [Google Scholar] [CrossRef]
- Stockl, K.; Vanderplas, A.; Tafesse, E.; Chang, E. Costs of lower extremity ulcers among patients with diabetes. Diabetes Care 2004, 27, 2129–2134. [Google Scholar] [CrossRef]
- Tsourdi, E.; Barthel, A.; Rietzsch, H.; Reichel, A.; Bornstein, S.R. Current aspects in the pathophysiology and treatment of chronic wounds in diabetes mellitus. BioMed Res. Int. 2013, 2013, 385641. [Google Scholar] [CrossRef]
- Berendt, A.R.; Peters, E.J.G.; Bakker, K.; Embil, J.M.; Eneroth, M.; Hinchliffe, R.J.; Jeffcoate, W.J.; Lipsky, B.A.; Senneville, E.; The, J.; et al. Diabetic foot osteomyelitis: A progress report on diagnosis and a systematic review of treatment. Diabetes Metab. Res. Rev. 2008, 24 (Suppl. 1), S145–S161. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.G.; Waller, J.; Palestro, C.J.; Schwartz, M.; Klein, M.J.; Hermann, G.; Harrington, E.; Harrington, M.; Roman, S.H.; Stagnaro-Green, A. Unsuspected osteomyelitis in diabetic foot ulcers: Diagnosis and monitoring by leukocyte scanning with indium 111 oxyquinoline. JAMA 1991, 266, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Rabjohn, L.; Roberts, K.; Troiano, M.; Schoenhaus, H. Diagnostic and prognostic value of erythrocyte sedimentation rate in contiguous osteomyelitis of the foot and ankle. J. Foot Ankle Surg. 2007, 46, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Demirdal, T.; Sen, P. The significance of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and lymphocyte-monocyte ratio in predicting peripheral arterial disease, peripheral neuropathy, osteomyelitis and amputation in diabetic foot infection. Diabetes Res. Clin. Pract. 2018, 144, 118–125. [Google Scholar] [CrossRef]
- Ertugrul, B.M.; Savk, O.; Ozturk, B.; Cobanoglu, M.; Oncu, S.; Sakarya, S. The diagnosis of diabetic foot osteomyelitis: Examination findings and laboratory values. Med. Sci. Monit. 2009, 15, CR307–CR312. [Google Scholar]
- Fleischer, A.E.; Didyk, A.A.; Woods, J.B.; Burns, S.E.; Wrobel, J.S.; Armstrong, D.G. Combined clinical and laboratory testing improves diagnostic accuracy for osteomyelitis in the diabetic foot. J. Foot Ankle Surg. 2009, 48, 39–46. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Perales, T.A.; Murff, R.T.; Edelson, G.W.; Welchon, J.G. Value of white blood cell count with differential in the acute diabetic foot infection. J. Am. Podiatr. Med. Assoc. 1996, 86, 224–227. [Google Scholar] [CrossRef]
- Eneroth, M.; Apelqvist, J.; Stenström, A. Clinical characteristics and outcome in 223 diabetic patients with deep foot infections. Foot Ankle Int. 1997, 18, 716–722. [Google Scholar] [CrossRef]
- Lauri, C.; Tamminga, M.; Glaudemans, A.W.J.M.; Juárez Orozco, L.E.; Erba, P.A.; Jutte, P.C.; Lipsky, B.A.; IJzerman, M.J.; Signore, A.; Slart, R.H.J.A. Detection of osteomyelitis in the diabetic foot by imaging techniques: A systematic review and meta-analysis comparing MRI, white blood cell scintigraphy, and FDG-PET. Diabetes Care 2017, 40, 1111–1120. [Google Scholar] [CrossRef]
- Llewellyn, A.; Jones-Diette, J.; Kraft, J.; Holton, C.; Harden, M.; Simmonds, M. Imaging tests for the detection of osteomyelitis: A systematic review. Health Technol. Assess. 2019, 23, 1–128. [Google Scholar] [CrossRef]
- Coye, T.L.; Crisologo, P.A.; Suludere, M.A.; Malone, M.; Oz, O.K.; Lavery, L.A. The infected diabetic foot: Modulation of traditional biomarkers for osteomyelitis diagnosis in the setting of diabetic foot infection and renal impairment. Int. Wound J. 2024, 21, e14770. [Google Scholar] [CrossRef] [PubMed]
- Michail, M.; Jude, E.; Liaskos, C.; Karamagiolis, S.; Makrilakis, K.; Dimitroulis, D.; Michail, O.; Tentolouris, N. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int. J. Low Extrem. Wounds 2013, 12, 94–99. [Google Scholar] [CrossRef]
- Lavery, L.A.; Ahn, J.; Ryan, E.C.; Bhavan, K.; Oz, O.K.; La Fontaine, J.; Wukich, D.K. What are the optimal cutoff values for ESR and CRP to diagnose osteomyelitis in patients with diabetes-related foot infections? Clin. Orthop. Relat. Res. 2019, 477, 1594–1602. [Google Scholar] [CrossRef]
- Moallemi, S.K.; Niroomand, M.; Tadayon, N.; Forouzanfar, M.M.; Fatemi, A. Diagnostic value of erythrocyte sedimentation rate and C-reactive protein in detecting diabetic foot osteomyelitis: A cross-sectional study. Arch. Acad. Emerg. Med. 2020, 8, e71. [Google Scholar] [PubMed]
- Xu, J.; Cheng, F.; Li, Y.; Zhang, J.; Feng, S.; Wang, P. Erythrocyte sedimentation rate combined with the probe-to-bone test for fast and early diagnosis of diabetic foot osteomyelitis. Int. J. Low Extrem. Wounds 2021, 20, 227–231. [Google Scholar] [CrossRef]
- Bathon, J.; Graves, J.; Jens, P.; Hamrick, R.; Mayes, M. The erythrocyte sedimentation rate in end-stage renal failure. Am. J. Kidney Dis. 1987, 10, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Brouillard, M.; Reade, R.; Boulanger, E.; Cardon, G.; Dracon, M.; Dequiedt, P.; Pagniez, D. Erythrocyte sedimentation rate, an underestimated tool in chronic renal failure. Nephrol. Dial. Transplant. 1996, 11, 2244–2247. [Google Scholar] [CrossRef]
- Bilen, Y.; Cankaya, E.; Keles, M.; Gulcan, E.; Uyanik, A.; Turkeli, M.; Albayrak, B.; Yildirim, R. Does decreased mean platelet volume predict inflammation in chronic renal failure, dialysis, and transplanted patients? Ren. Fail. 2014, 36, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Alsomaili, M.I.; Yousuf, M.; Hejaili, F.; Almotairi, W.; Al-Sayyari, A.A. Erythrocyte sedimentation rate in stable patients on chronic hemodialysis. Saudi J. Kidney Dis. Transpl. 2015, 26, 1149–1153. [Google Scholar] [CrossRef]
- Cao, X.; Gong, X.; Ma, X. Diabetic nephropathy versus diabetic retinopathy in a Chinese population: A retrospective study. Med. Sci. Monit. 2019, 25, 6446–6453. [Google Scholar] [CrossRef]
- Chen, X.; Shen, Y.; Wang, Y.; Li, Y.; Guo, S.; Liang, Y.; Wang, X.; Zhou, S.; Hu, X.; Ma, K.; et al. Decreased accuracy of erythrocyte sedimentation rate in diagnosing osteomyelitis in diabetic foot infection patients with severe renal impairment: A retrospective cross-sectional study. PLoS ONE 2022, 17, e0265769. [Google Scholar] [CrossRef]
- Chou, Y.Y.; Hou, C.C.; Wu, C.W.; Huang, D.W.; Tsai, S.L.; Liu, T.H.; Ding, L.M.; Chang, C.K.; Ou, K.L.; Chiu, Y.L.; et al. Risk factors that predict major amputations and amputation time intervals for hospitalized diabetic patients with foot complications. Int. Wound J. 2022, 19, 1329–1338. [Google Scholar] [CrossRef]
- Roine, I.; Faingezicht, I.; Arguedas, A.; Herrera, J.F.; Rodríguez, F. Serial serum C-reactive protein to monitor recovery from acute hematogenous osteomyelitis in children. Pediatr. Infect. Dis. J. 1995, 14, 40–44. [Google Scholar] [CrossRef]
- Sharma, H.; Sharma, S.; Krishnan, A.; Yuan, D.; Vangaveti, V.N.; Malabu, U.H.; Haleagrahara, N. The efficacy of inflammatory markers in diagnosing infected diabetic foot ulcers and diabetic foot osteomyelitis: Systematic review and meta-analysis. PLoS ONE 2022, 17, e0267412. [Google Scholar] [CrossRef]
- Coye, T.L.; Suludere, M.A.; Kang, G.E.; Crisologo, P.A.; Malone, M.; Rogers, L.C.; Lavery, L.A. The infected diabetes-related foot: Comparison of ESR/albumin and CRP/albumin ratios with ESR and CRP to differentiate bone and soft tissue infections. Wound Repair Regen. 2023, 31, 738–744. [Google Scholar] [CrossRef]
- Saleh, A.; George, J.; Faour, M.; Klika, A.K.; Higuera, C.A. Serum biomarkers in periprosthetic joint infections. Bone Joint Res. 2018, 7, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Carli, A.V.; Abdelbary, H.; Ahmadzai, N.; Cheng, W.; Shea, B.; Hutton, B.; Sniderman, J.; Philip Sanders, B.S.; Esmaeilisaraji, L.; Skidmore, B.; et al. Diagnostic accuracy of serum, synovial, and tissue testing for chronic periprosthetic joint infection after hip and knee replacements: A systematic review. J. Bone Joint Surg. Am. 2019, 101, 635–649. [Google Scholar] [CrossRef]
- Littlejohn, E.; Marder, W.; Lewis, E.; Francis, S.; Jackish, J.; McCune, W.J.; Somers, E.C. The ratio of erythrocyte sedimentation rate to C-reactive protein is useful in distinguishing infection from flare in systemic lupus erythematosus patients presenting with fever. Lupus 2018, 27, 1123–1129. [Google Scholar] [CrossRef]
- Hage, J.E.; Cunha, B.A. Are ESR/CRP ratios helpful in differentiating West Nile encephalitis from non-West Nile virus meningitis/encephalitis? Scand. J. Infect. Dis. 2013, 45, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.P.; Klemt, C.; Tirumala, V.; Oganesyan, R.; van den Kieboom, J.; Kwon, Y.M. Elevated ESR/CRP ratio is associated with reinfection after debridement, antibiotics, and implant retention in chronic periprosthetic joint infections. J. Arthroplasty 2020, 35, 3254–3260. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chen, H.A.; Liao, H.T.; Liu, C.H.; Tsai, C.Y.; Chou, C.T. The clinical usefulness of ESR, CRP, and disease duration in ankylosing spondylitis: The product of these acute-phase reactants and disease duration is associated with patient’s poor physical mobility. Rheumatol. Int. 2015, 35, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Volaco, A.; Chantelau, E.; Richter, B.; Luther, B. Outcome of critical foot ischemia in longstanding diabetic patients: A retrospective cohort study in a specialized tertiary care centre. Vasa 2004, 33, 36–41. [Google Scholar]
- Surriah, M.H.; Al-Imari, A.N.K.; Bakkour, A.M.; Jallod Al-Asadi, R.R. Predictive value of risk factors for amputation of lower extremity in patients with diabetic foot in Al-Karama teaching hospital. Int. Surg. J. 2019, 6, 1549–1555. [Google Scholar] [CrossRef]
- Jeon, B.J.; Choi, H.J.; Kang, J.S.; Tak, M.S.; Park, E.S. Comparison of five systems of classification of diabetic foot ulcers and predictive factors for amputation. Int. Wound J. 2017, 14, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Gürlek, A.; Bayraktar, M.; Savaş, C.; Gedik, O. Amputation rate in 147 Turkish patients with diabetic foot: The Hacettepe University Hospital experience. Exp. Clin. Endocrinol. Diabetes 1998, 106, 404–409. [Google Scholar] [CrossRef]
- Won, S.H.; Chung, C.Y.; Park, M.S.; Lee, T.; Sung, K.H.; Lee, S.Y.; Kim, T.G.; Lee, K.M. Risk factors associated with amputation-free survival in patients with diabetic foot ulcers. Yonsei Med. J. 2014, 55, 1373–1378. [Google Scholar] [CrossRef]
- Radišić Biljak, V.; Tomas, M.; Lapić, I.; Saračević, A. Are shortened aPTT values always to be attributed only to preanalytical problems? Diagnosis 2024, 11, 430–434. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T. Inflammation and coagulation. Crit. Care Med. 2010, 38 (Suppl. 2), S26–S34. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T. The interactions between inflammation and coagulation. Br. J. Haematol. 2005, 131, 417–430. [Google Scholar] [CrossRef]
- Gerber, G.F. Anemia of Chronic Disease (Anemia of Chronic Inflammation); Merck Manual Professional Version; Merck & Co., Inc.: Rahway, NJ, USA, 2024. [Google Scholar]
- Fertrin, K.Y. Diagnosis and management of iron deficiency in chronic inflammatory conditions (CIC): Is too little iron making your patient sick? Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 478–486. [Google Scholar] [CrossRef]
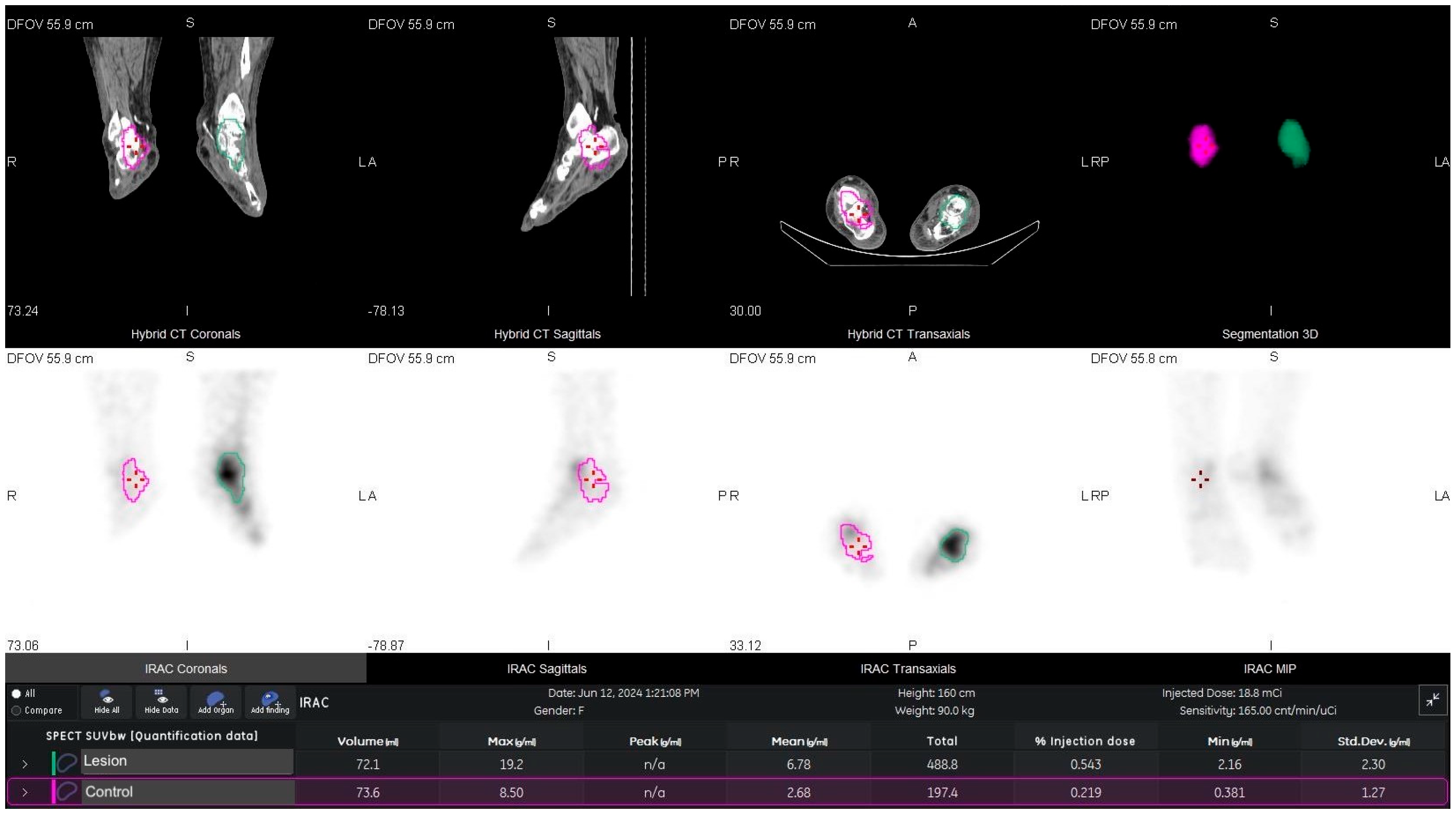


| Patients (n = 117) | |
|---|---|
| Age (years) | 61.37 ± 12.20 |
| BMI (kg/m2) | 26.88 ± 3.29 |
| SPECT/CT Findings | |
| SUVmax Lesion | 10.94 ± 7.36 |
| SUVmean Lesion | 4.38 ± 3.63 |
| SUVmax Control | 3.62 ± 1.70 |
| SUVmean Control | 0.93 ± 0.50 |
| Laboratory Data | |
| CRP (mg/L) | 39.77 ± 31.02 |
| Platelets (%) | 289.43 ± 106.69 |
| Lymphocytes (%) | 24.30 ± 9.31 |
| Neutrophil (%) | 65.12 ± 9.98 |
| WBC (%) | 8.82 ± 3.45 |
| MCH (pg) | 27.70 ± 2.27 |
| FBG (mg/dL) | 174.25 ± 85.63 |
| HbA1c (%) | 8.68 ± 2.34 |
| Albumin (g/dL) | 4.36 ± 4.01 |
| PT (s) | 10.10 ± 1.99 |
| aPTT (s) | 28.66 ± 3.51 |
| INR | 1.05 ± 0.18 |
| Infection variables | |
| CRP/Albumin | 8.70 ± 11.97 |
| Neutrophil/Lymphocytes | 3.56 ± 3.06 |
| ESR × CRP | 4228.14 ± 2246.19 |
| Comparison | Spearman ρ | 95% CI | p-Value |
|---|---|---|---|
| SUVmax vs. CRP | 0.25 | 0.06–0.42 | 0.008 |
| SUVmax vs. ESR | 0.28 | 0.09–0.45 | 0.004 |
| SUVmean vs. CRP | 0.29 | 0.08–0.11 | 0.003 |
| SUVmean vs. ESR | 0.3 | 0.09–0.12 | 0.002 |
| SUVmean vs. ESR × CRP | 0.35 | 0.20–0.51 | 0.0002 |
| SUVmean vs. CRP/Albumin | 0.28 | 0.07–0.46 | 0.007 |
| SUVmean vs. MCH | −0.2 | −0.37–0.07 | 0.039 |
| SUVmean vs. aPTT | −0.37 | −0.58–0.12 | 0.0028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalcin, H.P.; Gunduz, P.A.; Samsum, M.; Samsum, E.C.; Erol, A.; Turan, U.M.; Turgut, G.G.; Yalci, A.; Yesildag, N.; Yalcin, M.F.; et al. Quantitative Bone SPECT/CT in Diabetic Foot Osteomyelitis: Diagnostic Performance Within-Patient Lesion–Contralateral Separation and Associations with Inflammatory Burden. Diagnostics 2025, 15, 2907. https://doi.org/10.3390/diagnostics15222907
Yalcin HP, Gunduz PA, Samsum M, Samsum EC, Erol A, Turan UM, Turgut GG, Yalci A, Yesildag N, Yalcin MF, et al. Quantitative Bone SPECT/CT in Diabetic Foot Osteomyelitis: Diagnostic Performance Within-Patient Lesion–Contralateral Separation and Associations with Inflammatory Burden. Diagnostics. 2025; 15(22):2907. https://doi.org/10.3390/diagnostics15222907
Chicago/Turabian StyleYalcin, Hulya Peker, Pınar Akkus Gunduz, Mehmet Samsum, Emel Colak Samsum, Aysenur Erol, Umut Mert Turan, Gulsah Gedikli Turgut, Aysun Yalci, Nihal Yesildag, Musa Fatih Yalcin, and et al. 2025. "Quantitative Bone SPECT/CT in Diabetic Foot Osteomyelitis: Diagnostic Performance Within-Patient Lesion–Contralateral Separation and Associations with Inflammatory Burden" Diagnostics 15, no. 22: 2907. https://doi.org/10.3390/diagnostics15222907
APA StyleYalcin, H. P., Gunduz, P. A., Samsum, M., Samsum, E. C., Erol, A., Turan, U. M., Turgut, G. G., Yalci, A., Yesildag, N., Yalcin, M. F., & Eryavuz, N. Z. (2025). Quantitative Bone SPECT/CT in Diabetic Foot Osteomyelitis: Diagnostic Performance Within-Patient Lesion–Contralateral Separation and Associations with Inflammatory Burden. Diagnostics, 15(22), 2907. https://doi.org/10.3390/diagnostics15222907







