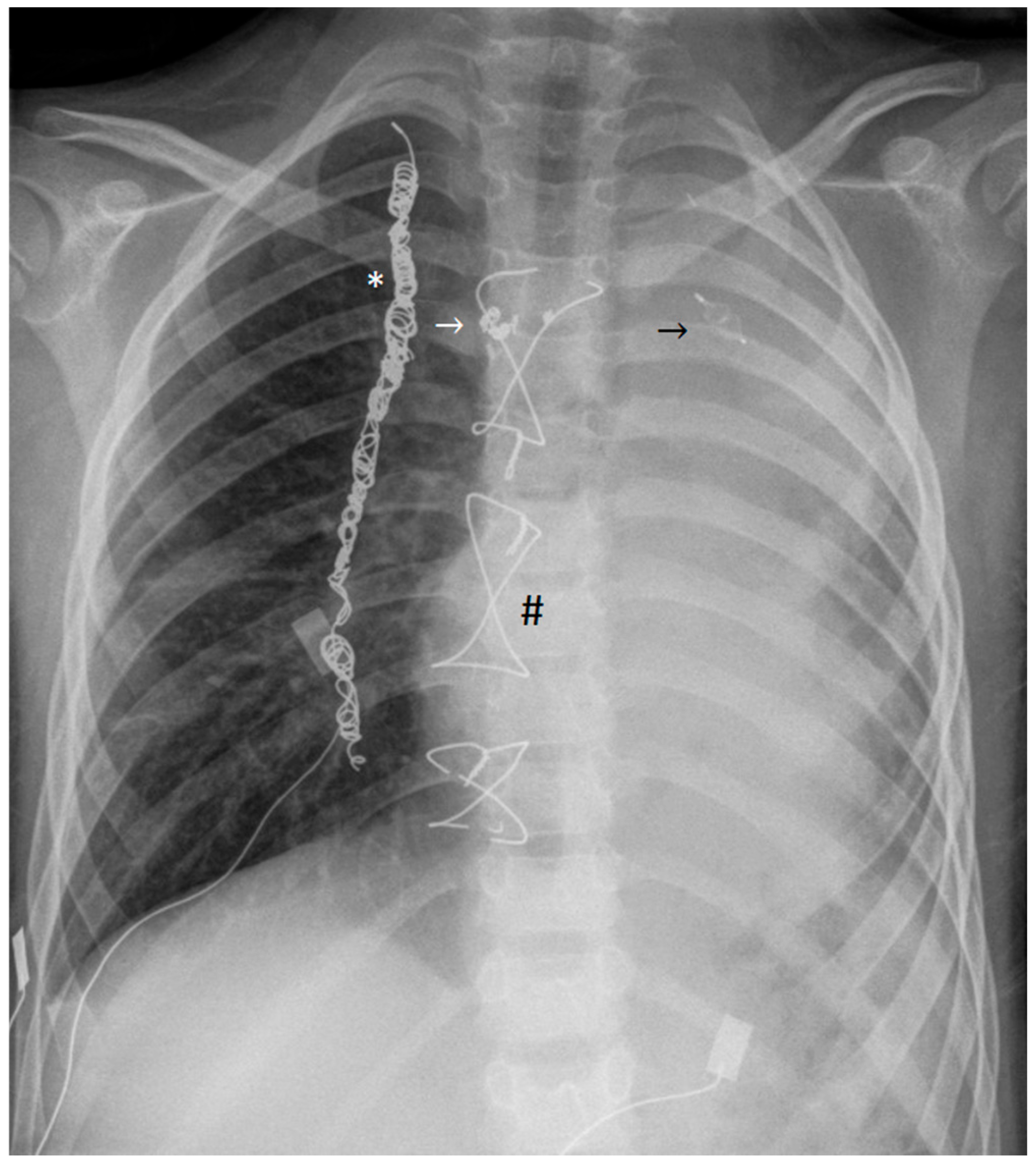
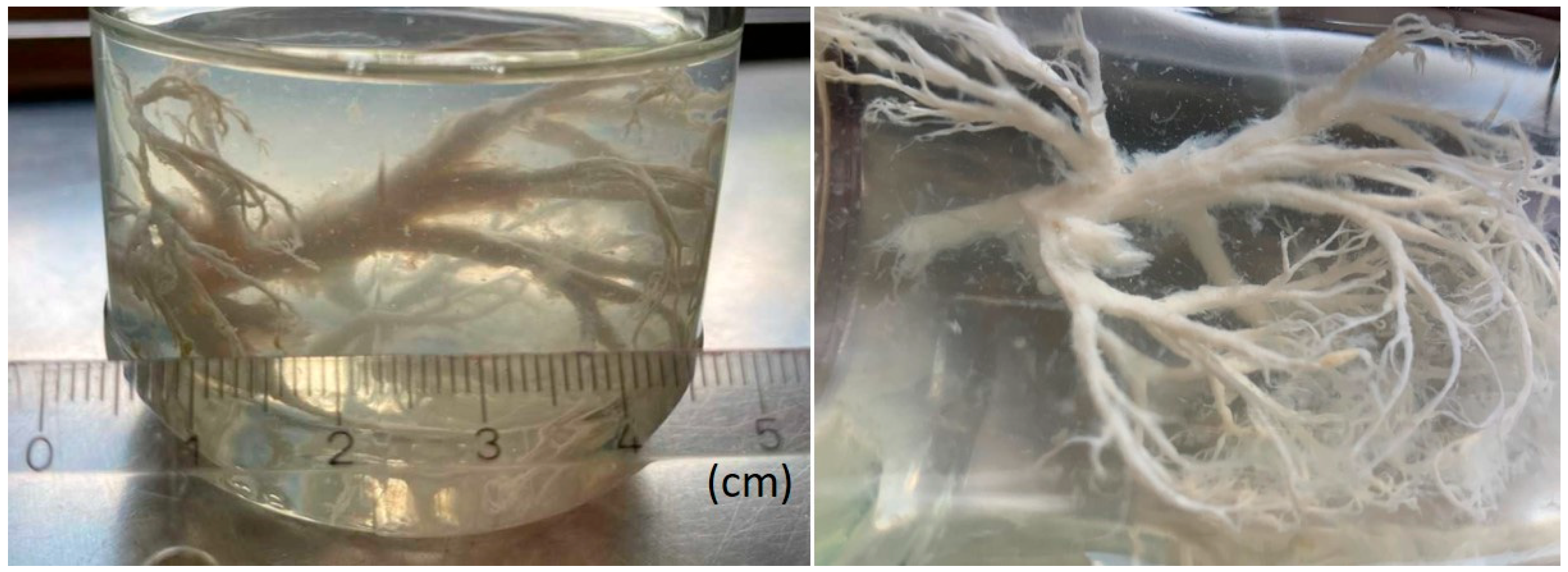
Plastic Bronchitis: Extensive Cast Expectoration in a 6-Year-Old Boy with Fontan Circulation
Abstract
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caruthers, R.L.; Kempa, M.; Loo, A.; Gulbransen, E.; Kelly, E.; Erickson, S.R.; Hirsch, J.C.; Schumacher, K.R.; Stringer, K.A. Demographic characteristics and estimated prevalence of Fontan-associated plastic bronchitis. Pediatr. Cardiol. 2013, 34, 256–261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maqsood, A.; Imel, L.R. Plastic Bronchitis. N. Engl. J. Med. 2022, 386, 780. [Google Scholar] [CrossRef] [PubMed]
- Mehta, I.; Patel, K. Lymphatic Plastic Bronchitis. N. Engl. J. Med. 2022, 386, e19. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Song, H.; Li, J.; Ren, Y. Plastic Bronchitis in a Child. Indian J. Pediatr. 2023, 90, 829–830. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, M.; Stumper, O. Plastic bronchitis after Fontan operation: Treatment with stent fenestration of the Fontan circuit. Heart 2004, 90, 801. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singhi, A.K.; Vinoth, B.; Kuruvilla, S.; Sivakumar, K. Plastic bronchitis. Ann. Pediatr. Cardiol. 2015, 8, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Verghese, S.; Jackson, M.; Vaughns, J.; Preciado, D. Plastic bronchitis in a child with Fontan’s physiology presenting for urgent rigid bronchoscopy. Anesth. Analg. 2008, 107, 1446–1447. [Google Scholar] [CrossRef] [PubMed]
- Tzifa, A.; Robards, M.; Simpson, J.M. Plastic bronchitis; a serious complication of the Fontan operation. Int. J. Cardiol. 2005, 101, 513–514. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A.S.; Veldtman, G.R.; Thorup, L.; Hjortdal, V.E.; Dori, Y. Plastic Bronchitis and Protein-Losing Enteropathy in the Fontan Patient: Evolving Understanding and Emerging Therapies. Can. J. Cardiol. 2022, 38, 988–1001. [Google Scholar] [CrossRef] [PubMed]
- Nayır Büyükşahin, H.; Emiralioglu, N.; Sekerel, B.E.; Soyer, T.; Oguz, B.; Güzelkaş, I.; Sunman, B.; Alboğa, D.; Akgül Erdal, M.; Yalcın, E.; et al. Plastic bronchitis during childhood: Diversity of presentation, etiology, treatment, and outcomes. Pediatr. Pulmonol. 2023, 58, 2559–2567. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.A.; Gribaudo, E.; Agnoletti, G. The pathophysiology and complications of Fontan circulation. Acta Biomed. 2021, 92, e2021260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- RochéRodríguez, M.; DiNardo, J.A. The Lymphatic System in the Fontan Patient-Pathophysiology, Imaging, and Interventions: What the Anesthesiologist Should Know. J. Cardiothorac. Vasc. Anesth. 2022, 36 Pt A, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, K.R.; Singh, T.P.; Kuebler, J.; Aprile, K.; O’Brien, M.; Blume, E.D. Risk factors and outcome of Fontan-associated plastic bronchitis: A case-control study. J. Am. Heart Assoc. 2014, 3, e000865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harteveld, L.M.; Blom, N.A.; Hazekamp, M.G.; Ten Harkel, A.D.J. Treatment and outcome of plastic bronchitis in single ventricle patients: A systematic review. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Patel, M.; Inja, R.; Krvavac, A.; Lechner, A.J. Plastic Bronchitis in Adult and Pediatric Patients: A Review of its Presentation, Diagnosis, and Treatment. Mo. Med. 2021, 118, 363–373. [Google Scholar] [PubMed] [PubMed Central]
- Xiong, L.; Rao, X.; Peng, X.; Zhang, G.; Liu, H. Management of Plastic Bronchitis Using α-Chymotrypsin: A Novel Treatment Modality. Cureus 2021, 13, e13551. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pfeifer, J.; Poryo, M.; Gheibeh, A.; Rentzsch, A.; Abdul-Khaliq, H. Transcatheter Embolization of Systemic-to-Pulmonary Collaterals: A New Approach Using Concerto™ Helix Nylon-Fibered Microcoils. J. Clin. Med. 2024, 14, 113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pfeifer, J.; Gheibeh, A.; Fries, P.; Poryo, M.; Rentzsch, A.; Abdul-Khaliq, H. Transcatheter Embolization in Congenital Cardiovascular Malformations-Variable Use of Vascular Plugs. Cardiovasc. Ther. 2024, 2024, 4778469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maleux, G.; Storme, E.; Cools, B.; Heying, R.; Boshoff, D.; Louw, J.J.; Frerich, S.; Malekzadeh-Milanii, S.; Hubrechts, J.; Brown, S.C.; et al. Percutaneous embolization of lymphatic fistulae as treatment for protein-losing enteropathy and plastic bronchitis in patients with failing Fontan circulation. Catheter. Cardiovasc. Interv. 2019, 94, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Dori, Y.; Keller, M.S.; Rome, J.J.; Gillespie, M.J.; Glatz, A.C.; Dodds, K.; Goldberg, D.J.; Goldfarb, S.; Rychik, J.; Itkin, M. Percutaneous Lymphatic Embolization of Abnormal Pulmonary Lymphatic Flow as Treatment of Plastic Bronchitis in Patients with Congenital Heart Disease. Circulation 2016, 133, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.N.; Dautry, R.; Bonnet, D.; Malekzadeh-Milani, S. Transvenous retrograde thoracic duct embolization for effective treatment of recurrent plastic bronchitis after fontan palliation. Catheter. Cardiovasc. Interv. 2023, 101, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Drinkwater, D.C.; Christian, K.G. Plastic bronchitis: Is thoracic duct ligation a real surgical option? Ann. Thorac. Surg. 2006, 81, 2281–2283. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.; Rahbar, R.; Fynn-Thompson, F.; Farasat, E.; Pugi, J.; Samadizadeh, S.; Mohammadi-Brenjegani, A.; Ghaedsharaf, S.; Zojaji, M. Plastic bronchitis in pediatrics: A systematic review of etiologies, clinical presentations, treatments, and prognosis. Int. J. Pediatr. Otorhinolaryngol. 2025, 195, 112422. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Shen, C.; Lu, Z.; Hou, T.; Niu, F.; Wang, Y.; Ning, J.; Liu, R. Clinical features and risk factors of plastic bronchitis caused by Mycoplasma pneumoniae pneumonia in children. BMC Pulm. Med. 2023, 23, 468, Erratum in BMC Pulm. Med. 2023, 23, 499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfeifer, J.; Poryo, M.; Fries, P.; Abdul-Khaliq, H. Plastic Bronchitis: Extensive Cast Expectoration in a 6-Year-Old Boy with Fontan Circulation. Diagnostics 2025, 15, 2864. https://doi.org/10.3390/diagnostics15222864
Pfeifer J, Poryo M, Fries P, Abdul-Khaliq H. Plastic Bronchitis: Extensive Cast Expectoration in a 6-Year-Old Boy with Fontan Circulation. Diagnostics. 2025; 15(22):2864. https://doi.org/10.3390/diagnostics15222864
Chicago/Turabian StylePfeifer, Jochen, Martin Poryo, Peter Fries, and Hashim Abdul-Khaliq. 2025. "Plastic Bronchitis: Extensive Cast Expectoration in a 6-Year-Old Boy with Fontan Circulation" Diagnostics 15, no. 22: 2864. https://doi.org/10.3390/diagnostics15222864
APA StylePfeifer, J., Poryo, M., Fries, P., & Abdul-Khaliq, H. (2025). Plastic Bronchitis: Extensive Cast Expectoration in a 6-Year-Old Boy with Fontan Circulation. Diagnostics, 15(22), 2864. https://doi.org/10.3390/diagnostics15222864




