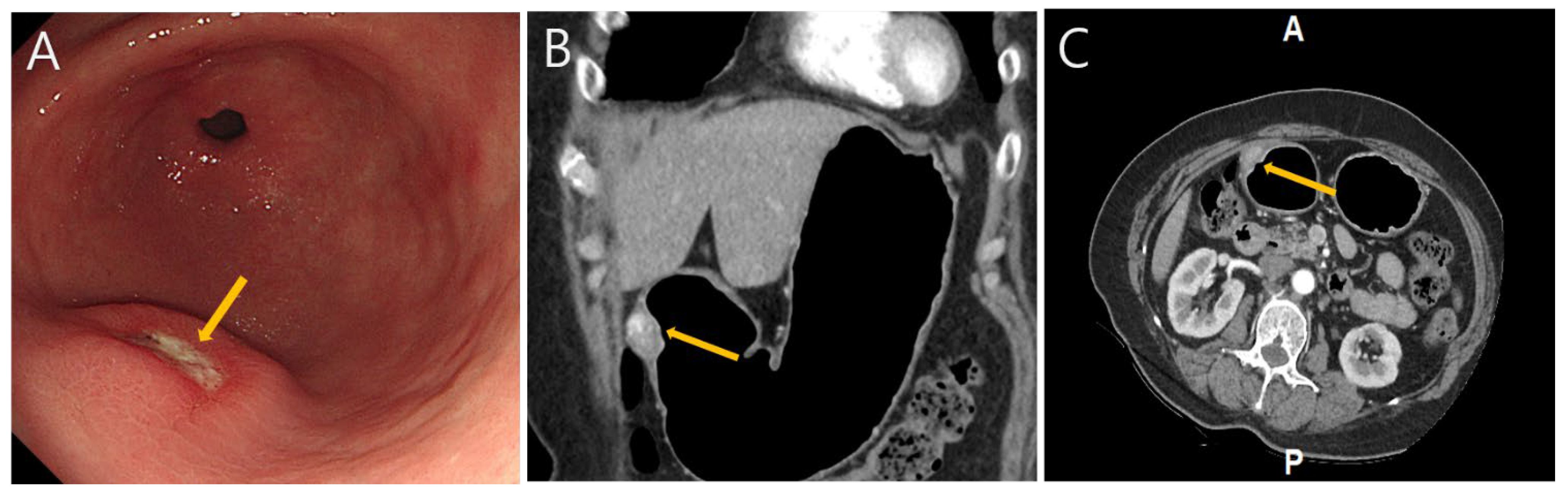
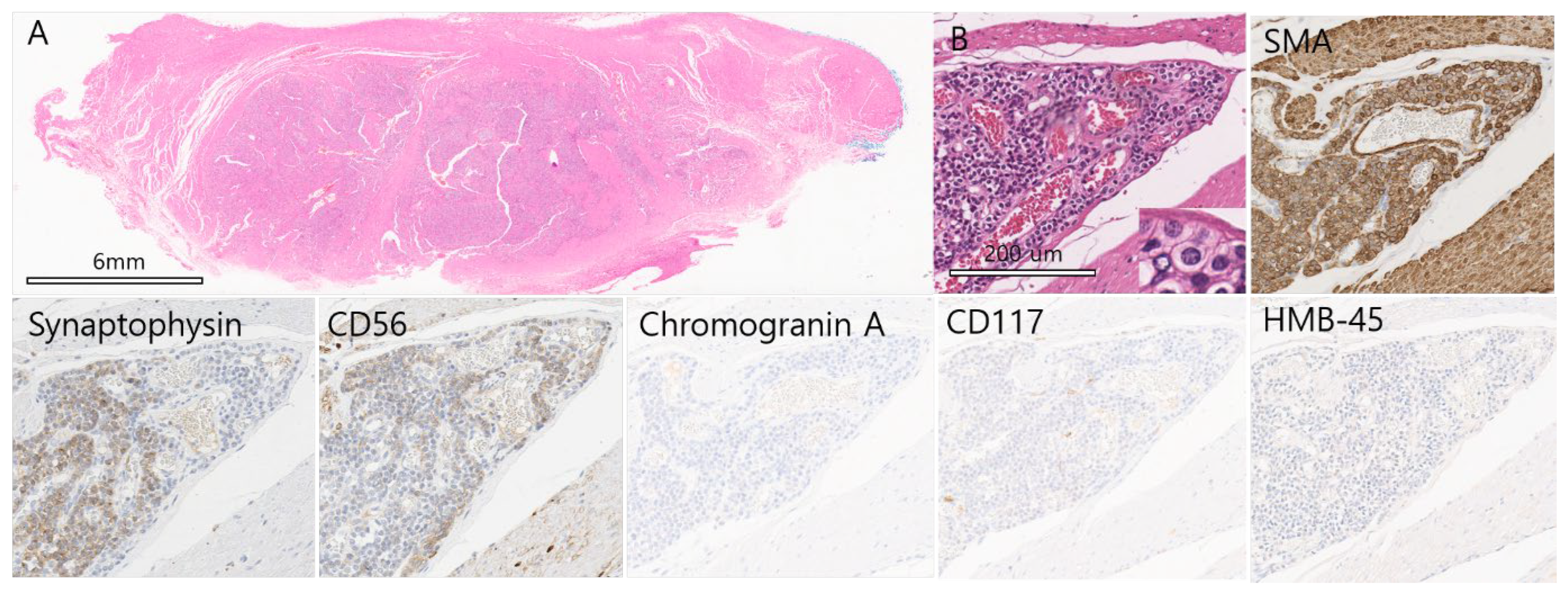
Gastric Glomus Tumor with Neuroendocrine Features: A Diagnostic Pitfall for Neuroendocrine Tumors
Abstract
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsagkataki, E.S.; Flamourakis, M.E.; Gkionis, I.G.; Giakoumakis, M.I.; Delimpaltadakis, G.N.; Kazamias, G.M.; Giannikaki, E.S.; Christodoulakis, M.S. Gastric glomus tumor: A case report and review of the literature. J. Med. Case Rep. 2021, 15, 415. [Google Scholar] [CrossRef] [PubMed]
- Pansa, A.; Sama, L.; Ruspi, L.; Sicoli, F.; Cananzi, F.C.M.; Quagliuolo, V. Glomus Tumor of the Stomach: A Systematic Review and Illustrative Case Report. Dig. Dis. 2023, 41, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Cramer, H.M.; Dave, N.; Segura, S. Gastric glomus tumor mimicking neuroendocrine tumor on fine needle aspiration: A diagnostic pitfall. Am. J. Clin. Pathol. 2023, 160, S22–S23. [Google Scholar] [CrossRef]
- Dhorajiya, P.; Samuel, J.; Mahabir, R.; Sharabi, A. Gastric Glomus Tumor: A Rare Gastric Tumor Featuring Small Round Blue Cell; Diagnostic Pitfall for Carcinoid Tumor. Am. J. Clin. Pathol. 2018, 150, S29. [Google Scholar] [CrossRef]
- Ezeh, K.J.; Boateng, W.; Paudel, B.; Ezeudemba, O.; Botros, Y. A Case of Gastric Glomus Tumor Misdiagnosed as Carcinoid Tumor. Cureus 2023, 15, e34316. [Google Scholar] [CrossRef] [PubMed]
- Koseoglu, H.; Duzenli, T.; Sezikli, M. Gastric neuroendocrine neoplasms: A review. World J. Clin. Cases 2021, 9, 7973–7985. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Yuan, J.; Shi, H.Y. Features of gastric glomus tumor: A clinicopathologic, immunohistochemical and molecular retrospective study. Int. J. Clin. Exp. Pathol. 2014, 7, 1438–1448. [Google Scholar] [PubMed]
- Miettinen, M.; Paal, E.; Lasota, J.; Sobin, L.H. Gastrointestinal glomus tumors: A clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am. J. Surg. Pathol. 2002, 26, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Nalbant, O.A.; Temiz, P.; Vural, S.; Keles, M.C. Glomus Tumor of the Stomach: Case Report. Turk. J. Pathol. 2010, 26, 74–77. [Google Scholar] [CrossRef]
- Lin, J.; Shen, J.; Yue, H.; Li, Q.; Cheng, Y.; Zhou, M. Gastric Glomus Tumor: A Clinicopathologic and Immunohistochemical Study of 21 Cases. Biomed. Res. Int. 2020, 2020, 5637893. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.C.; Husain, S.; Zhang, Z.; Yuan, L. Gastric glomus tumor on EUS-FNA-based cytology: Clinicopathologic and immunohistochemical features of 4 cases, including 1 case with associated MIR143HG-NOTCH2 fusion gene. J. Am. Soc. Cytopathol. 2023, 12, 296–306. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.H.; Kim, T.-H.; An, H.J. Gastric Glomus Tumor with Neuroendocrine Features: A Diagnostic Pitfall for Neuroendocrine Tumors. Diagnostics 2025, 15, 2865. https://doi.org/10.3390/diagnostics15222865
Song DH, Kim T-H, An HJ. Gastric Glomus Tumor with Neuroendocrine Features: A Diagnostic Pitfall for Neuroendocrine Tumors. Diagnostics. 2025; 15(22):2865. https://doi.org/10.3390/diagnostics15222865
Chicago/Turabian StyleSong, Dae Hyun, Tae-Han Kim, and Hyo Jung An. 2025. "Gastric Glomus Tumor with Neuroendocrine Features: A Diagnostic Pitfall for Neuroendocrine Tumors" Diagnostics 15, no. 22: 2865. https://doi.org/10.3390/diagnostics15222865
APA StyleSong, D. H., Kim, T.-H., & An, H. J. (2025). Gastric Glomus Tumor with Neuroendocrine Features: A Diagnostic Pitfall for Neuroendocrine Tumors. Diagnostics, 15(22), 2865. https://doi.org/10.3390/diagnostics15222865





