Myxoid Glomus Tumors Showing CD34 Expression: A Series of Eight Cases
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gombos, Z.; Zhang, P.J. Glomus tumor. Arch. Pathol. Lab. Med. 2008, 132, 1448–1452. [Google Scholar] [CrossRef] [PubMed]
- Brenn, T.; Goodlad, J.; Mentzel, T. AFIP Series 5 Fasc. N. 7-Non Melanocytic Tumors of the Skin; American Registry of Pathology: Arlington, VA, USA, 2021. [Google Scholar]
- Jackett, L.A.; Folpe, A.L. (Eds.) Chapter 7: Glomus tumour. In Skin Tumours, 5th ed.; WHO Classification of Tumours Editorial Board; WHO Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2025; Volume 12, Available online: https://publications.iarc.fr (accessed on 4 September 2025).
- Amyere, M.; Aerts, V.; Brouillard, P.; McIntyre, B.A.; Duhoux, F.P.; Wassef, M.; Enjolras, O.; Mulliken, J.B.; Devuyst, O.; Antoine-Poirel, H.; et al. Somatic uniparental isodisomy explains multifocality of glomuvenous malformations. Am. J. Hum. Genet. 2013, 92, 188–196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goldblum, J.R.; Folpe, A.L.; Weiss, S.W. (Eds.) Enzinger and Weiss’s Soft Tissue Tumors, 7th ed.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Ezeh, K.J.; Rajwana, Y.; Paudel, B.; Shen, T.; Botros, Y. Gastric Glomus Tumor Presenting With Gastrointestinal Bleed and Pulmonary Embolism: A Rare Entity With Management Dilemma. Cureus 2022, 14, e25632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masson, P. Le glomus neuromyoarterial des regions tactiles et ses tumeurs. Lyon Chir. 1924, 21, 257–280. [Google Scholar]
- Folpe, A.L.; Fanburg–Smith, J.C.; Miettinen, M.; Weiss, S.W. Atypical and malignant glomus tumors: Analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am. J. Surg. Pathol. 2001, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Paal, E.; Lasota, J.; Sobin, L.H. Gastrointestinal glomus tumors: A clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am. J. Surg. Pathol. 2002, 26, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Calduch, L.; Monteagudo, C.; Martínez-Ruiz, E.; Ramón, D.; Pinazo, I.; Cardá, C.; Jordá, E. Familial generalized multiple glomangiomyoma: Report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr. Dermatol. 2002, 19, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Tajima, Y.; Weathers, D.R.; Neville, B.W.; Benoit, P.W.; Pedley, D.M. Glomus tumor (golomangioma) of the tongue. A light and electron microscopic study. Oral Surg. Oral Med. Oral Pathol. 1981, 52, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Kaye, V.M.; Dehner, L.P. Cutaneous glomus tumor. A comparative immunohistochemical study with pseudoangiomatous intradermal melanocytic nevi. Am. J. Dermatopathol. 1991, 13, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Bertalot, G.; Falchetti, M.; Parafioriti, A. Glomus tumour: The immunohistochemical characteristics of twenty-three cases. Pathologica 1994, 86, 509–512. [Google Scholar] [PubMed]
- Jundi, M.; Lack, E.E.; Brun, E.A.; Esquivel, J.; Kumar, D. Glomus tumor of the duodenum: A case report. Int. J. Surg. Pathol. 2004, 12, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Negri, G.; Schulte, M.; Mohr, W. Glomus tumor with diffuse infiltration of the quadriceps muscle: A case report. Hum. Pathol. 1997, 28, 750–752. [Google Scholar] [CrossRef] [PubMed]
- Mentzel, T.; Hügel, H.; Kutzner, H. CD34-positive glomus tumor: Clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J. Cutan. Pathol. 2002, 29, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Hatori, M.; Aiba, S.; Kato, M.; Kamiya, N.; Kokubun, S. Expression of CD34 in glomus tumors. Tohoku J. Exp. Med. 1997, 182, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Edwards, J.R.; Espinosa, O.; Banerji, S.; Jackson, D.G.; Athanasou, N.A. Expression of a lymphatic endothelial cell marker in benign and malignant vascular tumors. Hum. Pathol. 2004, 35, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Moody, G.H.; Myskow, M.; Musgrove, C. Glomus tumor of the lip. A case report and immunohistochemical study. Oral Surg. Oral Med. Oral Pathol. 1986, 62, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Tsuneyoshi, M.; Enjoji, M. Glomus tumor: A clinicopathologic and electron microscopic study. Cancer 1982, 50, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Dervan, P.A.; Tobbia, I.N.; Casey, M.; O’Loughlin, J.; O’Brien, M. Glomus tumours: An immunohistochemical profile of 11 cases. Histopathology 1989, 14, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Young, M.R.; Cigal, M. Tumor skewing of CD34+ cell differentiation from a dendritic cell pathway into endothelial cells. Cancer Immunol. Immunother. 2006, 55, 558–568. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chu, P.G.; Chang, K.L.; Wu, A.Y.; Weiss, L.M. Nasal glomus tumors: Report of two cases with emphasis on immunohistochemical features and differential diagnosis. Hum. Pathol. 1999, 30, 1259–1261. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.B.; Chen, L. Glomus tumor in the stomach: A case report and review of the literature. Oncol. Lett. 2014, 7, 1790–1792. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pabuççuoğlu, U.; Lebe, B. Matrix metalloproteinase-9 expression in a CD34-positive glomus tumor with myxoid stromal change. Indian. J. Dermatol. Venereol. Leprol. 2008, 74, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Skelton, H.G.; Smith, K.J. Infiltrative glomus tumor arising from a benign glomus tumor: A distinctive immunohistochemical pattern in the infiltrative component. Am. J. Dermatopathol. 1999, 21, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Jaoude, J.A.; Farah, A.R.; Sargi, Z.; Khairallah, S.; Fakih, C. Glomus tumors: Report on eleven cases and a review of the literature. Chir. Main. 2000, 19, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Slone, S.P.; Moore, G.D.; Parker, L.P.; Rickard, K.A.; Nixdorf-Miller, A.S. Glomus tumor of the ovary masquerading as granulosa cell tumor: Case report. Int. J. Gynecol. Pathol. 2010, 29, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Liapi-Avgeri, G.; Karabela-Bouropoulou, V.; Agnanti, N. Glomus tumor. A histological, histochemical and immunohistochemical study of the various types. Pathol. Res. Pract. 1994, 190, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.R.; Gaddis, K.J.; Hess, S.; Rubin, A.I. Nail Unit Glomus Tumor with Myxoid and Symplastic Change Presenting with Longitudinal Erythronychia. Dermatopathology 2018, 5, 74–78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodríguez, J.M.; Idoate, M.A.; Pardo-Mindán, F.J. The role of mast cells in glomus tumours: Report of a case of an intramuscular glomus tumour with a prominent mastocytic component. Histopathology 2003, 42, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Fetsch, J.F.; Laskin, W.B.; Miettinen, M. Superficial acral fibromyxoma: A clinicopathologic and immunohistochemical analysis of 37 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes. Hum. Pathol. 2001, 32, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, A.; Hisaoka, M.; Hashimoto, H. Angioleiomyoma: A clinicopathologic and immunohistochemical reappraisal with special reference to the correlation with myopericytoma. Hum. Pathol. 2007, 38, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.; Ceci, F.M.; Yousefian, F.; Nikakis, J.; Elias, M.; Goodman, M.B. A Rare Case of Myxoid Solitary Fibrous Tumor of the Upper Lip: The Key Role of STAT6 in Diagnosis. Cureus 2025, 17, e81510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doyle, L.A.; Möller, E.; Cin, P.D.; Fletcher, C.D.M.; Mertens, F.; Hornick, J.L. MUC4 is a highly sensitive and specific marker for low-grade fibromyxoid sarcoma. Am. J. Surg. Pathol. 2011, 35, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Tfayli, Y.; Baydoun, A.; Naja, A.S.; Saghieh, S. Management of myxoid liposarcoma of the extremity. Oncol. Lett. 2021, 22, 596. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
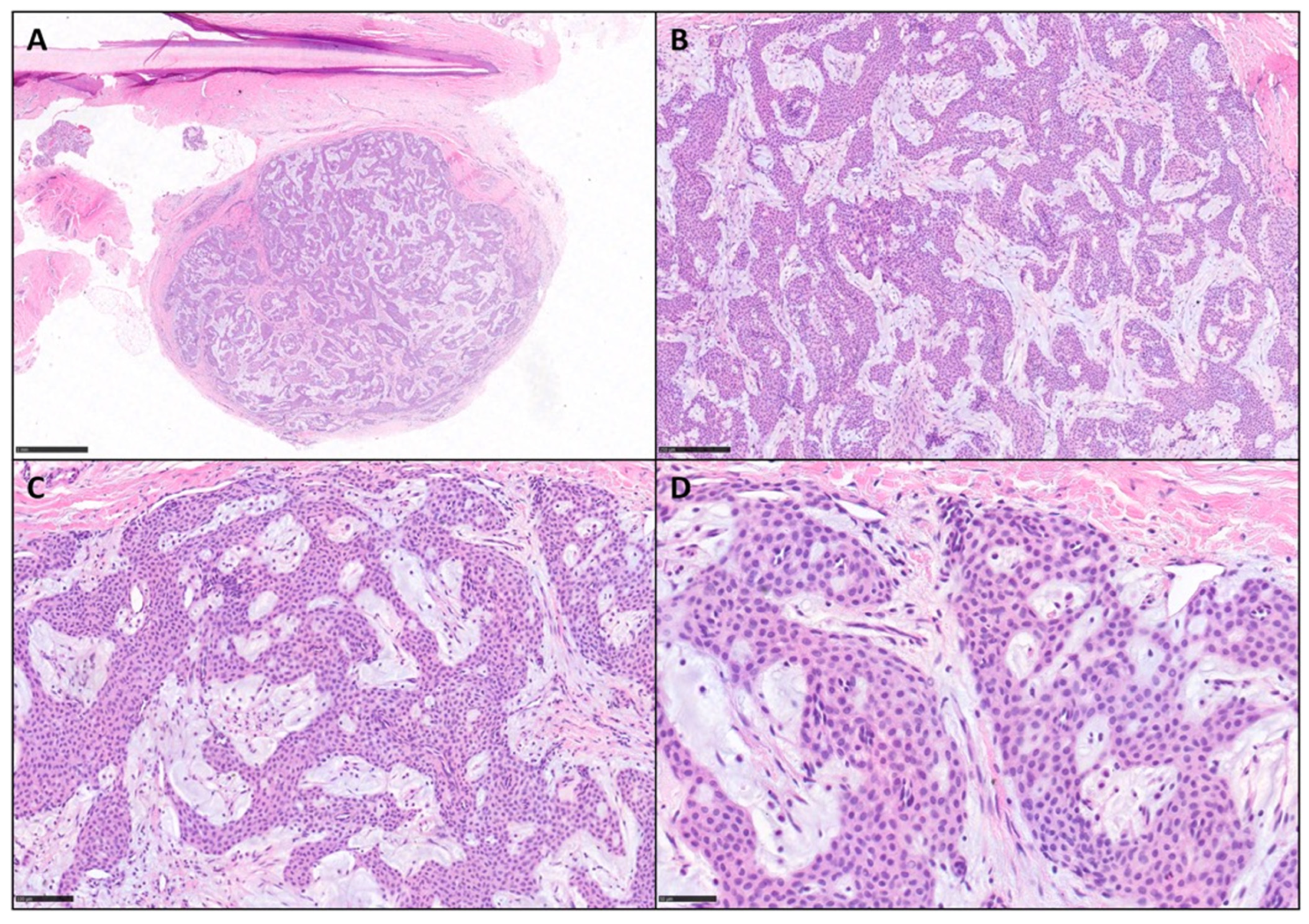
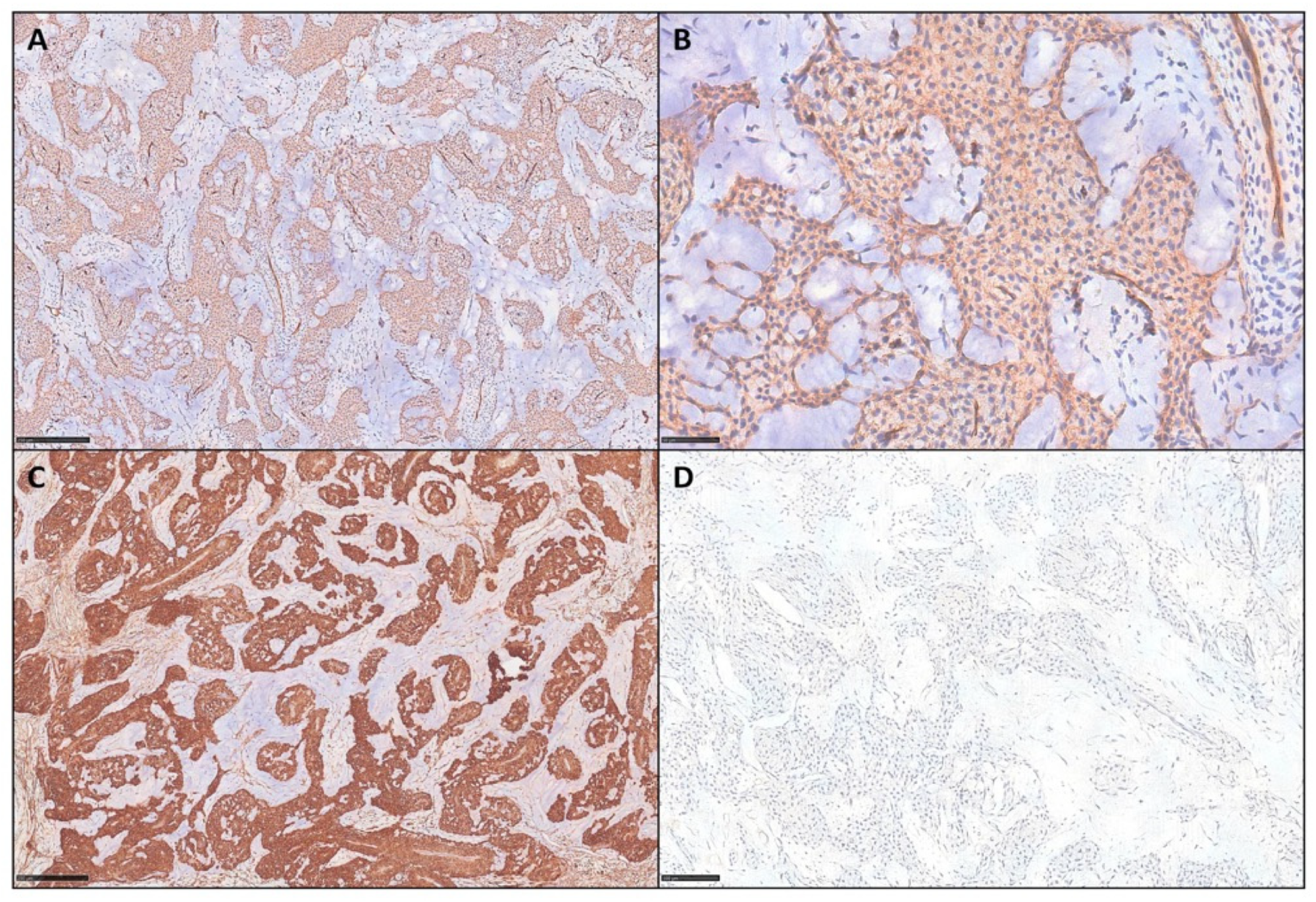
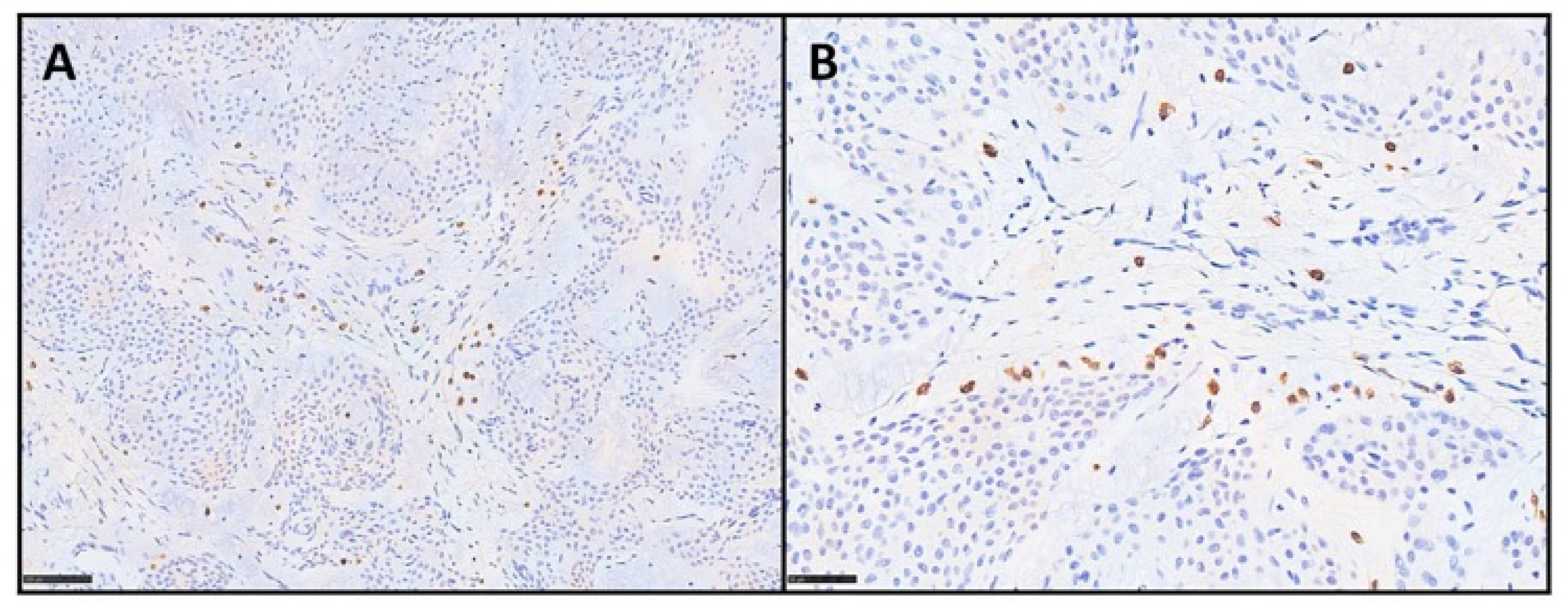
| Case Number | Age | Sex | Site | IHC | Mastocytes Count (Tryptase) |
|---|---|---|---|---|---|
| Case 1 | 33 | M | IV left toe, distal phalanx | SMA+, CD34+ (focal), S100− | 20/HPF |
| Case 2 | 55 | F | I left toe, distal phalax | SMA+, CD34+, S100− | 15/HPF |
| Case 3 | 26 | F | I left finger, distal phalanx | SMA+, CD34+, S100− | 12/HPF |
| Case 4 | 53 | F | III right finger | SMA+, CD34+, S100− | NP |
| Case 5 | 71 | M | II left finger, nail bed | SMA+, CD34+, S100− | NP |
| Case 6 | 32 | F | III left finger, nail bed | SMA+, CD34+, S100− | NP |
| Case 7 | 23 | F | II right finger, distal phalanx | SMA+, CD34+, S100− | NP |
| Case 8 | 69 | F | IV left finger, nail bed | SMA+, CD34+, S100− | NP |
| Authors (Year of Publication) | No. of Cases | Location | Histologic Description | Immunohistochemistry |
|---|---|---|---|---|
| Tsuneyoshi and Enjoji (1982) [20] | 23 | Fingers | Glomus epithelioid cells around vessels within myxoid matrix and numerous mast cells | NR |
| Pabuççuoğlu & Lebe (2008) [25] | 1 | Finger | Typical glomus cells in myxoid stroma containing mast cells | SMA+, CD34+, MMP-9+ |
| Mentzel et al. (2002) [16] | 6 | Fingers | Solid, glomangioma and glomangiomyoma types with prominent myxoid change | SMA+, CD34+ |
| Da Silva et al. (2018) [30] | 1 | Finger | Glomus cells with symplastic features within myxoid stroma | SMA+ |
| Our cases (2025) | 8 | Fingers | Typical glomus cells in myxoid stroma containing numerous mast cells | SMA+, CD34+, tryptase+, S100− |
| Entity | CD34 | SMA | Desmin | S100 | Other Markers |
|---|---|---|---|---|---|
| Myxoidglomus tumor | Variable/focal to diffuse positivity | Positive | Negative | Negative | MSA+, vimentin+ |
| Superficial acral fibromyxoma | Diffuse positivity | Negative or focal | Negative | Negative | EMA+ |
| Angioleiomyoma | Negative | Positive | Positive | Negative | Calponin+, SMA+, HMB45/Melan-A− |
| Myopericytoma | Rarely positive or negative | Positive | Positive | Negative | h-caldesmon+ |
| Solitary fibrous tumor (myxoid variant) | Diffuse positivity | Negative | Negative | Negative | STAT6+ |
| Low-grade fibromyxoid sarcoma | Negative | Negative | Negative | Negative | MUC4+ |
| Myxoid liposarcoma | Negative | Negative | Negative | Negative | MDM2+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorino, J.; Della Mura, M.; Colagrande, A.; Ricci, C.; Ingravallo, G.; Fanelli, F.; Fortarezza, F.; Giubellino, A.; Cazzato, G. Myxoid Glomus Tumors Showing CD34 Expression: A Series of Eight Cases. Diagnostics 2025, 15, 2852. https://doi.org/10.3390/diagnostics15222852
Sorino J, Della Mura M, Colagrande A, Ricci C, Ingravallo G, Fanelli F, Fortarezza F, Giubellino A, Cazzato G. Myxoid Glomus Tumors Showing CD34 Expression: A Series of Eight Cases. Diagnostics. 2025; 15(22):2852. https://doi.org/10.3390/diagnostics15222852
Chicago/Turabian StyleSorino, Joana, Mario Della Mura, Anna Colagrande, Costantino Ricci, Giuseppe Ingravallo, Francesco Fanelli, Francesco Fortarezza, Alessio Giubellino, and Gerardo Cazzato. 2025. "Myxoid Glomus Tumors Showing CD34 Expression: A Series of Eight Cases" Diagnostics 15, no. 22: 2852. https://doi.org/10.3390/diagnostics15222852
APA StyleSorino, J., Della Mura, M., Colagrande, A., Ricci, C., Ingravallo, G., Fanelli, F., Fortarezza, F., Giubellino, A., & Cazzato, G. (2025). Myxoid Glomus Tumors Showing CD34 Expression: A Series of Eight Cases. Diagnostics, 15(22), 2852. https://doi.org/10.3390/diagnostics15222852









