Soluble Urokinase Plasminogen Activator Receptor (suPAR) Predicts 28-Day and 90-Day Mortality in Emergency Department Patients with Chest Pain, Dyspnoea, or Abdominal Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Design
2.3. suPAR Measurement
2.4. Endpoints
2.5. Statistical Analysis
2.5.1. Sample Size
2.5.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. suPAR Levels at Baseline
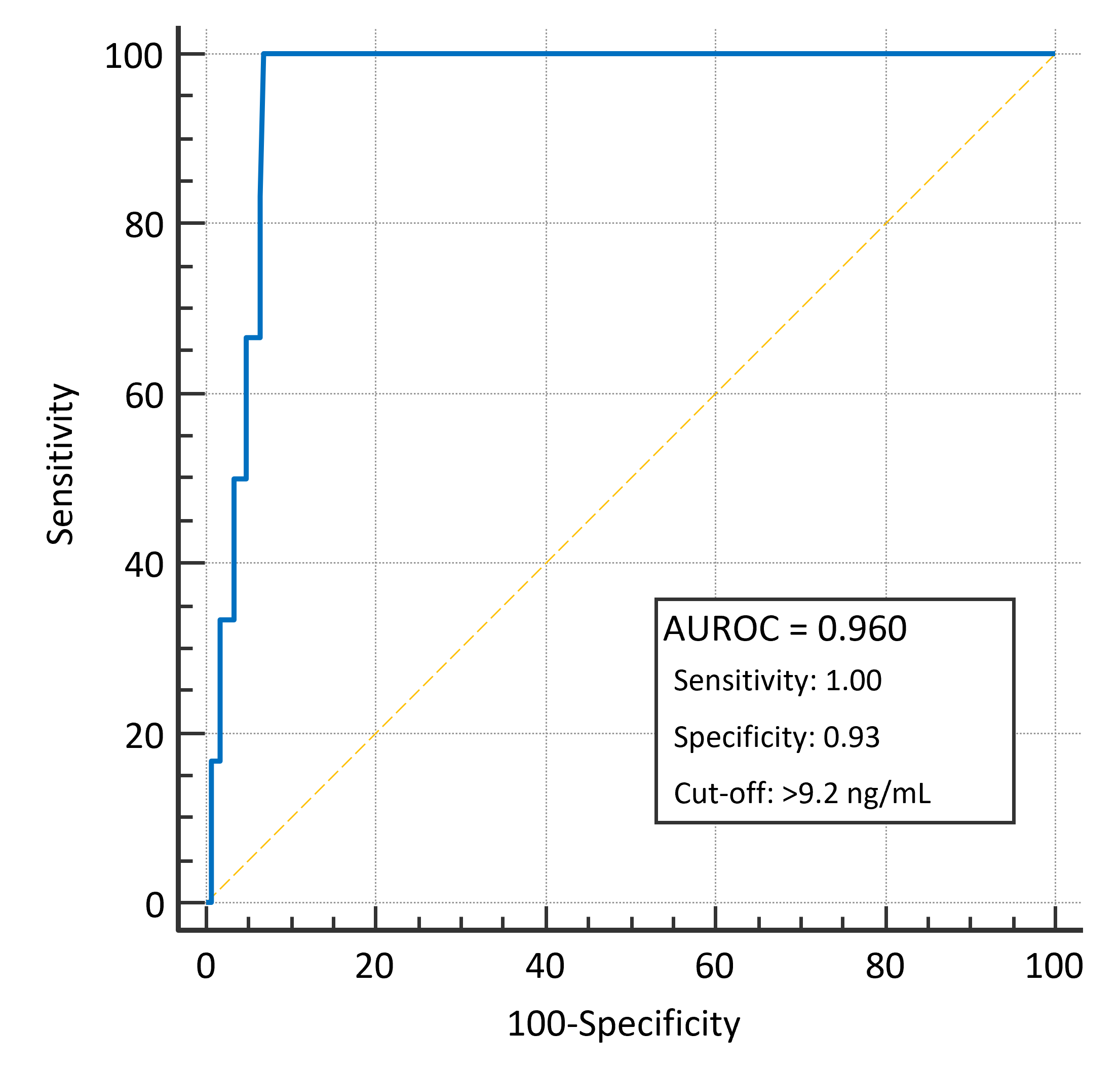
3.3. 28-Day Mortality
3.4. Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUROC | area under the ROC curve |
| BMI | body mass index |
| ED | emergency department |
| RDW | red cells distribution width |
| ROC | receiver operating characteristic |
| suPAR | soluble urokinase plasminogen activator receptor |
References
- CDC—Centre for Disease Control; National Center for Health Statistics (U.S.). National Hospital Ambulatory Medical Care Survey: 2021 Emergency Department Summary Tables. Available online: https://www.cdc.gov/nchs/fastats/emergency-department.htm (accessed on 24 August 2025).
- Castello, L.M.; Gavelli, F. Sepsis Scoring Systems: Mindful Use in Clinical Practice. Eur. J. Intern. Med. 2024, 125, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Lee, C.M.Y.; Begg, S.; Crombie, A.; Mnatzaganian, G. The Use of Early Warning System Scores in Prehospital and Emergency Department Settings to Predict Clinical Deterioration: A Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0265559. [Google Scholar] [CrossRef] [PubMed]
- Usman, O.A.; Usman, A.A.; Ward, M.A. Comparison of SIRS, qSOFA, and NEWS for the Early Identification of Sepsis in the Emergency Department. Am. J. Emerg. Med. 2019, 37, 1490–1497. [Google Scholar] [CrossRef]
- Bruhn, R.; Skjøt-Arkil, H.; Skovsted, T.A.; Brasen, C.L.; Andersen, E.S.; Heltborg, A.; Hertz, M.A.; Petersen, E.R.B.; Mogensen, C.B.; Torres, A.; et al. Biomarker Profiling for Infection Diagnosis in Emergency Departments: A Diagnostic Study Evaluating C-Reactive Protein, Procalcitonin, Club Cell Protein 16, Interleukin-6, Chitinase-like Protein, and Soluble Urokinase-Type Plasminogen Activator Receptor. Clin. Biochem. 2025, 138, 110943. [Google Scholar] [CrossRef]
- Rasmussen, L.J.H.; Petersen, J.E.V.; Eugen-Olsen, J. Soluble Urokinase Plasminogen Activator Receptor (suPAR) as a Biomarker of Systemic Chronic Inflammation. Front. Immunol. 2021, 12, 780641. [Google Scholar] [CrossRef]
- Rehan, S.T.; Hussain, H.U.; Ali, E.; Kumar, K.A.; Tabassum, S.; Hasanain, M.; Shaikh, A.; Ali, G.; Yousaf, Z.; Asghar, M.S. Role of Soluble Urokinase Type Plasminogen Activator Receptor (suPAR) in Predicting Mortality, Readmission, Length of Stay and Discharge in Emergency Patients: A Systematic Review and Meta Analysis. Medicine 2023, 102, e35718. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, C.; Han, Y.; Li, D.; Huang, Y.; Shi, K.; Xia, S.; Wei, J.; Liu, H.; Sun, L.; et al. The Predictive Value of suPAR for Glomerular Segmental Sclerosis Lesions in Renal Pathology. Ren. Fail. 2025, 47, 2498628. [Google Scholar] [CrossRef] [PubMed]
- Artusa, F.; Lamatsch, S.; Phan, M.D.; Özdirik, B.; Berger, H.; Egerer, M.; Knorr-Klocke, J.; Fischer, J.; Veelken, R.; van Bömmel, F.; et al. Soluble Urokinase Plasminogen Activator Receptor Predicts Survival and Hepatic Decompensation in Advanced Hepatocellular Carcinoma. Liver Int. 2025, 45, e70121. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, F.; Chen, C.; Xu, L.; Lin, W.; Huang, C.; Yang, Y.; Wu, S.; Qi, J.; Cao, H.; et al. Soluble Urokinase Plasminogen Activator Receptor Is Associated with Short-Term Mortality and Enhanced Reactive Oxygen Species Production in Acute-on-Chronic Liver Failure. BMC Gastroenterol. 2021, 21, 429. [Google Scholar] [CrossRef]
- Wisborg, F.D.; El Caidi, N.O.; Taraldsen, I.A.; Tonning, S.; Kandiah, A.; El-Sheikh, M.; Bahrami, H.S.Z.; Andersen, O.; Rasmussen, L.J.H.; Hove, J.; et al. Soluble Urokinase Plasminogen Activator Receptor (suPAR) as a Prognostic Biomarker in Acutely Admitted Patients with Atrial Fibrillation. J. Arrhythm. 2025, 41, e70077. [Google Scholar] [CrossRef]
- Turan, C.; Yurtseven, A.; Ozkaya, P.Y.; Azarsiz, E.; Saz, E.U. The Role of Soluble Urokinase Plasminogen Activator Receptor (suPAR) as an Early Indicator of Mortality in Pediatric Septic Shock. J. Clin. Lab. Anal. 2024, 38, e25040. [Google Scholar] [CrossRef] [PubMed]
- Belvederi, F.; Leggeri, S.; Urbani, A.; Baroni, S. suPAR as a Biomarker of Support in Different Clinical Settings. Clin. Chim. Acta 2025, 573, 120303. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Ning, P.; Zheng, Y.; Shang, Y.; Zhou, B.; Gao, Z. Serum suPAR and Syndecan-4 Levels Predict Severity of Community-Acquired Pneumonia: A Prospective, Multi-Centre Study. Crit. Care 2018, 22, 15. [Google Scholar] [CrossRef]
- Huang, Q.; Xiong, H.; Yan, P.; Shuai, T.; Liu, J.; Zhu, L.; Lu, J.; Yang, K.; Liu, J. The Diagnostic and Prognostic Value of suPAR in Patients with Sepsis: A Systematic Review and Meta-Analysis. Shock 2020, 53, 416–425. [Google Scholar] [CrossRef]
- Aronen, A.; Aittoniemi, J.; Huttunen, R.; Nikkola, A.; Nikkola, J.; Limnell, O.; Nordback, I.; Sand, J.; Laukkarinen, J. Plasma Level of Soluble Urokinase Plasminogen Activator Receptor (suPAR) Predicts Long-Term Mortality after First Acute Alcohol-Induced Pancreatitis. Eur. J. Intern. Med. 2019, 64, 72–75. [Google Scholar] [CrossRef]
- Ohnewein, B.; Shomanova, Z.; Jirak, P.; Paar, V.; Topf, A.; Pylypenko, L.; Schäbinger, M.; Volg, F.; Hoppe, U.C.; Pistulli, R.; et al. Dynamics of the Novel Cardiac Biomarkers sST2, H-FABP, GDF-15 and suPAR in HFrEF Patients Undergoing Heart Failure Therapy, a Pilot Study. J. Clin. Med. 2025, 14, 5668. [Google Scholar] [CrossRef]
- Bahrami, H.S.Z.; Jørgensen, P.G.; Hove, J.D.; Dixen, U.; Rasmussen, L.J.H.; Eugen-Olsen, J.; Rossing, P.; Jensen, M.T. Soluble Urokinase Plasminogen Activator Receptor and Interleukin-6 Improves Prediction of All-Cause Mortality and Major Adverse Cardiovascular Events in Type 1 Diabetes. J. Intern. Med. 2025, 298, 188–199. [Google Scholar] [CrossRef]
- Hessels, L.; Duijkers, R.; Schoorl, M.; Terpstra, L.; Thijs, W.; Boersma, W. The Value of Soluble Urokinase Plasminogen Activator Receptor (suPAR) as Predictive Tool in Hospitalised Patients With Community-Acquired Pneumonia (CAP). Clin. Respir. J. 2025, 19, e70089. [Google Scholar] [CrossRef]
- Haupt, T.H.; Kallemose, T.; Ladelund, S.; Rasmussen, L.J.; Thorball, C.W.; Andersen, O.; Pisinger, C.; Eugen-Olsen, J. Risk Factors Associated with Serum Levels of the Inflammatory Biomarker Soluble Urokinase Plasminogen Activator Receptor in a General Population. Biomark. Insights 2014, 9, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Haupt, T.H.; Petersen, J.; Ellekilde, G.; Klausen, H.H.; Thorball, C.W.; Eugen-Olsen, J.; Andersen, O. Plasma suPAR Levels Are Associated with Mortality, Admission Time, and Charlson Comorbidity Index in the Acutely Admitted Medical Patient: A Prospective Observational Study. Crit. Care 2012, 16, R130. [Google Scholar] [CrossRef]
- Rasmussen, L.J.H.; Ladelund, S.; Haupt, T.H.; Ellekilde, G.; Poulsen, J.H.; Iversen, K.; Eugen-Olsen, J.; Andersen, O. Soluble Urokinase Plasminogen Activator Receptor (suPAR) in Acute Care: A Strong Marker of Disease Presence and Severity, Readmission and Mortality. A Retrospective Cohort Study. Emerg. Med. J. 2016, 33, 769–775. [Google Scholar] [CrossRef]
- Rasmussen, L.J.H.; Ladelund, S.; Haupt, T.H.; Ellekilde, G.E.; Eugen-Olsen, J.; Andersen, O. Combining National Early Warning Score With Soluble Urokinase Plasminogen Activator Receptor (suPAR) Improves Risk Prediction in Acute Medical Patients: A Registry-Based Cohort Study. Crit. Care Med. 2018, 46, 1961–1968. [Google Scholar] [CrossRef]
- Schultz, M.; Rasmussen, L.J.H.; Kallemose, T.; Kjøller, E.; Lind, M.N.; Ravn, L.; Lange, T.; Køber, L.; Rasmussen, L.S.; Eugen-Olsen, J.; et al. Availability of suPAR in Emergency Departments May Improve Risk Stratification: A Secondary Analysis of the TRIAGE III Trial. Scand. J. Trauma Resusc. Emerg. Med. 2019, 27, 43. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.; Rasmussen, L.J.H.; Høi-Hansen, T.; Kjøller, E.; Jensen, B.N.; Lind, M.N.; Ravn, L.; Kallemose, T.; Lange, T.; Køber, L.; et al. Early Discharge from the Emergency Department Based on Soluble Urokinase Plasminogen Activator Receptor (suPAR) Levels: A TRIAGE III Substudy. Dis. Markers 2019, 2019, 3403549. [Google Scholar] [CrossRef] [PubMed]
- Lyngbæk, S.; Marott, J.L.; Møller, D.V.; Christiansen, M.; Iversen, K.K.; Clemmensen, P.M.; Eugen-Olsen, J.; Jeppesen, J.L.; Hansen, P.R. Usefulness of Soluble Urokinase Plasminogen Activator Receptor to Predict Repeat Myocardial Infarction and Mortality in Patients with ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Intervention. Am. J. Cardiol. 2012, 110, 1756–1763. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Desai, S.R.; Ko, Y.-A.; Liu, C.; Dhindsa, D.S.; Nayak, A.; Hooda, A.; Martini, M.A.; Ejaz, K.; Sperling, L.S.; et al. Sex Differences in Circulating Soluble Urokinase-Type Plasminogen Activator Receptor (suPAR) Levels and Adverse Outcomes in Coronary Artery Disease. J. Am. Heart Assoc. 2020, 9, e015457. [Google Scholar] [CrossRef]
- Shell, A.; Vize, C.; Gianaros, P.; Rasmussen, L.J.H.; Marsland, A.L. Executive Function and Soluble Urokinase-Type Plasminogen Activator Receptor (suPAR): A Longitudinal Study of Midlife Adults. Brain Behav. Immun. 2025, 129, 537–546. [Google Scholar] [CrossRef]
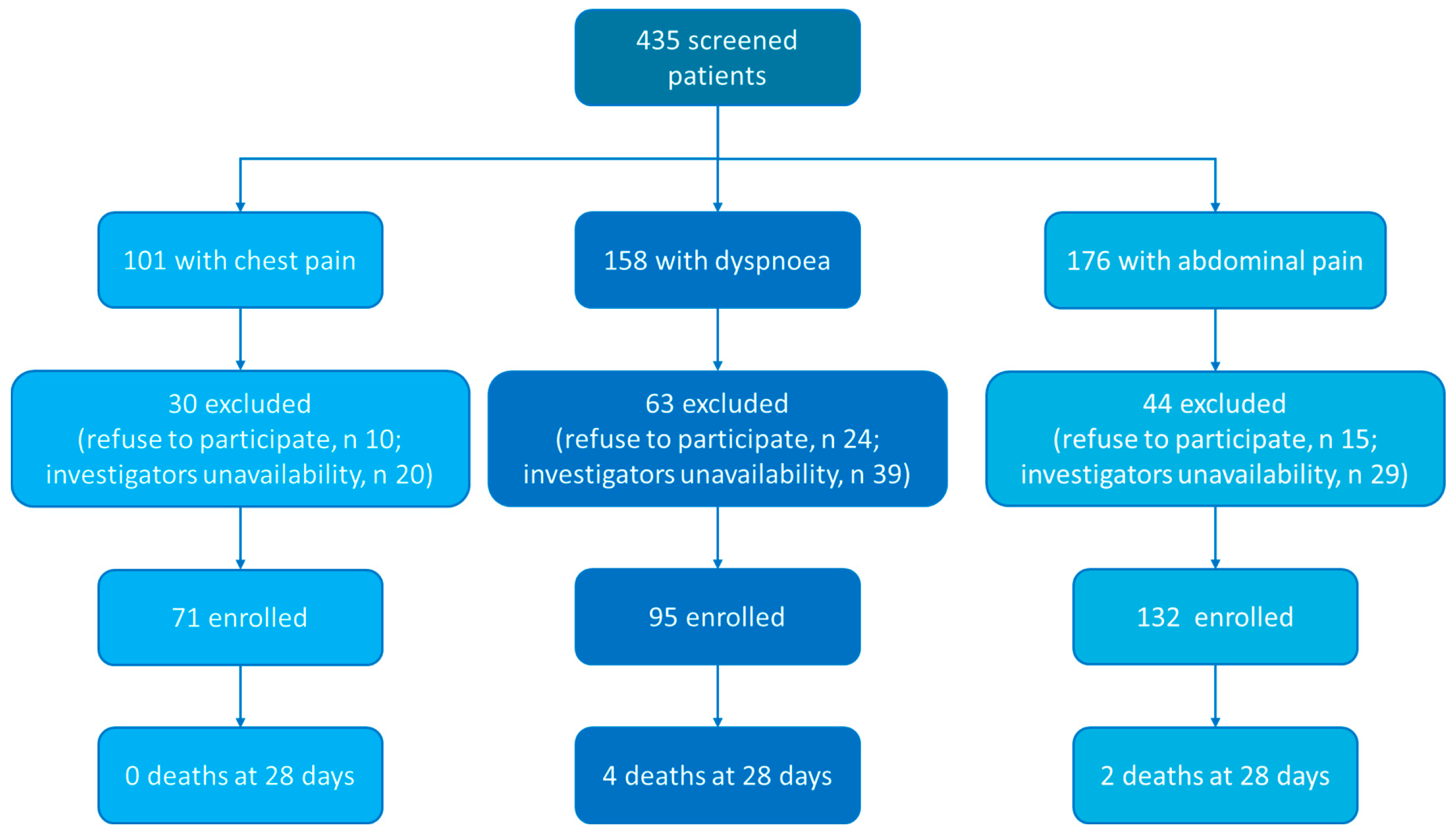

| Patients = 298 | |
| Male sex (n, %) | 164 (55%) |
| Age (years) | 59 [46–73] |
| Body mass index | 25.7 [23.1–28.4] |
| Peripheral oxygen saturation (%) | 98 [95–99] |
| Charlson comorbidity index | 2 [0–4] |
| Comorbidities (n, %) | |
| Arterial hypertension | 139 (46.6%) |
| Chronic heart failure | 11 (3.7%) |
| Atrial fibrillation/flutter | 34 (11.4%) |
| Acute coronary syndrome | 46 (15.4%) |
| Chronic obstructive pulmonary disease | 8 (2.7%) |
| Stroke/transient ischemic attack | 10 (3.3%) |
| Neoplasm | 29 (9.7%) |
| Diabetes | 34 (11.4%) |
| Presenting symptom (n, %) | |
| Chest pain | 71 (23.8%) |
| Dyspnoea | 95 (31.9%) |
| Abdominal pain | 132 (44.3%) |
| Patients’ destination (n, %) | |
| Discharge | 170 (57.0%) |
| Admission to low-intensity ward | 95 (31.9%) |
| Admission to semi-intensive/intensive care unit | 33 (11.1%) |
| 28-day mortality (n, %) | 6 (2%) |
| 28-day hospital readmission (n, %) | 20 (6.7%) |
| 90-day mortality (n, %) | 8 (2.7%) |
| 90-day hospital readmission (n, %) | 46 (15.4%) |
| White blood cells count (×103/µL) | 8.59 [6.50–10.93] |
| Haemoglobin (g/dL) | 14.0 [12.8–15.4] |
| RDW (%) | 13.3 [12.7–14.2] |
| Creatinine (mg/dL) | 0.81 [0.64–1.02] |
| Blood glucose (mg/dL) | 111 [97–131] |
| C-reactive protein (mg/L) | 7.4 [1.3–53.0] |
| Lactate (mmol/L) | 1.0 [0.76–1.40] |
| suPAR (ng/mL) | 3.70 [2.60–5.55] |
| Dead | Survived | p Value | |
|---|---|---|---|
| Age (years) | 83 [71–87] | 59 [45–72] | 0.002 |
| Charlson comorbidity index | 4.5 [3.8–5.3] | 2.0 [0.0–4.0] | 0.007 |
| Blood lactate (mmol/L) | 2.0 [1.2–5.6] | 1.0 [0.7–1.4] | 0.024 |
| White blood cells (×10/µL) | 10.06 [6.42–17.73] | 8.57 [6.49–10.92] | 0.325 |
| Haemoglobin (g/dL) | 10.7 [8.9–12.6] | 14.1 [12.8–15.4] | 0.001 |
| RDW (%) | 17.9 [13.5–21.6] | 13.3 [12.7–14.2] | 0.004 |
| C-reactive protein (mg/L) | 107 [21–219] | 7 [1–47] | 0.005 |
| suPAR (ng/mL) | 12.65 [9.83–18.53] | 3.60 [2.60–5.48] | <0.001 |
| Creatinine (mg/dL) | 1.5 [1.0–2.0] | 0.8 [0.6–1.0] | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavelli, F.; Giolitti, F.M.; Vidali, M.; Montersino, M.; Bertoli, M.; Molinari, L.; Baldrighi, M.; Beltrame, M.; Sainaghi, P.P.; Bellan, M.; et al. Soluble Urokinase Plasminogen Activator Receptor (suPAR) Predicts 28-Day and 90-Day Mortality in Emergency Department Patients with Chest Pain, Dyspnoea, or Abdominal Pain. Diagnostics 2025, 15, 2851. https://doi.org/10.3390/diagnostics15222851
Gavelli F, Giolitti FM, Vidali M, Montersino M, Bertoli M, Molinari L, Baldrighi M, Beltrame M, Sainaghi PP, Bellan M, et al. Soluble Urokinase Plasminogen Activator Receptor (suPAR) Predicts 28-Day and 90-Day Mortality in Emergency Department Patients with Chest Pain, Dyspnoea, or Abdominal Pain. Diagnostics. 2025; 15(22):2851. https://doi.org/10.3390/diagnostics15222851
Chicago/Turabian StyleGavelli, Francesco, Francesca Maria Giolitti, Matteo Vidali, Marta Montersino, Matteo Bertoli, Luca Molinari, Marco Baldrighi, Michela Beltrame, Pier Paolo Sainaghi, Mattia Bellan, and et al. 2025. "Soluble Urokinase Plasminogen Activator Receptor (suPAR) Predicts 28-Day and 90-Day Mortality in Emergency Department Patients with Chest Pain, Dyspnoea, or Abdominal Pain" Diagnostics 15, no. 22: 2851. https://doi.org/10.3390/diagnostics15222851
APA StyleGavelli, F., Giolitti, F. M., Vidali, M., Montersino, M., Bertoli, M., Molinari, L., Baldrighi, M., Beltrame, M., Sainaghi, P. P., Bellan, M., Patrucco, F., Avanzi, G. C., & Castello, L. M. (2025). Soluble Urokinase Plasminogen Activator Receptor (suPAR) Predicts 28-Day and 90-Day Mortality in Emergency Department Patients with Chest Pain, Dyspnoea, or Abdominal Pain. Diagnostics, 15(22), 2851. https://doi.org/10.3390/diagnostics15222851






