1. Introduction
Bell’s palsy is the most common peripheral palsy of the seventh cranial nerve, with affected patients typically presenting with rapid onset of unilateral facial weakness or paralysis [
1,
2,
3]. Bell’s palsy is typically a diagnosis of exclusion and often managed on the basis of the clinical presentation when no other neurological deficits are present. In cases where patients exhibit typical features of Bell’s palsy, additional laboratory or radiological examinations are generally not required [
4,
5,
6]. Although it is often a benign disease with symptoms and signs resolving within 6 months, facial palsy can be associated with less common conditions such as stroke, neoplasm, infection, or pontine hemorrhage [
7,
8,
9,
10,
11].
The need to perform brain magnetic resonance imaging (MRI) for patients presenting with facial palsy remains a subject of ongoing debate. While routine MRI may be a standard procedure to rule out secondary causes in some settings, it may not be necessary for patients without additional neurological symptoms. From an economical perspective, the necessity of MRI for the diagnosis of Bell’s palsy should be carefully considered [
12].
Current clinical guidelines vary [
4,
5,
6], and it remains unclear whether routine MRI adds value to the diagnostic process or unnecessarily increases healthcare costs and resource utilization.
To address this gap, we investigated patients who presented with clinically isolated unilateral facial palsy and underwent brain MRI in the emergency department (ED) in order to evaluate the diagnostic yield of brain MRI. By analyzing the MRI results of these patients, we aimed to establish the necessity of routine MRI for confirming the diagnosis of Bell’s palsy and determine if it should be reserved for cases with specific clinical indications in ED.
2. Methods
2.1. Study Design and Participants
This single-center, retrospective study included adult patients (aged ≥18 years) who presented to the ED with facial palsy as the primary symptom from 1 January 2020 to 31 December 2022. Patients were excluded if they exhibited neurological abnormalities other than facial palsy during the initial examination or did not undergo MRI. The study protocol was approved by the institutional review board of our hospital (study no. 2023-0916), and the requirement for informed consent was waived due to the retrospective design. All procedures were conducted in accordance with the Declaration of Helsinki and local data privacy regulations. This study is an observational study conducted using an existing database and was carried out in accordance with the STROBE statement.
2.2. Variables
Data were extracted from medical records of the included patients. Demographic information, including sex, age, primary symptoms, and time of symptom onset, was collected. Comorbid conditions such as hypertension, diabetes, and a history of transient ischemic attack (TIA) or stroke were also recorded. Neurological findings were based on the initial assessment performed by emergency physicians (residents or attending physicians), and included the House−Brackmann facial nerve grading scale and other relevant neurological signs. All patients who presented to the ED with neurologic complaints underwent a structured neurological examination, which was recorded in the electronic medical record.
In accordance with the institutional protocol, physicians are required to assess and document at least four of the following seven neurological domains to qualify for procedural billing under the “simple neurologic examination” code: (1) level of consciousness and higher cortical function, (2) cranial nerve function, (3) limb motor strength, (4) limb sensory function, (5) deep tendon reflexes, (6) cerebellar and vestibular function, and (7) gait and balance.
For the purpose of this study, “clinically isolated unilateral facial palsy” was defined as unilateral facial weakness without any other documented neurological abnormalities on initial examination. MRI results were reviewed on the basis of the interpretations provided by a radiology specialist. All brain MRIs during the study period were performed using the same 1.5-Tesla scanner (MAGNETOM Avanto, Siemens Healthineers, Erlangen, Germany).
2.3. Outcomes
The primary outcome was the presence of positive MRI findings. Patients were categorized into two groups based on MRI results: those with positive findings and those with negative findings. An MRI was considered positive if it revealed a brain lesion deemed causally related to the facial palsy (e.g., ischemic stroke, tumor, and hemorrhage), based on anatomical correlation and interpretation by a board-certified radiologist. Findings unrelated to facial nerve pathways or considered incidental were classified as negative. In cases where brain MRI revealed structural abnormalities, the relevance of these findings to the patient’s presenting facial palsy was determined through consultation with a board-certified neurologist or neurosurgeon. If the lesion was deemed anatomically unrelated, contralateral to the symptoms or not clinically compatible with acute onset facial palsy, it was classified as incidental. These patients were ultimately diagnosed with idiopathic (Bell’s) palsy based on the specialist’s judgment. Radiologic enhancement of the facial nerve alone, in the absence of an alternative diagnosis, was not regarded as a positive finding. Baseline characteristics were compared between the two groups.
2.4. Statistical Analysis
Continuous variables are presented as mean with standard deviations or median with interquartile range (IQR), depending on distribution. Categorical variables are reported as number and percentage (%). Continuous variables were compared using the unpaired Student’s t-test for normally distributed data and the Mann–Whitney U test for non-normally distributed data. Categorical variables were compared using the chi-square test. To explore the factors associated with non-idiopathic facial palsy, univariate logistic regression was first performed. Variables with statistical significance were entered into a multivariable logistic regression model. Given the limited number of outcome events (n = 13), the events-per-variable (EPV) for our multivariable model was approximately 4.3. We acknowledge that this falls below the recommended threshold of 10 and have the risk of overfitting. We performed sensitivity analysis of Firth’s penalized likelihood logistic regression to account for small sample size and rare events. Odds ratios (ORs) were determined using logistic regression analysis with a 95% confidence interval (CI). All statistical analyses were performed using IBM SPSS Statistics, version 21 (IBM Corp., Armonk, NY, USA) and R software, version 4.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
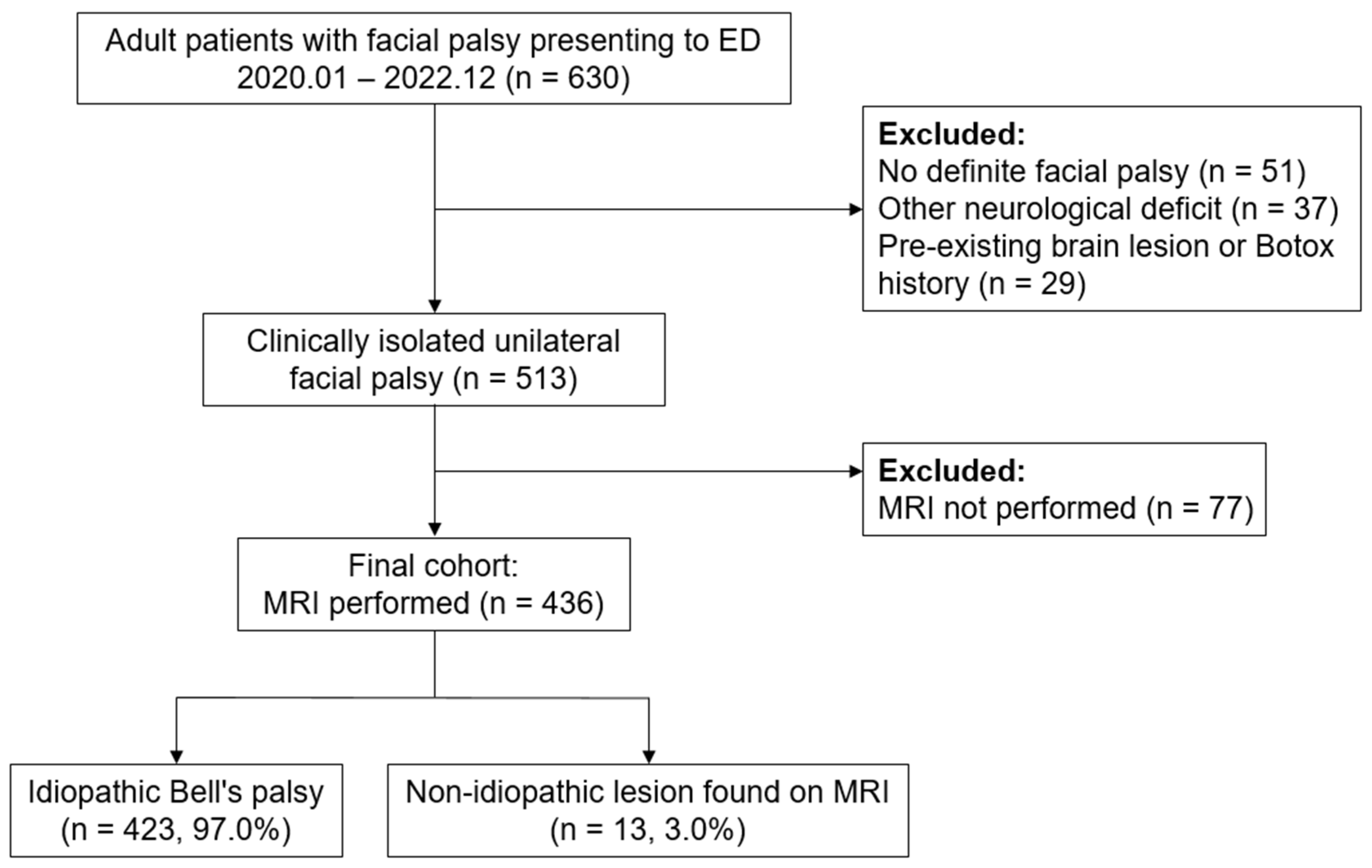
During the study period, 630 adult patients presented to the ED with facial palsy as the chief complaint. We excluded 51 patients without objective paralysis on neurological examination, 37 with additional neurological deficits, and 29 with pre-existing brain lesions or prior Botox injections. This left 513 patients with clinically isolated unilateral facial palsy, of whom 77 did not undergo brain MRI based on clinical judgment and were excluded from further analysis.
The final cohort included 436 patients who underwent brain MRI (
Figure 1). Among them, 13 (3.0%) had non-idiopathic lesions considered causative of their facial palsy (seven brain tumors or lymphomas, five ischemic strokes, and one pontine hemorrhage). Of the remaining 423 patients, eight had incidental findings (e.g., brain tumors, subdural hemorrhage, or infarction) deemed unrelated to their facial palsy after specialist review. These patients were ultimately diagnosed with Bell’s palsy.
Table 1 summarizes the baseline characteristics of the enrolled patients. The mean age of patients was 58 years, and 52.3% were women. The mean time from symptom onset to the ED presentation was 25 h. In total, 41.3% patients exhibited facial palsy with House–Brackmann grades 4–5. No significant differences were found between the Bell’s palsy group and the non-idiopathic facial palsy group in terms of these demographic and clinical characteristics. A history of TIA/stroke (
p = 0.02) and active malignancy (
p < 0.001) were significantly more frequent in the non-idiopathic facial palsy group.
Diffusion-weighted MRI was performed for 92% patients; 8% underwent MRI with contrast enhancement. In the Bell’s palsy group, 98.1% patients received a consultation with ear, nose, and throat (ENT) specialists, and the median ED stay was approximately 283 min. However, in the other facial palsy group, the median duration of the ED stay was 626 min.
Table 2 presents the results of logistic regression analyses. Univariate logistic regression analysis showed that the time of onset and the presence of comorbid diseases such as TIA/stroke and malignancy were significant factors, with ORs of 4.23 (95% CI: 1.10 to 16.26), 0.04 (95% CI: 1.10 to 16.26), and 18.57 (95% CI: 5.81 to 59.42), respectively. In multivariate logistic regression analysis that included variables with statistical significance in univariate analysis, the presence of TIA/stroke and active malignancy remained significant, with adjusted ORs of 12.08 (95% CI: 2.26 to 64.41) and 27.08 (6.32 to 116.08), respectively. To account for the small number of events and potential overfitting, we additionally performed Firth’s penalized logistic regression as a sensitivity analysis. The results were consistent in direction and magnitude, supporting the robustness of our findings. Full results are presented in
Supplementary Table S1.
Table 3 and
Figure 2 detail the positive MRI findings observed in the 13 patients whose final diagnosis differed from idiopathic Bell’s palsy. Brain tumors included newly diagnosed primary brain neoplasms, skull or brain parenchymal metastases from systemic malignancy, and leptomeningeal seeding. Ischemic strokes were found in the middle cerebral artery (MCA) territory, basal ganglia, internal capsule, corona radiata, and posterior circulation. One case involved pontine hemorrhage with an underlying cavernous malformation.
In each case, the anatomical relevance of the lesion to the patient’s presenting facial palsy was evaluated by board-certified neurologists, neurosurgeons, or oncologists. While most lesions clearly corresponded to known facial nerve pathways or brainstem structures, in two cases (Cases #7 and #12), the correlation was less direct. Nevertheless, after multidisciplinary consultation, these lesions were considered clinically relevant to the facial palsy and thus classified as positive MRI findings.
4. Discussions
During the study period, 3% patients with unilateral facial palsy underwent brain MRI and exhibited positive findings that correlated with their symptoms, including brain tumors, ischemic stroke, and pontine hemorrhage. This indicates the presence of cases where initial neurological examination alone may not be sufficient for accurate diagnosis.
Facial palsy may result from either central or peripheral lesions. Central lesions, such as strokes involving the motor cortex or corticobulbar tract, typically spare the forehead. In contrast, peripheral causes—including Bell’s palsy, tumors, infections, or trauma—affect the facial nerve nucleus or its distal course and often present with complete unilateral facial weakness. Recognizing these patterns is important for accurate diagnosis.
The diagnosis of Bell’s palsy is generally based on clinical examination alone, without the need for laboratory tests or imaging studies. However, the 2020 guideline from the French Society of ENT suggests, based on expert opinion, that MRI may be warranted in cases with atypical presentations in order to differentiate between Bell’s palsy and other conditions like stroke [
5]. Given the existence of several reports of cases, such as those of occult tumors or pontine strokes mimicking Bell’s palsy and leading to misdiagnosis [
13,
14,
15,
16,
17,
18,
19], there is a need to understand the frequency with which different diagnoses are established for patients who primarily exhibit facial palsy.
In this study, among 513 patients with clinically isolated unilateral facial palsy, 436 underwent MRI. This reflected both patient preferences and the high imaging rates intended to avoid misdiagnoses in tertiary hospitals. Among the 436 patients, 21 showed positive MRI findings. Of these, eight were considered incidental and unrelated to Bell’s palsy. The remaining 13 patients required reevaluation of the initial diagnosis. For example, for patient #1, initial examination was limited because of dementia; however, MRI revealed an infarct in the MCA territory. Patient #13 initially complained of facial palsy only during the initial examination, although MRI detected pontine hemorrhage. Further imaging with brain computed tomography confirmed the presence of a lesion correlating with the symptoms. Subsequent examinations revealed additional symptoms such as diplopia, nystagmus, extraocular muscle limitation, and dysarthria, which had not been reported initially. These cases revealed the challenges faced while diagnosing Bell’s palsy solely on the basis of the patient’s reported symptoms, especially when initial neurological examinations are limited or unreliable.
Additionally, patients #7 to #11 had active malignancies and underwent MRI for exclusion of brain metastasis, despite the absence of other abnormalities in the neurological examination. This further underscores the utility of MRI in cases where malignancy is a concern.
Thus, while MRI may be useful for diagnosing conditions that mimic Bell’s palsy, it may not be necessary for all patients with clinically isolated unilateral facial palsy if initial neurological evaluations are thorough and do not indicate other risk factors or neurological signs. A selective imaging strategy based on risk factors such as malignancy or TIA/stroke may improve diagnostic efficiency without compromising safety.
In support of this selective imaging strategy, we estimated the diagnostic yield and number needed to image (NNI) for different clinical subgroups. In the overall cohort of 436 patients, the NNI to identify one clinically relevant brain lesion was approximately 33.5. However, among patients with either a history of malignancy or prior TIA/stroke (
n = 63), 10 had clinically relevant findings, resulting in an NNI of just 6.3. These results suggest that risk factor-based triage can substantially improve diagnostic efficiency and reduce resource use in the ED. The corresponding estimates are summarized in
Supplementary Table S2.
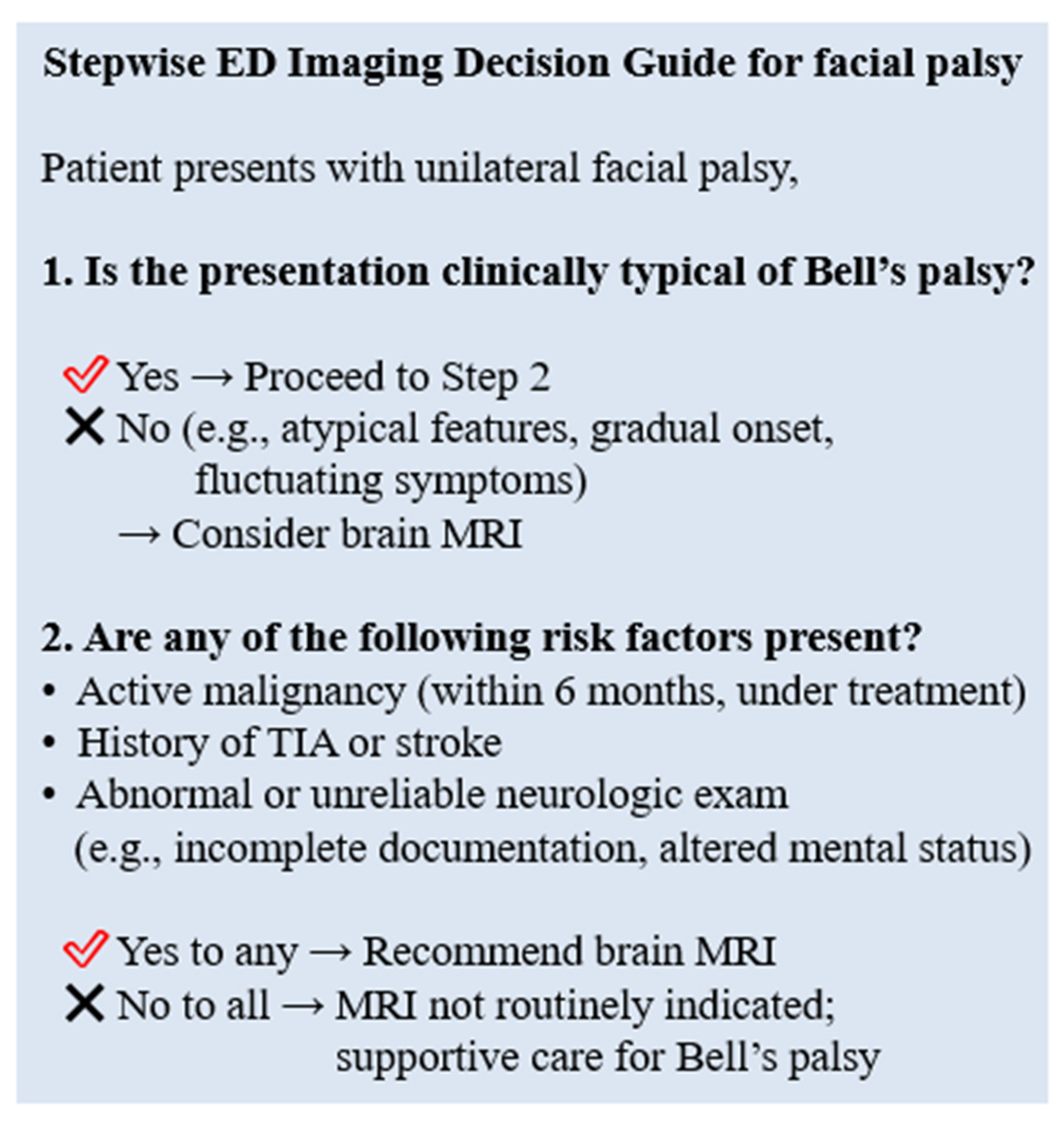
To translate these findings into clinical practice, we developed a stepwise ED imaging decision guide (
Figure 3). This guide incorporates both clinical presentation and patient-specific risk factors, such as active malignancy, prior TIA/stroke, or unreliable neurological examination. While not intended as an absolute protocol, it may serve as a practical tool to assist clinicians in making more efficient and targeted decisions regarding brain MRI in patients presenting with facial palsy.
5. Limitations
This study has several limitations. First, its retrospective design relied on chart documentation, which may have been incomplete or inconsistent, potentially leading to misclassification of clinically isolated facial palsy. Second, as a single-center study in a tertiary ED with high MRI availability and a patient population enriched with comorbidities, both selection and work-up bias may have influenced both MRI utilization and lesion detection rates. Imaging protocols were not standardized, and higher-risk or atypical patients were more likely to undergo contrast-enhanced imaging. The timing between symptom onset and MRI acquisition was also variable, which may have affected lesion detectability, particularly for ischemic or enhancing lesions. Third, only 13 patients had non-idiopathic facial palsy, resulting in a low EPV (4.3) and potential overfitting in multivariable analysis, despite sensitivity analyses using penalized regression. Finally, the high accessibility of MRI and the relatively high prevalence of malignancy in our cohort may limit the generalizability of these findings to community or resource-limited settings. These factors may have increased the positive predictive value of selective imaging strategies in this population. Therefore, our results should be interpreted with caution when applied to populations in other countries or healthcare environments.
6. Conclusions
In adults presenting to the ED with clinically isolated unilateral facial palsy and no additional neurological signs, the diagnostic yield of routine brain MRI was low (3%). A history of active malignancy or prior TIA/stroke appeared to increase the likelihood of alternative diagnoses. These findings suggest that a selective imaging approach—based on individual risk factors and the reliability of the initial neurological examination—may help optimize resource utilization while maintaining diagnostic safety.
Supplementary Materials
The following supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/diagnostics15172135/s1, Table S1: Results of Firth’s penalized logistic regression for predictors of positive MRI findings; Table S2: Diagnostic yield and number needed to image (NNI) for clinically relevant brain lesions in patients with clinically isolated facial palsy.
Author Contributions
Conceptualization, Y.-J.K. and W.Y.K.; data curation, H.P., Y.-J.K. and W.Y.K.; formal analysis, H.P.; investigation, H.P.; methodology, W.Y.K.; supervision, Y.-J.K. and W.Y.K.; validation, Y.-J.K. and W.Y.K.; writing—original draft, H.P.; writing—review and editing, H.P., Y.-J.K. and W.Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2024-00335934). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Asan Medical Center (protocol code 2023-0916, date of approval: 23 July 2023). The requirement for informed consent was waived due to the retrospective design of the study.
Informed Consent Statement
Patient consent was waived due to the retrospective design of the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Basic-Kes, V.; Dobrota, V.D.; Cesarik, M.; Matovina, L.Z.; Madzar, Z.; Zavoreo, I.; Demarin, V. Peripheral facial weakness (Bell’s palsy). Acta Clin. Croat. 2013, 52, 195–202. [Google Scholar] [PubMed]
- Holland, N.J.; Bernstein, J.M. Bell’s palsy. BMJ Clin. Evid. 2014. [Google Scholar]
- Warner, M.J.; Hutchison, J.; Varacallo, M. Bell Palsy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Baugh, R.F.; Basura, G.J.; Ishii, L.E.; Schwartz, S.R.; Drumheller, C.M.; Burkholder, R.; Deckard, N.A.; Dawson, C.; Driscoll, C.; Gillespie, M.B.; et al. Clinical Practice Guideline: Bell’s Palsy. Otolaryngol.–Head Neck Surg. 2013, 149, S1–S27. [Google Scholar] [CrossRef] [PubMed]
- Fieux, M.; Franco-Vidal, V.; Devic, P.; Bricaire, F.; Charpiot, A.; Darrouzet, V.; Denoix, L.; Gatignol, P.; Guevara, N.; Montava, M.; et al. French Society of ENT (SFORL) guidelines. Management of acute Bell’s palsy. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2020, 137, 483–488. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, J.R.; Guyatt, G.H.; Sud, S.; Dorion, J.; Hill, M.D.; Kolber, M.R.; Lea, J.; Reg, S.L.; Somogyi, B.K.; Westerberg, B.D.; et al. Management of Bell palsy: Clinical practice guideline. CMAJ 2014, 186, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, J.G.; Urban, P.P.; Pitz, S.; Guntinas-Lichius, O.; Gagyor, I. The Diagnosis and Treatment of Idiopathic Facial Paresis (Bell’s Palsy). Dtsch. Arztebl. Int. 2019, 116, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Jesse, S.; Kassubek, J.; Pinkhardt, E.; Ludolph, A.C. Differential diagnosis of peripheral facial nerve palsy: A retrospective clinical, MRI and CSF-based study. J. Neurol. 2019, 266, 2488–2494. [Google Scholar] [CrossRef] [PubMed]
- May, M.; Klein, S.R. Differential diagnosis of facial nerve palsy. Otolaryngol. Clin. N. Am. 1991, 24, 613–645. [Google Scholar] [CrossRef]
- George, E.; Richie, M.B.; Glastonbury, C.M. Facial Nerve Palsy: Clinical Practice and Cognitive Errors. Am. J. Med. 2020, 133, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Fahimi, J.; Navi, B.B.; Kamel, H. Potential misdiagnoses of Bell’s palsy in the emergency department. Ann. Emerg. Med. 2014, 63, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, E.; Schaffer, H.M.; Wozniak, A.; Leonetti, J.P. Economic Impact of Diagnostic Imaging in the Workup of Uncomplicated Bell’s Palsy. J. Neurol. Surg. B Skull Base 2022, 83, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, L.; Kaur, R.; Flossmann, E. Pontine stroke mimicking Bell’s palsy: A cautionary tale! BMJ Case Rep. 2021, 14, e238141. [Google Scholar] [CrossRef] [PubMed]
- Karadan, U.; Manappallil, R.G.; Jayakrishnan, C.; Supreeth, R.N. Pontine haemorrhage disguised as Bell’s palsy. BMJ Case Rep. 2018, 2018, bcr-2017223214. [Google Scholar] [CrossRef] [PubMed]
- Mabel, H.M.; Othman, N.B.; Cheah, W.K. Pontine stroke: A rare mimicker of Bell’s palsy. Med. J. Malays. 2022, 77, 403–405. [Google Scholar]
- Mower, S. Bell’s palsy: Excluding serious illness in urgent and emergency care settings. Emerg. Nurse 2017, 25, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wang, B.; Lu, F.; He, X.; Lu, G.; Zhang, S. Facial nerve in skullbase tumors: Imaging and clinical relevance. Eur. J. Med. Res. 2023, 28, 121. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.J.; Matic, D.; Fung, K.; MacNeil, S.D.; Nichols, A.C.; Kiwan, R.; Tay, K.; Yoo, J. Bell’s palsy misdiagnosis: Characteristics of occult tumors causing facial paralysis. J. Otolaryngol. Head Neck Surg. 2022, 51, 39. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.N.; Mallepally, N.; Tabilona, J.R.; Lu, L.B. Not So Benign Bell’s Palsy: Malignant Peripheral Nerve Sheath Tumor of the Facial Nerve Involving the Temporal Bone. J. Gen. Intern. Med. 2021, 36, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).