Photon-Counting Computed Tomography of the Paranasal Sinuses Improves Intraoperative Accuracy of Image-Guided Surgery
Abstract
1. Background
2. Methods
2.1. Study Design and Study Sample
2.2. Imaging Protocols
2.3. Setup of Image-Guided Surgery and Endoscopic Evaluation of Accuracy
2.4. Analysis of Image Quality
- -
- Visibility of the anterior skull base, lamina papyracea, orbital fat, anterior ethmoidal artery and bony coverage of the optic nerve and internal carotid artery (1 = not visible; 2 = vaguely visible, but not over the entire extent; 3 = vaguely visible, over the entire extent; 4 = unambiguously visible, but not over the entire extent; 5 = unambiguously visible, over the entire extent).
- -
- Overall image quality (1 = poor; 2 = acceptable; 3 = moderate; 4 = good; 5 = excellent).
- -
- Metal and motion artifacts (1 = severe artifacts, image interpretation impossible; 2 = severe artifacts, strong impairment of image interpretation; 3 = severe artifacts, slight impairment of image interpretation; 4 = slight artifacts, no impairment of image interpretation; 5 = no artifacts).
2.5. Statistical Analysis
3. Results
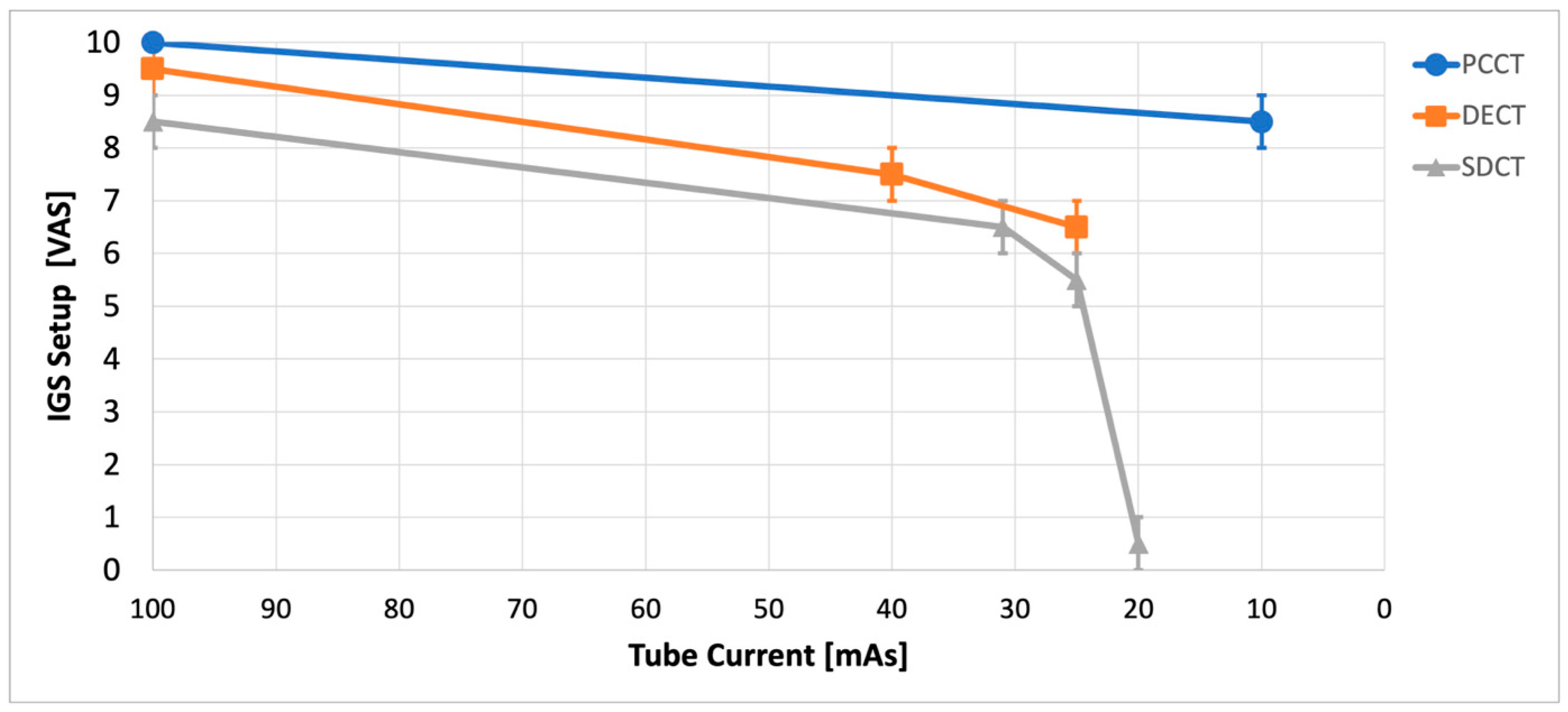
3.1. IGS Setup
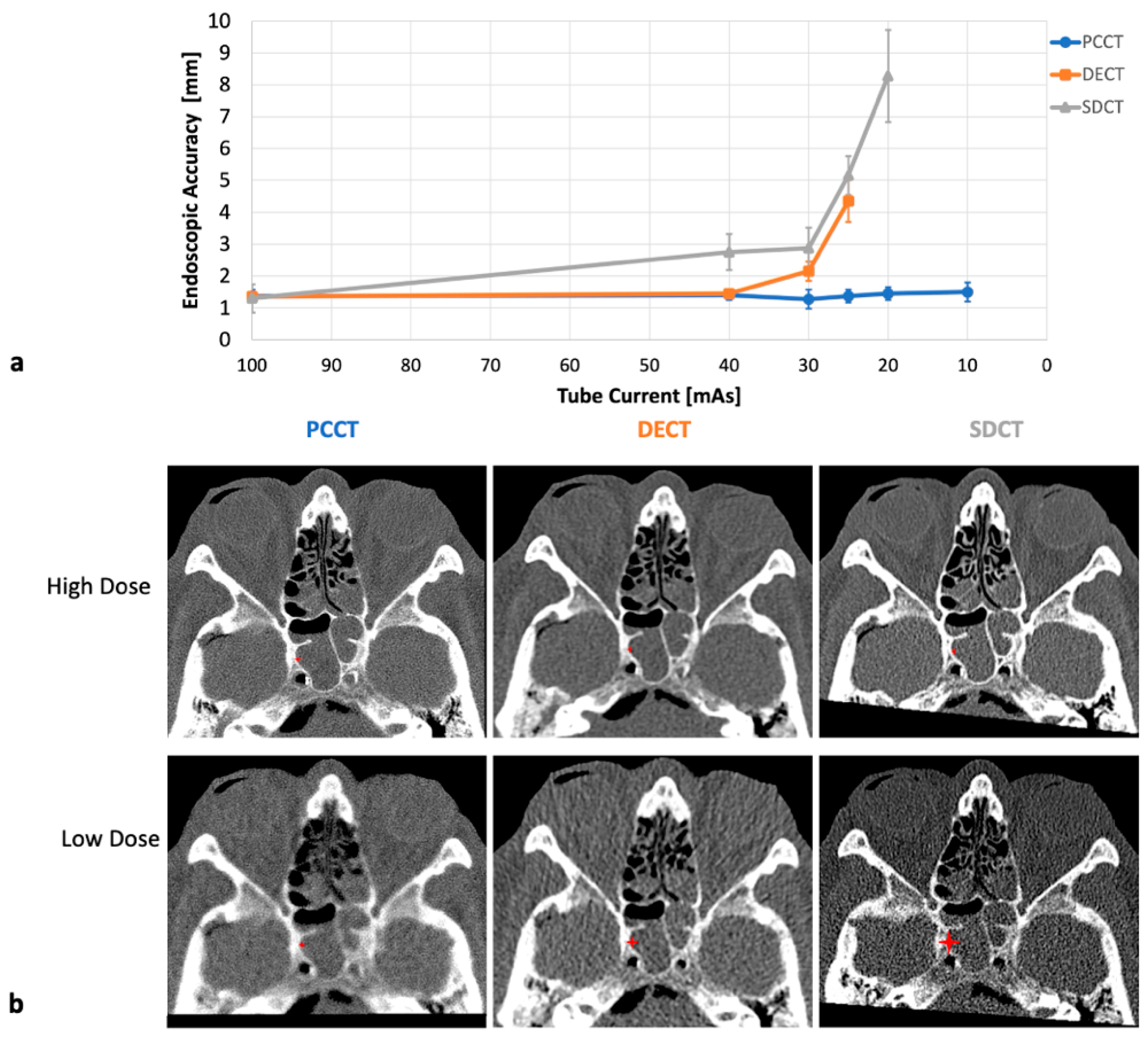
3.2. IGS Accuracy
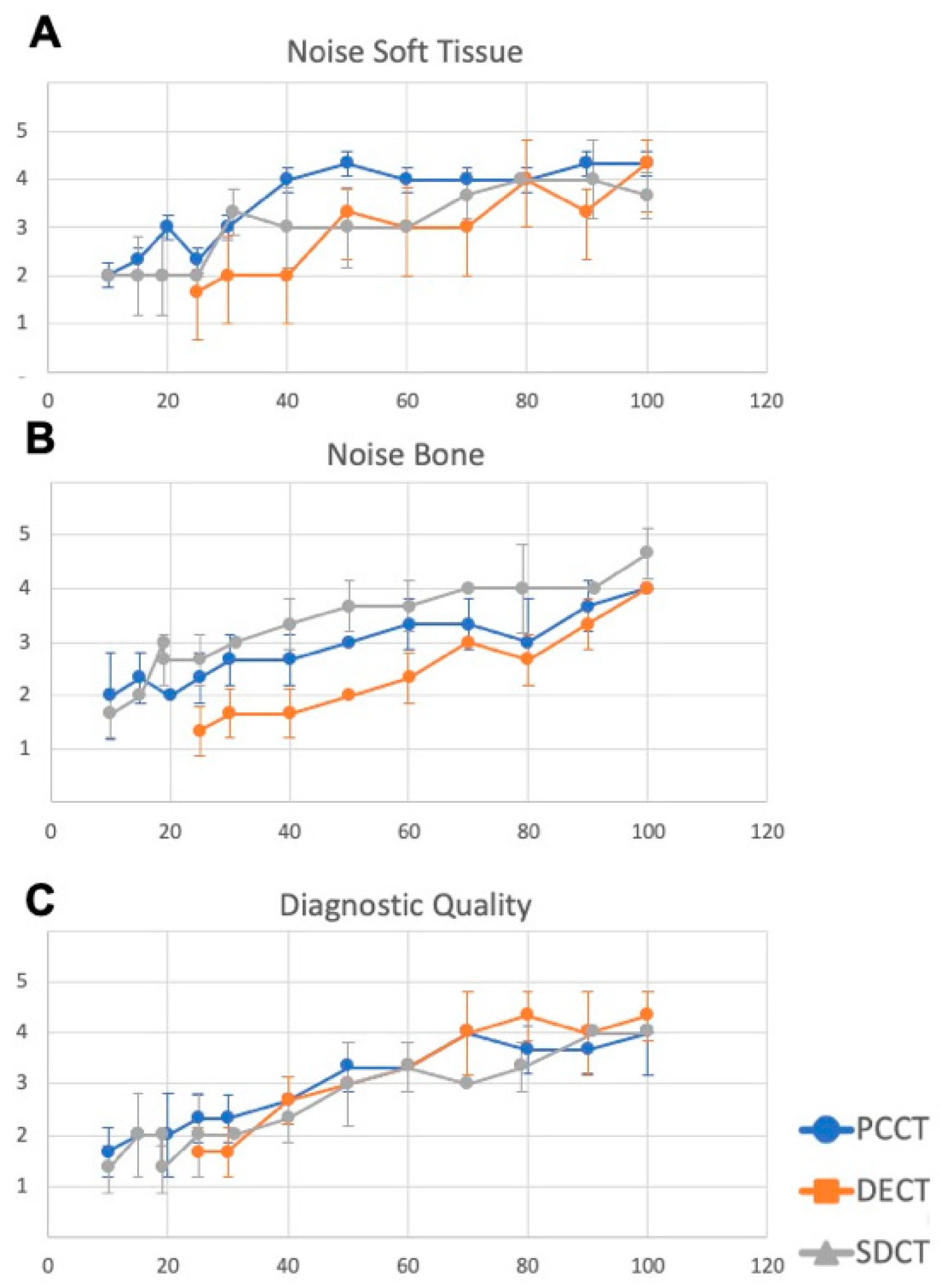
3.3. Analysis of Image Quality and Minimally Needed Radiation Dose
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTDI_vol | Computed Tomography Dose Index-Volume |
| CdTe | Cadmium Telluride |
| CNR | Contrast-to-Noise Ratio |
| CRS | Chronic Rhinosinusitis |
| CT | Computed Tomography |
| DECT | Dual-Energy Dual-Source Computed Tomography |
| DLP | Dose Length Product |
| EID | Energy-Integrating Detector |
| ESBS | Endoscopic Skull Base Surgery |
| FESS | Functional Endoscopic Sinus Surgery |
| IGS | Image-Guided Surgery |
| PCCT | Photon-Counting Computed Tomography |
| ROI | Region of Interest |
| SDCT | Spectral Detector Computed Tomography |
| SNR | Signal-to-Noise Ratio |
| VAS | Visual Analog Scale |
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef]
- Higgins, T.S.; Lane, A.P. Chapter 12: Surgery for sinonasal disease. Am. J. Rhinol. Allergy 2013, 27 (Suppl. 1), S42–S44. [Google Scholar] [CrossRef]
- Levine, C.G.; Casiano, R.R. Revision Functional Endoscopic Sinus Surgery. Otolaryngol. Clin. N. Am. 2017, 50, 143–164. [Google Scholar] [CrossRef]
- Mistry, S.G.; Strachan, D.R.; Loney, E.L. Improving paranasal sinus computed tomography reporting prior to functional endoscopic sinus surgery—An ENT-UK panel perspective. J. Laryngol. Otol. 2016, 130, 962–966. [Google Scholar] [CrossRef]
- Grevers, G. Anterior skull base trauma during endoscopic sinus surgery for nasal polyposis preferred sites for iatrogenic injuries. Rhinology 2001, 39, 1–4. [Google Scholar] [PubMed]
- Ledderose, G.J.; Stelter, K.; Betz, C.S.; Englhard, A.S.; Ledderose, C.; Leunig, A. Cerebrospinal fluid leaks during endoscopic sinus surgery in thirty-two patients. Clin. Otolaryngol. 2017, 42, 1105–1108. [Google Scholar] [CrossRef]
- Lum, S.G.; Gendeh, B.S.; Husain, S.; Gendeh, H.S.; Ismail, M.R.; Toh, C.J.; Izaham, A.; Tan, H.J. Internal carotid artery injury during endonasal sinus surgery: Our experience and review of the literature. Acta Otorhinolaryngol. Ital. 2019, 39, 130–136. [Google Scholar] [CrossRef]
- Wu, H.; Shen, T.; Chen, J.; Yan, J. Long-term therapeutic outcome of ophthalmic complications following endoscopic sinus surgery. Medicine 2016, 95, e4896. [Google Scholar] [CrossRef] [PubMed]
- Povolotskiy, R.; Cerasiello, S.Y.; Siddiqui, S.H.; Baredes, S.; Eloy, J.A.; Hsueh, W.D. Anemia and blood transfusion requirements in endoscopic sinus surgery: A propensity-matched analysis. Laryngoscope 2020, 130, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Sit, A.; Alvarado, R.; Earls, P.; Rimmer, J.; Kalish, L.; Campbell, R.; Sewell, W.; Harvey, R.J. CCAD or eCRS: Defining Eosinophilic Subpopulations in Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 2023, 37, 402–409. [Google Scholar] [CrossRef]
- Beswick, D.M.; Ramakrishnan, V.R. The Utility of Image Guidance in Endoscopic Sinus Surgery: A Narrative Review. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Ernst, B.P.; Reissig, M.R.; Strieth, S.; Eckrich, J.; Hagemann, J.H.; Döge, J.; Matthias, C.; Gouveris, H.; Rübenthaler, J.; Weiss, R.; et al. The role of structured reporting and structured operation planning in functional endoscopic sinus surgery. PLoS ONE 2020, 15, e0242804. [Google Scholar] [CrossRef]
- Becker, S.; Gonser, P.; Haas, M.; Sailer, M.; Froelich, M.F.; Betz, C.; Löwenheim, H.; Hirt, B.; Sommer, W.H.; Holderried, M.; et al. ENT Residents Benefit from a Structured Operation Planning Approach in the Training of Functional Endoscopic Sinus Surgery. Medicina 2021, 57, 1062. [Google Scholar] [CrossRef] [PubMed]
- Villemure-Poliquin, N.; Chretien, M.; Leclerc, J.E. Navigation and non-navigation CT scan of the sinuses: Comparison of the effective doses of radiation in children and adults. J. Otolaryngol. Head Neck Surg. 2021, 50, 66. [Google Scholar] [CrossRef] [PubMed]
- de Basea, M.B.; Thierry-Chef, I.; Harbron, R.; Hauptmann, M.; Byrnes, G.; Bernier, M.O.; Le Cornet, L.; Dabin, J.; Ferro, G.; Istad, T.S.; et al. Risk of hematological malignancies from CT radiation exposure in children, adolescents and young adults. Nat. Med. 2023, 29, 3111–3119. [Google Scholar] [CrossRef]
- Frush, D.P.; Donnelly, L.F.; Rosen, N.S. Computed tomography and radiation risks: What pediatric health care providers should know. Pediatrics 2003, 112, 951–957. [Google Scholar] [CrossRef]
- Hopkins, C.; Slack, R.; Lund, V.; Brown, P.; Copley, L.; Browne, J. Long-term outcomes from the English national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Laryngoscope 2009, 119, 2459–2465. [Google Scholar] [CrossRef]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-counting CT: Technical Principles and Clinical Prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef]
- Benson, J.C.; Rajendran, K.; Lane, J.I.; Diehn, F.E.; Weber, N.M.; Thorne, J.E.; Larson, N.B.; Fletcher, J.G.; McCollough, C.H.; Leng, S. A New Frontier in Temporal Bone Imaging: Photon-Counting Detector CT Demonstrates Superior Visualization of Critical Anatomic Structures at Reduced Radiation Dose. AJNR Am. J. Neuroradiol. 2022, 43, 579–584. [Google Scholar] [CrossRef]
- Rajendran, K.; Petersilka, M.; Henning, A.; Shanblatt, E.R.; Schmidt, B.; Flohr, T.G.; Ferrero, A.; Baffour, F.; Diehn, F.E.; Yu, L.; et al. First Clinical Photon-counting Detector CT System: Technical Evaluation. Radiology 2022, 303, 130–138. [Google Scholar] [CrossRef]
- Layer, Y.C.; Mesropyan, N.; Kupczyk, P.A.; Luetkens, J.A.; Isaak, A.; Dell, T.; Ernst, B.P.; Attenberger, U.I.; Kuetting, D. Use of virtual monoenergetic images for reduction of extensive dental implant associated artifacts in photon-counting detector CT. Sci. Rep. 2024, 14, 497. [Google Scholar] [CrossRef]
- Gudis, D.A.; Karnezis, T.K.; Soler, Z.M.; Schlosser, R.J. How I do It: The Sphenoethmoidectomy: With Illustration and Video. Am. J. Rhinol. Allergy 2015, 29, 457–458. [Google Scholar] [CrossRef]
- Haag, F.; Hokamp, N.G.; Overhoff, D.; Dasegowda, G.; Kuru, M.; Norenberg, D.; Schoenberg, S.O.; Kalra, M.K.; Froelich, M.F. Potential of photon counting computed tomography derived spectral reconstructions to reduce beam-hardening artifacts in chest CT. Eur. J. Radiol. 2024, 175, 111448. [Google Scholar] [CrossRef]
- Grosse Hokamp, N.; Neuhaus, V.; Abdullayev, N.; Laukamp, K.; Lennartz, S.; Mpotsaris, A.; Borggrefe, J. Reduction of artifacts caused by orthopedic hardware in the spine in spectral detector CT examinations using virtual monoenergetic image reconstructions and metal-artifact-reduction algorithms. Skelet. Radiol. 2018, 47, 195–201. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, C.; Nadolski, G.; Kovach, S.J., III; Zheng, J.; Cohen, A.; Kaplan, D.E.; Itkin, M. Thoracic Duct-Venous Junction Obstruction as Unknown Cause of Abdominal Pain: Diagnosis and Treatment. Radiology 2023, 308, e230380. [Google Scholar] [CrossRef] [PubMed]
- Rak, K.; Spahn, B.; Muller-Graff, F.T.; Engert, J.; Voelker, J.; Hackenberg, S.; Hagen, R.; Petritsch, B.; Grunz, J.P.; Bley, T.; et al. The Photon-Counting CT Enters the Field of Cochlear Implantation: Comparison to Angiography DynaCT and Conventional Multislice CT. Otol. Neurotol. 2024, 45, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.Z.; Talmadge, J.; Citardi, M.J. Semi-Quantitative Assessment of Surgical Navigation Accuracy During Endoscopic Sinus Surgery in a Real-World Environment. Ann. Otol. Rhinol. Laryngol. 2025, 134, 14–20. [Google Scholar] [CrossRef]
- Kaatsch, H.L.; Fulisch, F.; Dillinger, D.; Kubitscheck, L.; Becker, B.V.; Piechotka, J.; Brockmann, M.A.; Froelich, M.F.; Schoenberg, S.O.; Overhoff, D.; et al. Ultra-low-dose photon-counting CT of paranasal sinus: An in vivo comparison of radiation dose and image quality to cone-beam CT. Dentomaxillofac Radiol. 2024, 53, 103–108. [Google Scholar] [CrossRef]
- Stein, T.; Rau, A.; Russe, M.F.; Arnold, P.; Faby, S.; Ulzheimer, S.; Weis, M.; Froelich, M.F.; Overhoff, D.; Horger, M.; et al. Photon-Counting Computed Tomography—Basic Principles, Potenzial Benefits, and Initial Clinical Experience. Rofo 2023, 195, 691–698. [Google Scholar] [CrossRef]
- Siegel, M.J.; Ramirez-Giraldo, J.C. Photon counting detector computed tomography in pediatric cardiothoracic CT imaging. Radiol. Adv. 2024, 1, umae012. [Google Scholar] [CrossRef]
- Tao, W.; Goetti, R. Evaluation of ultra-low-dose CT with tin filter for craniosynostosis. J. Med. Imaging Radiat. Oncol. 2024, 69, 28–34. [Google Scholar] [CrossRef]
- Willershausen, I.; Evangeliou, S.; Fautz, H.P.; Amarteifio, P.; May, M.S.; Stroebel, A.; Zeilinger, M.; Uder, M.; Goelz, L.; Kopp, M. Low-Field MRI for Dental Imaging in Pediatric Patients With Supernumerary and Ectopic Teeth: A Comparative Study of 0.55 T and Ultra-Low-Dose CT. Investig. Radiol. 2024, 60, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Loftus, C.A.; Soler, Z.M.; Desiato, V.M.; Koochakzadeh, S.; Yoo, F.; Storck, K.A.; Schlosser, R.J. Factors impacting revision surgery in patients with chronic rhinosinusitis with nasal polyposis. Int. Forum Allergy Rhinol. 2020, 10, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.J.; Sajisevi, M.B.; McClennen, J.; Kaylie, D.M. Image-Guided Placement of Osseointegrated Implants for Challenging Auricular, Orbital, and Rhinectomy Defects. Ann. Otol. Rhinol. Laryngol. 2016, 125, 801–807. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ernst, B.P.; Burck, I.; Schliwa, S.; Becker, S.; Albrecht, T.; Vogl, T.J.; Scholtz, J.-E.; Levi, A.; Loth, A.G.; Bärhold, F.; et al. Photon-Counting Computed Tomography of the Paranasal Sinuses Improves Intraoperative Accuracy of Image-Guided Surgery. Diagnostics 2025, 15, 2777. https://doi.org/10.3390/diagnostics15212777
Ernst BP, Burck I, Schliwa S, Becker S, Albrecht T, Vogl TJ, Scholtz J-E, Levi A, Loth AG, Bärhold F, et al. Photon-Counting Computed Tomography of the Paranasal Sinuses Improves Intraoperative Accuracy of Image-Guided Surgery. Diagnostics. 2025; 15(21):2777. https://doi.org/10.3390/diagnostics15212777
Chicago/Turabian StyleErnst, Benjamin Philipp, Iris Burck, Stefanie Schliwa, Sven Becker, Tobias Albrecht, Thomas J. Vogl, Jan-Erik Scholtz, Anna Levi, Andreas German Loth, Friederike Bärhold, and et al. 2025. "Photon-Counting Computed Tomography of the Paranasal Sinuses Improves Intraoperative Accuracy of Image-Guided Surgery" Diagnostics 15, no. 21: 2777. https://doi.org/10.3390/diagnostics15212777
APA StyleErnst, B. P., Burck, I., Schliwa, S., Becker, S., Albrecht, T., Vogl, T. J., Scholtz, J.-E., Levi, A., Loth, A. G., Bärhold, F., Strieth, S., Froelich, M. F., Hertel, A., Layer, Y. C., Kuetting, D., & Eckrich, J. (2025). Photon-Counting Computed Tomography of the Paranasal Sinuses Improves Intraoperative Accuracy of Image-Guided Surgery. Diagnostics, 15(21), 2777. https://doi.org/10.3390/diagnostics15212777




