Effects of Noise Stress on Neuronal Activation in Rat Auditory Pathway-Related Brain Regions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Noise Exposure
- Initial Increase (0–5 m): The sound level increases linearly from 40 dB to 90 dB, representing a rapid onset of auditory stimulus.
- Slight Rise (5–15 m): A slower, continued linear increase occurs, reaching a peak at 105 dB.
- Drop in Intensity (15–18 m): The noise level then decreases linearly to 75 dB, representing a partial release from high-intensity noise.
- Logarithmic Rise (18–20 m): A nonlinear, more gradual logarithmic increase brings the level back to 95 dB.
- Fluctuations (20–30 m): The profile exhibits mild oscillations between 90 and 95 dB, simulating fluctuating environmental noise levels.
- Final Drop (30–31 m): A rapid drop occurs, with decibel levels falling sharply from around 90 dB to the baseline of 40 dB, marking the termination of the auditory stimulus.
2.3. Tissue Preparation
2.4. c-Fos Immunohistochemistry
2.5. Evaluated Brain Areas Cell Counting
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MGB | Medial geniculate body |
| SC | Superior colliculus |
| VCA | Ventral cochlear nucleus |
| Au1 | Primary auditory cortex |
| TZ | Trapezoid body |
References
- Joels, M.; Karst, H.; Sarabdjitsingh, R.A. The stressed brain of humans and rodents. Acta Physiol. 2018, 223, e13066. [Google Scholar] [CrossRef]
- Munhoz, C.D.; Garcia-Bueno, B.; Madrigal, J.L.; Lepsch, L.B.; Scavone, C.; Leza, J.C. Stress-induced neuroinflammation: Mechanisms and new pharmacological targets. Braz. J. Med. Biol. Res. 2008, 41, 1037–1046. [Google Scholar] [CrossRef]
- Bakay, W.M.H.; Cervantes, B.; Lao-Rodriguez, A.B.; Johannesen, P.T.; Lopez-Poveda, E.A.; Furness, D.N.; Malmierca, M.S. How ‘hidden hearing loss’ noise exposure affects neural coding in the inferior colliculus of rats. Hear. Res. 2024, 443, 108963. [Google Scholar] [CrossRef]
- Hesse, L.L.; Bakay, W.; Ong, H.C.; Anderson, L.; Ashmore, J.; McAlpine, D.; Linden, J.; Schaette, R. Non-Monotonic Relation between Noise Exposure Severity and Neuronal Hyperactivity in the Auditory Midbrain. Front. Neurol. 2016, 7, 133. [Google Scholar] [CrossRef]
- Kiefer, L.; Koch, L.; Merdan-Desik, M.; Gaese, B.H.; Nowotny, M. Comparing the electrophysiological effects of traumatic noise exposure between rodents. J. Neurophysiol. 2022, 127, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Ising, H.; Kruppa, B. Health effects caused by noise: Evidence in the literature from the past 25 years. Noise Health 2004, 6, 5–13. [Google Scholar] [PubMed]
- Kujawa, S.G.; Liberman, M.C. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef]
- Bakay, W.M.H.; Anderson, L.A.; Garcia-Lazaro, J.A.; McAlpine, D.; Schaette, R. Hidden hearing loss selectively impairs neural adaptation to loud sound environments. Nat. Commun. 2018, 9, 4298. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, L.; Lu, J.; Yang, G.; Laundrie, E.; Salvi, R. Noise exposure-induced enhancement of auditory cortex response and changes in gene expression. Neuroscience 2008, 156, 374–380. [Google Scholar] [CrossRef]
- Guo, L.; Li, P.H.; Li, H.; Colicino, E.; Colicino, S.; Wen, Y.; Zhang, R.; Feng, X.; Barrow, T.M.; Cayir, A.; et al. Effects of environmental noise exposure on DNA methylation in the brain and metabolic health. Environ. Res. 2017, 153, 73–82. [Google Scholar] [CrossRef]
- Themann, C.; Suter, A.H.; Stephenson, M.R. National Research Agenda for the Prevention of Occupational Hearing Loss—Part 1. Semin. Hear. 2013, 34, 145–207. [Google Scholar] [CrossRef]
- Salvi, R.J.; Wang, J.; Ding, D. Auditory plasticity and hyperactivity following cochlear damage. Hear. Res. 2000, 147, 261–274. [Google Scholar] [CrossRef]
- Münzel, T.; Schmidt, F.P.; Steven, S.; Herzog, J.; Daiber, A.; Sørensen, M. Environmental Noise and the Cardiovascular System. J. Am. Coll. Cardiol. 2018, 71, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Fink, D. Fiftieth anniversary of the Environmental Protection Agency’s “noise levels” monograph. J. Acoust. Soc. Am. 2024, 156, A44–A45. [Google Scholar] [CrossRef]
- Karki, T.; Manandhar, R.; Neupane, D.; Mahat, D.; Ban, P. Critical Analysis of Noise Pollution and Its Effect on Human Health. Int. J. Educ. Life Sci. 2024, 2, 161–176. [Google Scholar] [CrossRef]
- Jamos, A.M.; Hosier, B.; Davis, S.; Franklin, T.C. The Role of the Medial Olivocochlear Reflex in Acceptable Noise Level in Adults. J. Am. Acad. Audiol. 2021, 32, 137–143. [Google Scholar] [CrossRef]
- Feder, K.; Michaud, D.; McNamee, J.; Fitzpatrick, E.; Davies, H.; Leroux, T. Prevalence of Hazardous Occupational Noise Exposure, Hearing Loss, and Hearing Protection Usage Among a Representative Sample of Working Canadians. J. Occup. Environ. Med. 2017, 59, 92–113. [Google Scholar] [CrossRef]
- Kerns, E.; Masterson, E.A.; Themann, C.L.; Calvert, G.M. Cardiovascular conditions, hearing difficulty, and occupational noise exposure within US industries and occupations. Am. J. Ind. Med. 2018, 61, 477–491. [Google Scholar] [CrossRef]
- Tak, S.; Davis, R.R.; Calvert, G.M. Exposure to Hazardous Workplace Noise and Use of Hearing Protection Devices Among US Workers—NHANES, 1999–2004. Am. J. Ind. Med. 2009, 52, 358–371. [Google Scholar] [CrossRef]
- Williams, W. The epidemiology of noise exposure in the Australian workforce. Noise Health 2013, 15, 326–331. [Google Scholar] [CrossRef]
- Le, T.N.; Straatman, L.V.; Lea, J.; Westerberg, B. Current insights in noise-induced hearing loss: A literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J. Otolaryngol. Head Neck Surg. 2017, 46, 41. [Google Scholar] [CrossRef]
- Nelson, D.I.; Nelson, R.Y.; Concha-Barrientos, M.; Fingerhut, M. The global burden of occupational noise-induced hearing loss. Am. J. Ind. Med. 2005, 48, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Basner, M.; Babisch, W.; Davis, A.; Brink, M.; Clark, C.; Janssen, S.; Stansfeld, S. Auditory and non-auditory effects of noise on health. Lancet 2014, 383, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gutierrez, D.E.; Guthrie, O.W. Systemic health effects of noise exposure. J. Toxicol. Environ. Health B Crit. Rev. 2024, 27, 21–54. [Google Scholar] [CrossRef]
- Grocott, K.; Mansour, A.; Shiels, E.; Bentley, R.; Mason, K. Mental health effects of exposure to environmental noise at home: A systematic review of potential mediating pathways. Noise Health 2025, 27, 255–267. [Google Scholar] [CrossRef]
- Standring, S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 40th ed.; Elsevier Limited: New York, NY, USA, 2008; pp. xviii, 291–292. [Google Scholar]
- Ito, S.; Feldheim, D.A. The Mouse Superior Colliculus: An Emerging Model for Studying Circuit Formation and Function. Front. Neural Circuits 2018, 12, 10. [Google Scholar] [CrossRef]
- Zhang, C.; Beebe, N.L.; Schofield, B.R.; Pecka, M.; Burger, R.M. Endogenous Cholinergic Signaling Modulates Sound-Evoked Responses of the Medial Nucleus of the Trapezoid Body. J. Neurosci. 2021, 41, 674–688. [Google Scholar] [CrossRef]
- Pilz, P.K.; Schnitzler, H.U. Habituation and sensitization of the acoustic startle response in rats: Amplitude, threshold, and latency measures. Neurobiol. Learn. Mem. 1996, 66, 67–79. [Google Scholar] [CrossRef]
- LeDoux, J.E.; Ruggiero, D.A.; Reis, D.J. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J. Comp. Neurol. 1985, 242, 182–213. [Google Scholar] [CrossRef]
- Weinberger, N.M. Auditory associative memory and representational plasticity in the primary auditory cortex. Hear. Res. 2007, 229, 54–68. [Google Scholar] [CrossRef]
- Carrive, P. The periaqueductal gray and defensive behavior: Functional representation and neuronal organization. Behav. Brain Res. 1993, 58, 27–47. [Google Scholar] [CrossRef]
- Herman, J.P.; Ostrander, M.M.; Mueller, N.K.; Figueiredo, H. Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 1201–1213. [Google Scholar] [CrossRef]
- Bramhall, N.F. Use of the auditory brainstem response for assessment of cochlear synaptopathy in humans. J. Acoust. Soc. Am. 2021, 150, 4440–4451. [Google Scholar] [CrossRef]
- Eggermont, J.J. Hearing loss, hyperacusis, or tinnitus: What is modeled in animal research? Hear. Res. 2013, 295, 140–149. [Google Scholar] [CrossRef]
- Hickox, A.E.; Liberman, M.C. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J. Neurophysiol. 2014, 111, 552–564. [Google Scholar] [CrossRef]
- Sergeyenko, Y.; Lall, K.; Liberman, M.C.; Kujawa, S.G. Age-related cochlear synaptopathy: An early-onset contributor to auditory functional decline. J. Neurosci. 2013, 33, 13686–13694. [Google Scholar] [CrossRef] [PubMed]
- Weuve, J.; D’Souza, J.; Beck, T.; Evans, D.A.; Kaufman, J.D.; Rajan, K.B.; de Leon, C.F.M.; Adar, S.D. Long-term community noise exposure in relation to dementia, cognition, and cognitive decline in older adults. Alzheimers Dement. 2021, 17, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Ramchander, P.V.; Salvi, R.; Seigel, G.M. Synaptic Reorganization Response in the Cochlear Nucleus Following Intense Noise Exposure. Neuroscience 2019, 399, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Malfatti, T.; Ciralli, B.; Hilscher, M.M.; Leao, R.N.; Leao, K.E. Decreasing dorsal cochlear nucleus activity ameliorates noise-induced tinnitus perception in mice. BMC Biol. 2022, 20, 102. [Google Scholar] [CrossRef]
- Shore, S.E.; Wu, C. Mechanisms of Noise-Induced Tinnitus: Insights from Cellular Studies. Neuron 2019, 103, 8–20. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Zangenehpour, S.; Rahbar-Dehgan, F.; Ye, F. Molecular maps of neural activity and quiescence. Acta Neurobiol. Exp. 2000, 60, 403–410. [Google Scholar] [CrossRef]
- Kovacs, K.J. c-Fos as a transcription factor: A stressful (re)view from a functional map. Neurochem. Int. 1998, 33, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Campeau, S.; Watson, S.J. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J. Neuroendocr. 1997, 9, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Dedic, N.; Kuhne, C.; Gomes, K.S.; Hartmann, J.; Ressler, K.J.; Schmidt, M.V.; Deussing, J.M. Deletion of CRH From GABAergic Forebrain Neurons Promotes Stress Resilience and Dampens Stress-Induced Changes in Neuronal Activity. Front. Neurosci. 2019, 13, 986. [Google Scholar] [CrossRef]
- Fernandez-Quezada, D.; Garcia-Zamudio, A.; Ruvalcaba-Delgadillo, Y.; Luquin, S.; Garcia-Estrada, J.; Huerta, F.J. Male rats exhibit higher pro-BDNF, c-Fos and dendritic tree changes after chronic acoustic stress. Biosci. Trends 2019, 13, 546–555. [Google Scholar] [CrossRef]
- Palkovits, M.; Helfferich, F.; Dobolyi, A.; Usdin, T.B. Acoustic stress activates tuberoinfundibular peptide of 39 residues neurons in the rat brain. Brain Struct. Funct. 2009, 214, 15–23. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.S.; Kaltenbach, J.A.; Wang, J.; Kim, S.A. Fos-like immunoreactivity in auditory and nonauditory brain structures of hamsters previously exposed to intense sound. Exp. Brain Res. 2003, 153, 655–660. [Google Scholar] [CrossRef]
- Hoffman, G.E.; Smith, M.S.; Verbalis, J.G. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front. Neuroendocr. 1993, 14, 173–213. [Google Scholar] [CrossRef]
- Becker, J.B.; Arnold, A.P.; Berkley, K.J.; Blaustein, J.D.; Eckel, L.A.; Hampson, E.; Herman, J.P.; Marts, S.; Sadee, W.; Steiner, M.; et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 2005, 146, 1650–1673. [Google Scholar] [CrossRef]
- Burow, A.; Day, H.E.; Campeau, S. A detailed characterization of loud noise stress: Intensity analysis of hypothalamo-pituitary-adrenocortical axis and brain activation. Brain Res. 2005, 1062, 63–73. [Google Scholar] [CrossRef]
- Lee, J.J.; Cho, Y.W.; Huh, Y.; Cha, C.I.; Yeo, S.G. Effect of nitric oxide on auditory cortical neurons of aged rats. Neurosci. Lett. 2008, 447, 37–41. [Google Scholar] [CrossRef]
- Masini, C.V.; Day, H.E.; Campeau, S. Long-term habituation to repeated loud noise is impaired by relatively short interstressor intervals in rats. Behav. Neurosci. 2008, 122, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain Stereotaxic Co-Ordinates; Academic Press: Cambridge, MA, USA, 2007; p. 456. [Google Scholar]
- Eriksson, M.; Ceccatelli, S.; Uvnas-Moberg, K.; Iadarola, M.; Hokfelt, T. Expression of Fos-related antigens, oxytocin, dynorphin and galanin in the paraventricular and supraoptic nuclei of lactating rats. Neuroendocrinology 1996, 63, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Schrode, K.M.; Muniak, M.A.; Kim, Y.H.; Lauer, A.M. Central Compensation in Auditory Brainstem after Damaging Noise Exposure. eNeuro 2018, 5. Erratum in eNeuro 2019, 6. https://doi.org/10.1523/ENEURO.0093-19.2019. [Google Scholar] [CrossRef]
- Trabanco, J.C.; Morita, B.; Matas, C.G.; de Paiva, K.M.; Moreira, R.R.; Sanches, S.G.; Samelli, A.G. Effects of Noise and Chemical Exposure on Peripheral and Central Auditory Pathways in Normal-hearing Workers. Noise Health 2022, 24, 182–190. [Google Scholar] [CrossRef]
- Groschel, M.; Manchev, T.; Frohlich, F.; Jansen, S.; Ernst, A.; Basta, D. Neurodegeneration after repeated noise trauma in the mouse lower auditory pathway. Neurosci. Lett. 2024, 818, 137571. [Google Scholar] [CrossRef]
- Frohlich, F.; Basta, D.; Strubing, I.; Ernst, A.; Groschel, M. Time course of cell death due to acoustic overstimulation in the mouse medial geniculate body and primary auditory cortex. Noise Health 2017, 19, 133–139. [Google Scholar] [CrossRef]
- Frohlich, F.; Ernst, A.; Strubing, I.; Basta, D.; Groschel, M. Apoptotic mechanisms after repeated noise trauma in the mouse medial geniculate body and primary auditory cortex. Exp. Brain Res. 2017, 235, 3673–3682. [Google Scholar] [CrossRef]
- Almasabi, F.; van Zwieten, G.; Alosaimi, F.; Smit, J.V.; Temel, Y.; Janssen, M.L.F.; Jahanshahi, A. The Effect of Noise Trauma and Deep Brain Stimulation of the Medial Geniculate Body on Tissue Activity in the Auditory Pathway. Brain Sci. 2022, 12, 1099. [Google Scholar] [CrossRef]
- Zare, A.; van Zwieten, G.; Kotz, S.A.; Temel, Y.; Schultz, B.G.; Schwartze, M.; Janssen, M.L.F. The effect of noise trauma and high-frequency stimulation on thalamic sensory gating in rodents. bioRxiv 2020. [Google Scholar] [CrossRef]
- Salehi, M.S.; Namavar, M.R.; Tamadon, A.; Bahmani, R.; Jafarzadeh Shirazi, M.R.; Khazali, H.; Dargahi, L.; Pandamooz, S.; Mohammad-Rezazadeh, F.; Rashidi, F.S. The Effects of Acoustic White Noise on the Rat Central Auditory System During the Fetal and Critical Neonatal Periods: A Stereological Study. Noise Health 2017, 19, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Campeau, S.; Akil, H.; Watson, S.J. Lesions of the medial geniculate nuclei specifically block corticosterone release and induction of c-fos mRNA in the forebrain associated with audiogenic stress in rats. J. Neurosci. 1997, 17, 5979–5992. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Chew, G.L.; Biggin, M.D. Quantitating translational control: mRNA abundance-dependent and independent contributions and the mRNA sequences that specify them. Nucleic Acids Res. 2017, 45, 11821–11836. [Google Scholar] [CrossRef] [PubMed]
- Bialy, M.; Kaczmarek, L. c-Fos expression as a tool to search for the neurobiological base of the sexual behaviour of males. Acta Neurobiol. Exp. 1996, 56, 567–577. [Google Scholar] [CrossRef]
- Nikolaeva, S.D.; Ivlev, A.P.; Naumova, A.A.; Kulikov, A.A.; Glazova, M.V.; Chernigovskaya, E.V. Dysregulation of GABAergic System in the Inferior Colliculi of Rats during the Development of Audiogenic Epilepsy. J. Evol. Biochem. Physiol. 2023, 59, 1252–1261. [Google Scholar] [CrossRef]
- Wang, X.; Bi, S.; Yue, Z.; Chen, X.; Liu, Y.; Deng, T.; Shao, L.; Jing, X.; Wang, C.; Wang, Y.; et al. GABAergic neurons in central amygdala contribute to orchestrating anxiety-like behaviors and breathing patterns. Nat. Commun. 2025, 16, 3544. [Google Scholar] [CrossRef]
- Simler, S.; Vergnes, M.; Marescaux, C. Spatial and temporal relationships between C-Fos expression and kindling of audiogenic seizures in Wistar rats. Exp. Neurol. 1999, 157, 106–119. [Google Scholar] [CrossRef]
- Musumeci, S.; Bosco, P.; Calabrese, G.; Bakker, C.; Sarro, G.; Elia, M.; Ferri, R.; Oostra, B. Audiogenic Seizures Susceptibility in Transgenic Mice with Fragile X Syndrome. Epilepsia 2000, 41, 19–23. [Google Scholar] [CrossRef]
- Dewey, R.S.; Francis, S.T.; Guest, H.; Prendergast, G.; Millman, R.E.; Plack, C.J.; Hall, D.A. The association between subcortical and cortical fMRI and lifetime noise exposure in listeners with normal hearing thresholds. Neuroimage 2020, 204, 116239. [Google Scholar] [CrossRef]
- Benarroch, E. What Are the Functions of the Superior Colliculus and Its Involvement in Neurologic Disorders? Neurology 2023, 100, 784–790. [Google Scholar] [CrossRef]
- Ritter, A.; Habusha, S.; Givon, L.; Edut, S.; Klavir, O. Prefrontal control of superior colliculus modulates innate escape behavior following adversity. Nat. Commun. 2024, 15, 2158. [Google Scholar] [CrossRef]
- Zhou, J.; Hormigo, S.; Busel, N.; Castro-Alamancos, M.A. The Orienting Reflex Reveals Behavioral States Set by Demanding Contexts: Role of the Superior Colliculus. J. Neurosci. 2023, 43, 1778–1796. [Google Scholar] [CrossRef]
- Almada, R.C.; Genewsky, A.J.; Heinz, D.E.; Kaplick, P.M.; Coimbra, N.C.; Wotjak, C.T. Stimulation of the Nigrotectal Pathway at the Level of the Superior Colliculus Reduces Threat Recognition and Causes a Shift From Avoidance to Approach Behavior. Front. Neural Circuits 2018, 12, 36. [Google Scholar] [CrossRef]
- Cohen, J.D.; Castro-Alamancos, M.A. Neural correlates of active avoidance behavior in superior colliculus. J. Neurosci. 2010, 30, 8502–8511. [Google Scholar] [CrossRef]
- Lynch, E.; Dempsey, B.; Saleeba, C.; Monteiro, E.; Turner, A.; Burke, P.G.R.; Allen, A.M.; Dampney, R.A.L.; Hildreth, C.M.; Cornish, J.L.; et al. Descending pathways from the superior colliculus mediating autonomic and respiratory effects associated with orienting behaviour. J. Physiol. 2022, 600, 5311–5332. [Google Scholar] [CrossRef]

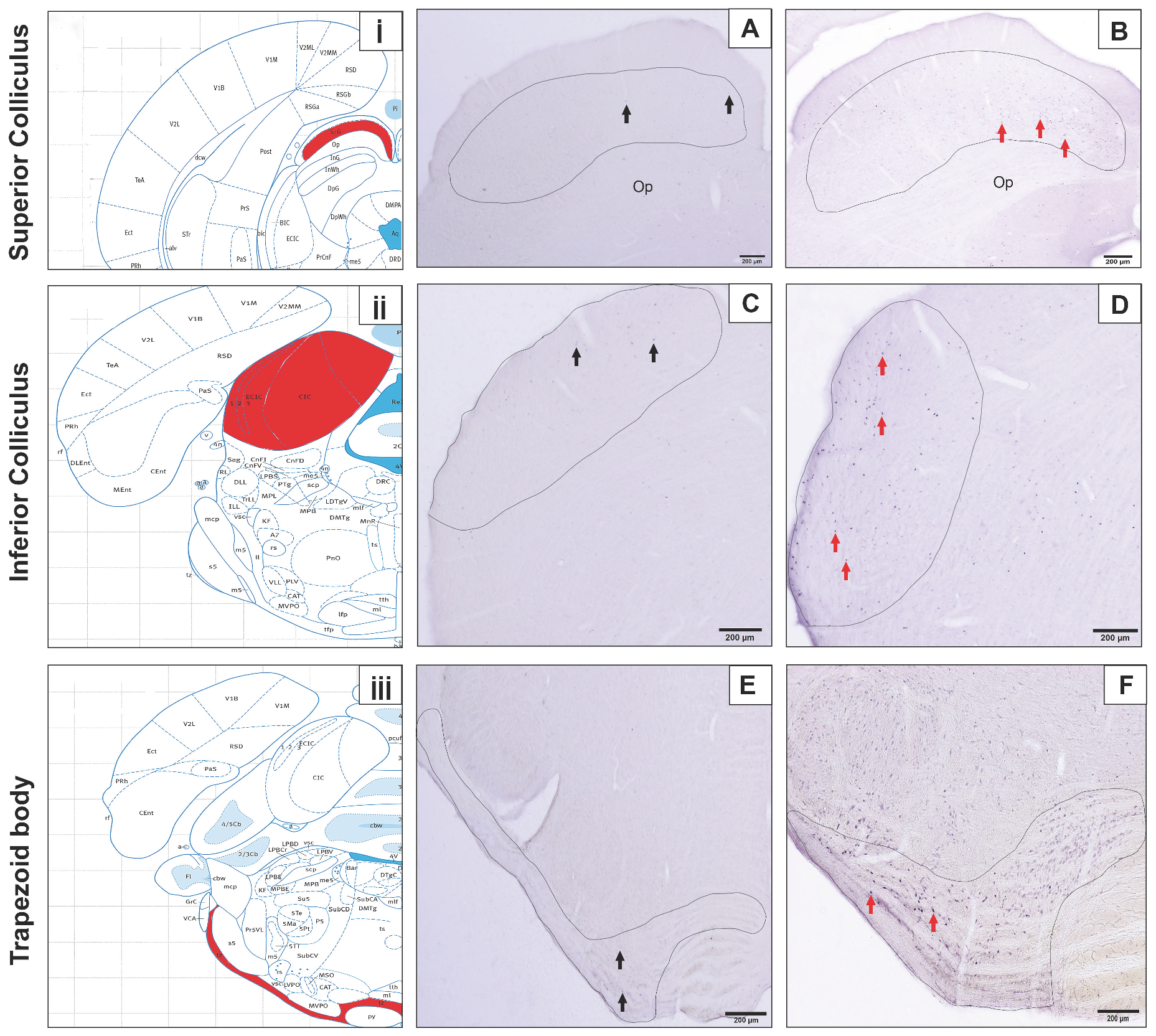
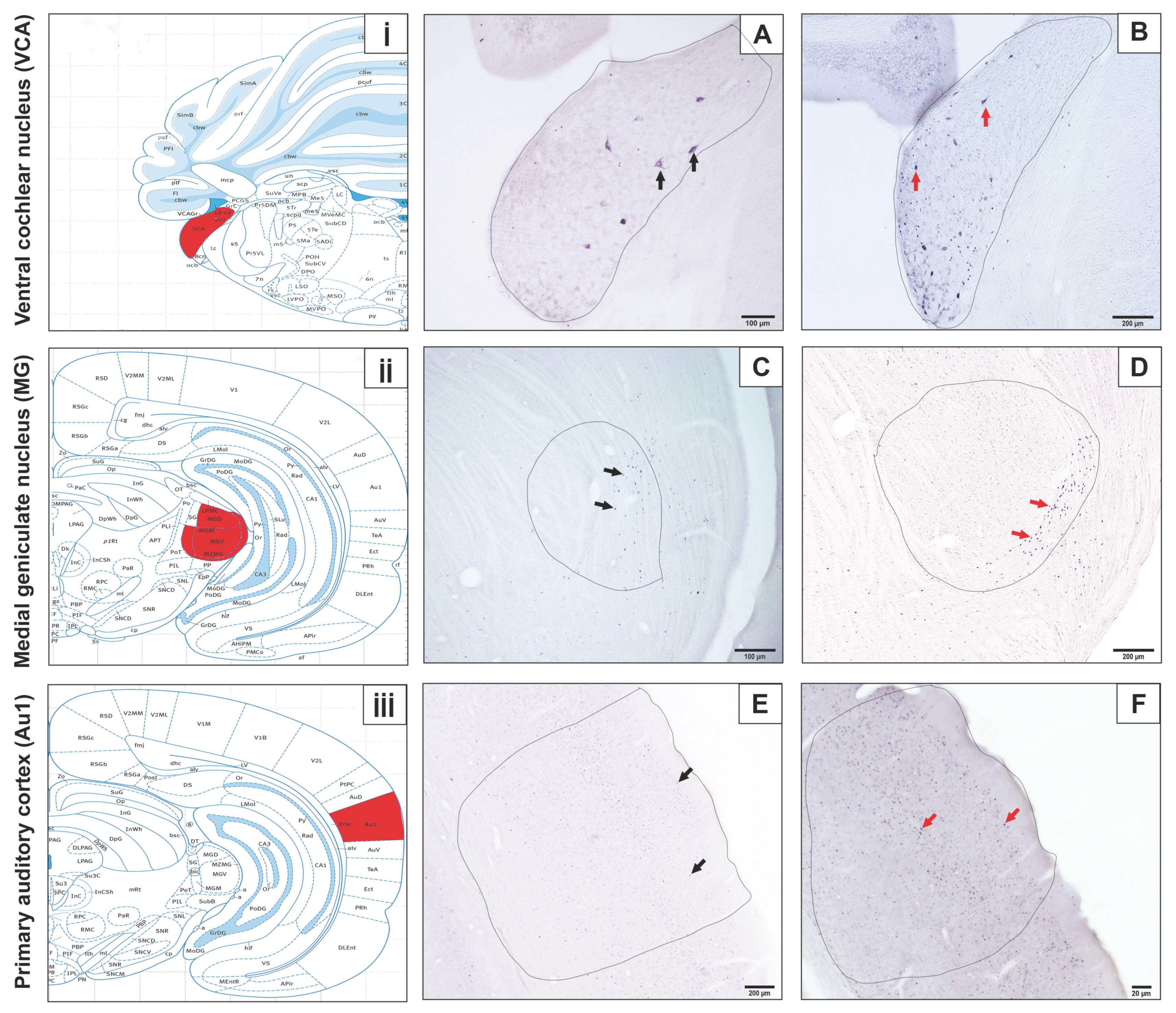
| Control Group | Experiment Group | ||
|---|---|---|---|
| Brain Area | Mean Value (With SD, Min and Max Numbers) | Mean Value (With SD, Min and Max Numbers) | p-Value |
| Auditory Cortex | 441.5 ± 176.08 (171 to 664) | 1113.13 ± 475.25 (584 to 1839) | <0.001 |
| Medial Geniculate Body | 308.83 ± 122.75 (191 to 471) | 373.33 ± 155.91 (199 to 654) | 0.68 |
| Superior Colliculus | 508.16 ± 186.23 (214 to 703) | 1515.16 ± 743.09 (684 to 2822) | <0.001 |
| Inferior Colliculus | 233.83 ± 115.39 (78 to 380) | 1478.33 ± 824.1 (689 to 2905) | <0.001 |
| Medial Nucleus of the Trapezoid Body | 43.16 ± 37.43 (2 to 93) | 772.16 ± 373.05 (255 to 1167) | <0.001 |
| Cochlear Nucleus | 345.33 ± 192.33 (84 to 633) | 706.5 ± 172.32 (557 to 1022) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gök Yurtseven, D.; Vatansever, A.; Topal, G.; Mergen, Ş.; Özdemir, Ö.F.; Kafa, İ.M.; Göktalay, G.; Eyigör, Ö. Effects of Noise Stress on Neuronal Activation in Rat Auditory Pathway-Related Brain Regions. Diagnostics 2025, 15, 2720. https://doi.org/10.3390/diagnostics15212720
Gök Yurtseven D, Vatansever A, Topal G, Mergen Ş, Özdemir ÖF, Kafa İM, Göktalay G, Eyigör Ö. Effects of Noise Stress on Neuronal Activation in Rat Auditory Pathway-Related Brain Regions. Diagnostics. 2025; 15(21):2720. https://doi.org/10.3390/diagnostics15212720
Chicago/Turabian StyleGök Yurtseven, Duygu, Alper Vatansever, Gonca Topal, Şule Mergen, Ömer Faruk Özdemir, İlker Mustafa Kafa, Gökhan Göktalay, and Özhan Eyigör. 2025. "Effects of Noise Stress on Neuronal Activation in Rat Auditory Pathway-Related Brain Regions" Diagnostics 15, no. 21: 2720. https://doi.org/10.3390/diagnostics15212720
APA StyleGök Yurtseven, D., Vatansever, A., Topal, G., Mergen, Ş., Özdemir, Ö. F., Kafa, İ. M., Göktalay, G., & Eyigör, Ö. (2025). Effects of Noise Stress on Neuronal Activation in Rat Auditory Pathway-Related Brain Regions. Diagnostics, 15(21), 2720. https://doi.org/10.3390/diagnostics15212720






