MOC31 for the Diagnosis of Metastatic Carcinoma and Mesothelial Lesions in Effusion Fluid—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Literature Search
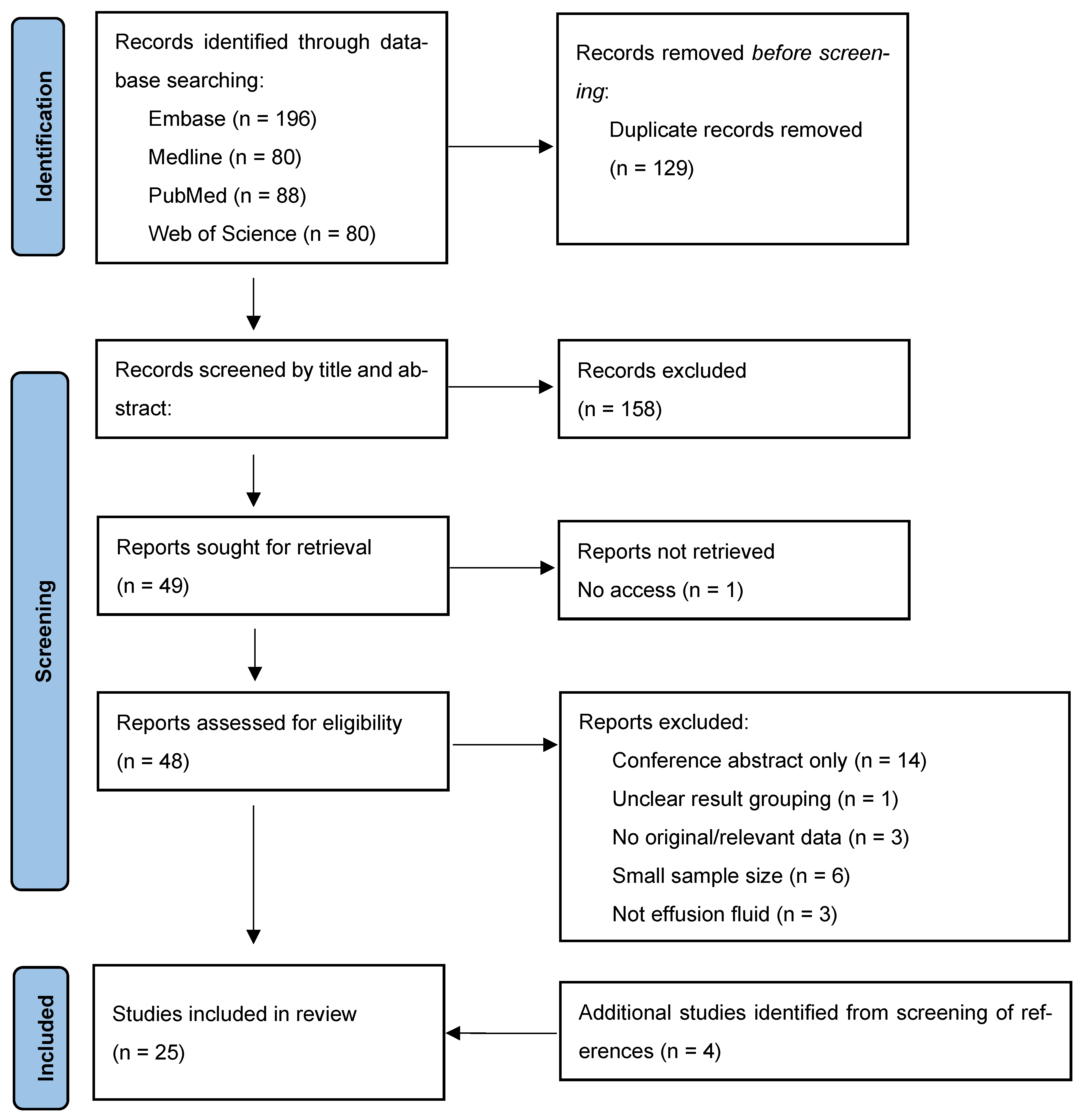
2.3. Study Selection
2.4. Data Extraction and Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Inclusion
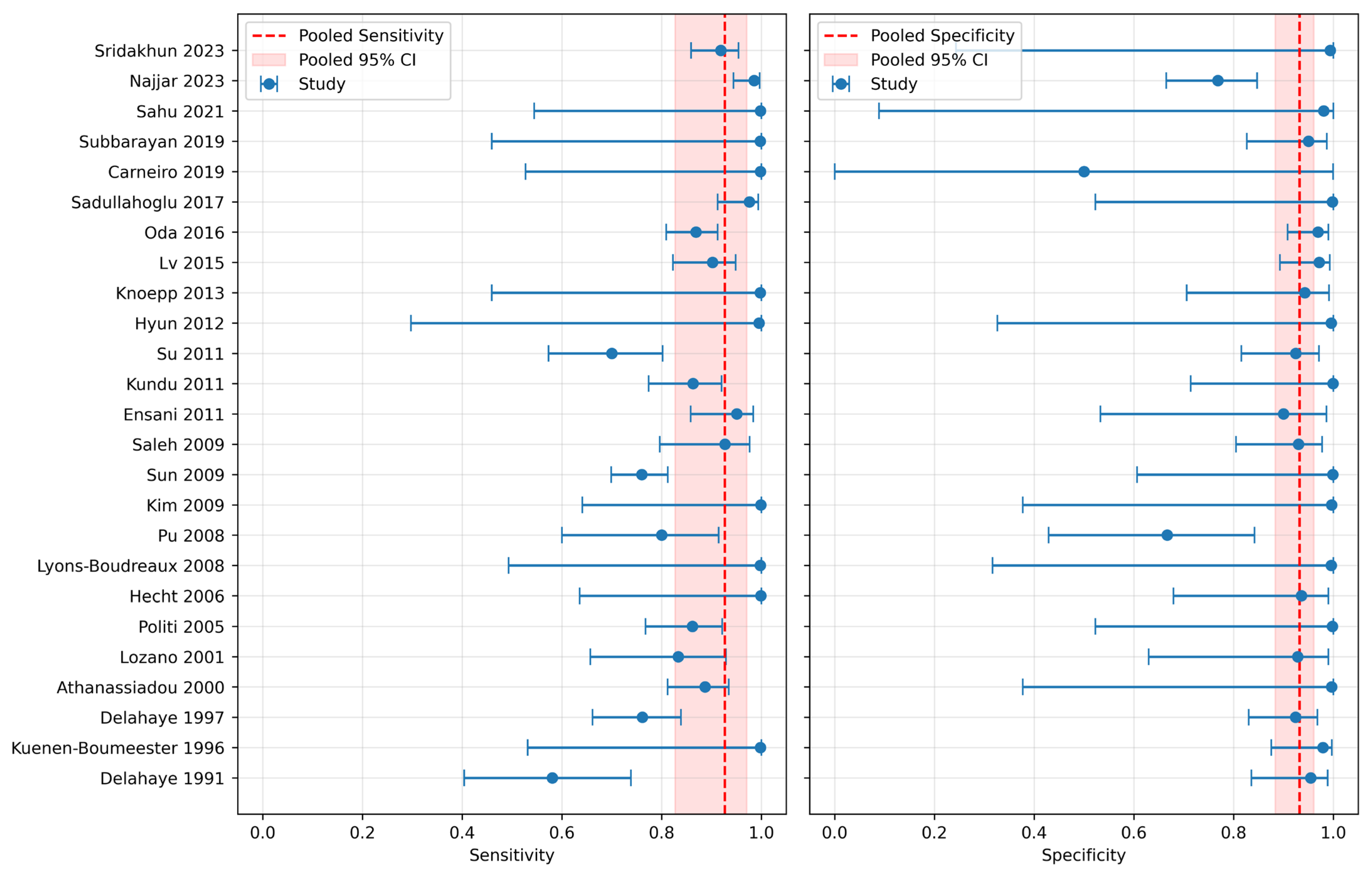
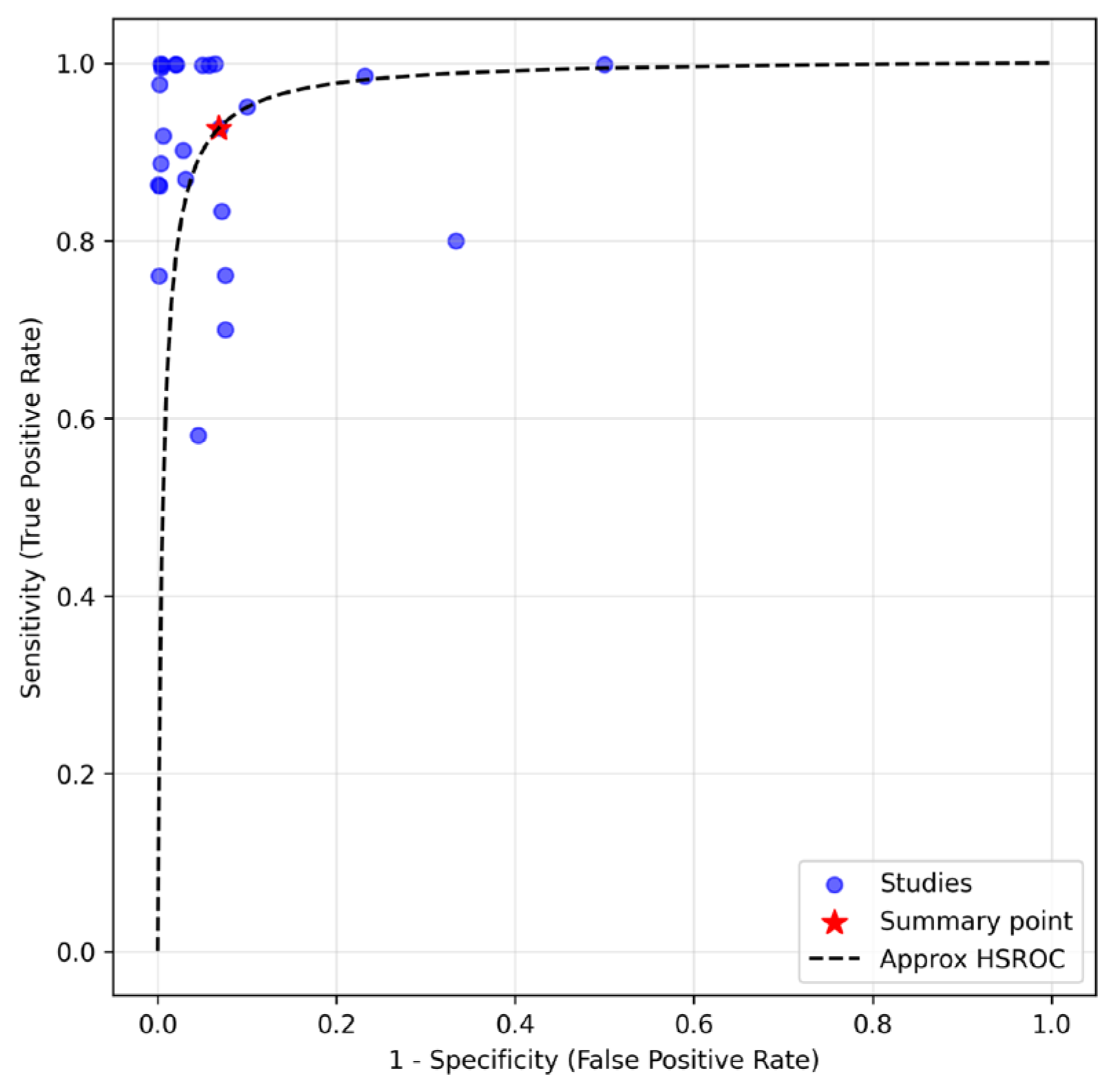
3.2. Diagnostic Metrics
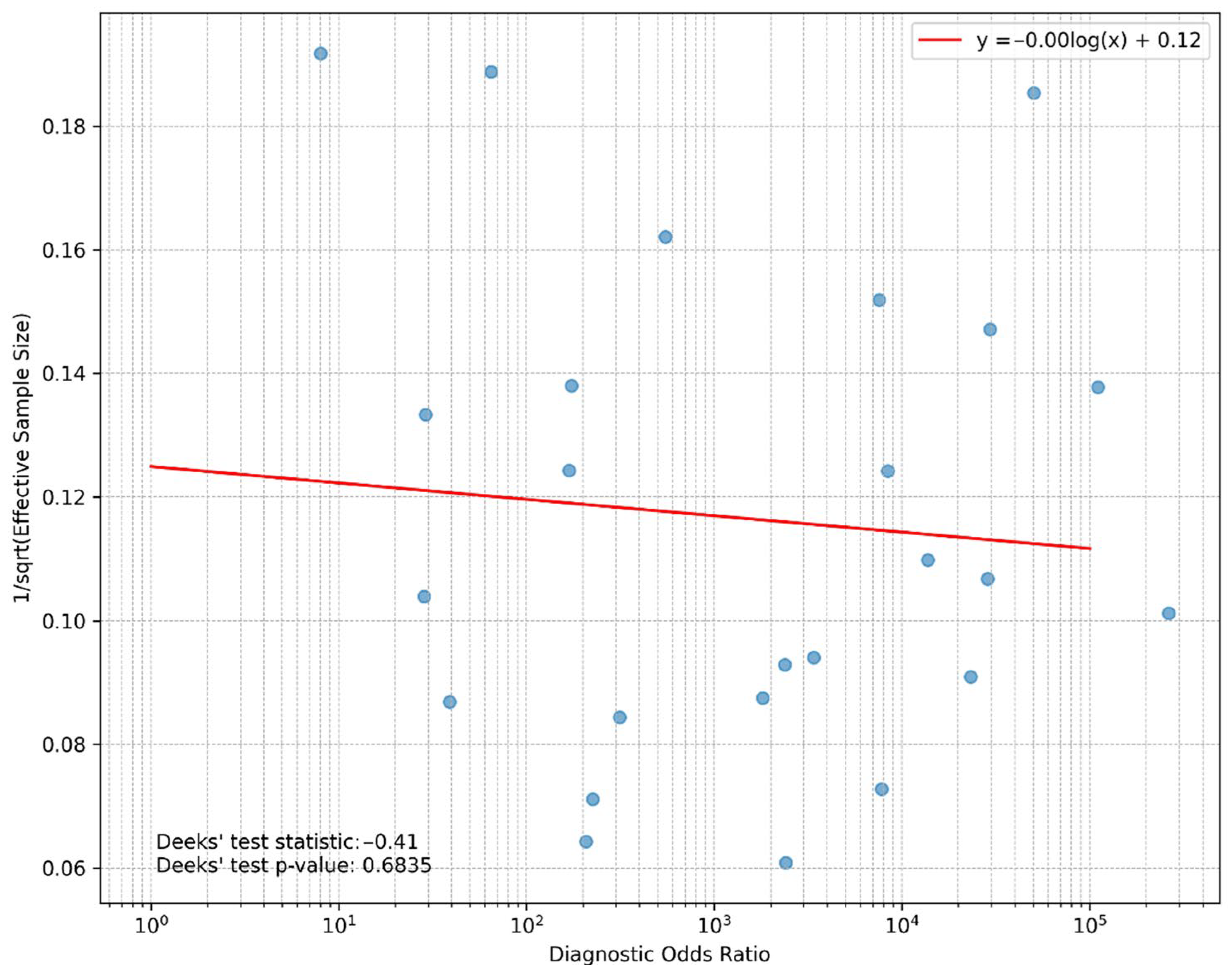
3.3. Publication Bias
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zimmermann, S.; Wels, W.; Froesch, B.A.; Gerstmayer, B.; Stahel, R.A.; Zangemeister-Wittke, U. A novel immunotoxin recognising the epithelial glycoprotein-2 has potent antitumoural activity on chemotherapy-resistant lung cancer. Cancer Immunol. Immunother. 1997, 44, 1–9. [Google Scholar] [CrossRef]
- Pairman, L.; Beckert, L.E.L.; Dagger, M.; Maze, M.J. Evaluation of pleural fluid cytology for the diagnosis of malignant pleural effusion: A retrospective cohort study. Intern. Med. J. 2022, 52, 1154–1159. [Google Scholar] [CrossRef]
- Shital, P.; Mirza, M.; Gondhali, G. Pleural fluid ‘cell block’ analysis in malignant pleural effusion: Sensitive, superior over fluid cytology and suitable for immunohistochemistry analysis (IHC), will decrease need for thoracoscopy guided procedures. Eur. Respir. J. 2017, 50 (Suppl. S61), PA4308. [Google Scholar] [CrossRef]
- Poon, I.K.; Chan, R.C.K.; Choi, J.S.H.; Ng, J.K.M.; Tang, K.T.; Wong, Y.Y.H.; Chan, K.P.; Yip, W.H.; Tse, G.M.; Li, J.J.X. A comparative study of diagnostic accuracy in 3026 pleural biopsies and matched pleural effusion cytology with clinical correlation. Cancer Med. 2023, 12, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.X.; Ng, J.K.M.; Tsang, J.Y.; Tsang, Y.T.; Mak, K.F.; Tse, G.M. Comparison of Claudin-4, BerEP4, Carcinoembryonic Antigen and MOC31 in Serous Fluids Metastases Demonstrate High Sensitivity of Claudin-4 at Low Cellularity. Diagn. Cytopathol. 2024, 52, 731–737. [Google Scholar] [CrossRef]
- Li, J.J.X.; Cheng, H.Y.; Lee, C.H.C.; Ng, J.K.M.; Tsang, J.Y.; Tse, G.M. Single-cell multiplex immunocytochemistry in cell block preparations of metastatic breast cancer confirms sensitivity of GATA-binding protein 3 over gross cystic disease fluid protein 15 and mammaglobin. Cancer Cytopathol. 2025, 133, e22910. [Google Scholar] [CrossRef]
- Lin, A.H.; Hsu, M.; Ng, J.K.M.; Farahani, S.J.; Li, J.J.X.; Nano, J.; Raeisi-Dehkordi, H.; Tang, W.; Vielh, P.; Muka, T. Performance of the Monoclonal Antibody B72.3 in Diagnosis of Malignant Carcinomatous Serous Effusions-A Systematic Review and Meta-Analysis of Diagnostic Performance. Cytopathology 2025, 36, 399–407. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; Hooft, L.; et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Sridakhun, N.; Intarawichian, P.; Thanee, M.; Watcharadetwittaya, S. Diagnosis of Metastatic Carcinoma Using Body Cavity Fluid Specimens: A Comparison of Diagnostic Panels. Acta Cytol. 2023, 67, 257–264. [Google Scholar] [CrossRef]
- Najjar, S.; Gan, Q.; Stewart, J.; Sneige, N. The utility of claudin-4 versus MOC-31 and Ber-EP4 in the diagnosis of metastatic carcinoma in cytology specimens. Cancer Cytopathol. 2023, 131, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Sharma, S.; Gupta, P.; Dey, P. MOC31 Immunostaining in the Diagnosis of Metastatic Adenocarcinoma in Serous Fluid: Special Emphasis on Atypical Cytological Cases. Acta Cytol. 2021, 65, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Subbarayan, D.; Bhattacharya, J.; Rani, P.; Khuraijam, B.; Jain, S. Use of Panel of Markers in Serous Effusion to Distinguish Reactive Mesothelial Cells from Adenocarcinoma. J. Cytol. 2019, 36, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, F.P.; Muniz-Junqueira, M.I.; De Vasconcelos Carneiro, M.; De Araújo Oliveira, Í.; Soares, A.C.; De Vargas Haar, N.; Takano, G.H.S.; De Sousa Vianna, L.M.; De Carvalho Caldas, G.; Vieira, D.L.M.; et al. Anti-EpCAM antibodies for detection of metastatic carcinoma in effusions and peritoneal wash. Oncol. Lett. 2019, 18, 2019–2024. [Google Scholar] [CrossRef]
- Sadullahoglu, C.; Nart, D.; Veral, A. The importance of EZH2 and MOC-31 expression in the differential diagnosis of benign and malignant effusions. Diagn. Cytopathol. 2017, 45, 118–124. [Google Scholar] [CrossRef]
- Oda, T.; Ogata, S.; Kawaguchi, S.; Minabe, S.; Dokyu, M.; Takahashi, H.; Kumazawa, F.; Shimazaki, H.; Takano, M.; Hase, K.; et al. Immunocytochemical utility of claudin-4 versus those of Ber-EP4 and MOC-31 in effusion cytology. Diagn. Cytopathol. 2016, 44, 499–504. [Google Scholar] [CrossRef]
- Lv, M.; Leng, J.H.; Hao, Y.Y.; Sun, Y.; Cha, N.; Wu, G.P. Expression and significance of MOC-31 and calretinin in pleural fluid of patients with lung cancer. Diagn. Cytopathol. 2015, 43, 527–531. [Google Scholar] [CrossRef]
- Knoepp, S.M.; Placido, J.; Fields, K.L.; Thomas, D.; Roh, M.H. The application of immunocytochemistry to direct smears in the diagnosis of effusions. Diagn. Cytopathol. 2013, 41, 425–430. [Google Scholar] [CrossRef]
- Hyun, T.S.; Barnes, M.; Tabatabai, Z.L. The diagnostic utility of D2-40, calretinin, CK5/6, desmin and MOC-31 in the differentiation of mesothelioma from adenocarcinoma in pleural effusion cytology. Acta Cytol. 2012, 56, 527–532. [Google Scholar] [CrossRef]
- Su, X.Y.; Li, G.D.; Liu, W.P.; Xie, B.; Jiang, Y.H. Cytological differential diagnosis among adenocarcinoma, epithelial mesothelioma, and reactive mesothelial cells in serous effusions by immunocytochemistry. Diagn. Cytopathol. 2011, 39, 900–908. [Google Scholar] [CrossRef]
- Kundu, U.R.; Krishnamurthy, S. Use of the monoclonal antibody MOC-31 as an immunomarker for detecting metastatic adenocarcinoma in effusion cytology. Cancer Cytopathol. 2011, 119, 272–278. [Google Scholar] [CrossRef]
- Ensani, F.; Nematizadeh, F.; Irvanlou, G. Accuracy of immunohistochemistry in evaluation of malignant pleural and peritoneal effusions. Pol. J. Pathol. 2011, 62, 95–100. [Google Scholar]
- Saleh, H.A.; El-Fakharany, M.; Makki, H.; Kadhim, A.; Masood, S. Differentiating reactive mesothelial cells from metastatic adenocarcinoma in serous effusions: The utility of immunocytochemical panel in the differential diagnosis. Diagn. Cytopathol. 2009, 37, 324–332. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, G.P.; Fang, C.Q.; Liu, S.L. Diagnostic utility of MOC-31, HBME-1 and MOC-31 mRNA in distinguishing between carcinoma cells and reactive mesothelial cells in pleural effusions. Acta Cytol. 2009, 53, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, G.E.; Choi, Y.D.; Lee, J.S.; Lee, J.H.; Nam, J.H.; Choi, C. Immunocytochemical panel for distinguishing between adenocarcinomas and reactive mesothelial cells in effusion cell blocks. Diagn. Cytopathol. 2009, 37, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Pu, R.T.; Pang, Y.; Michael, C.W. Utility of WT-1, p63, MOC31, mesothelin, and cytokeratin (K903 and CK5/6) immunostains in differentiating adenocarcinoma, squamous cell carcinoma, and malignant mesothelioma in effusions. Diagn. Cytopathol. 2008, 36, 20–25. [Google Scholar] [CrossRef]
- Lyons-Boudreaux, V.; Mody, D.R.; Zhai, J.; Coffey, D. Cytologic malignancy versus benignancy: How useful are the “newer” markers in body fluid cytology? Arch. Pathol. Lab. Med. 2008, 132, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.L.; Pinkus, J.L.; Pinkus, G.S. Monoclonal antibody MOC-31 reactivity as a marker for adenocarcinoma in cytologic preparations. Cancer 2006, 108, 56–59. [Google Scholar] [CrossRef]
- Politi, E.; Kandaraki, C.; Apostolopoulou, C.; Kyritsi, T.; Koutselini, H. Immunocytochemical panel for distinguishing between carcinoma and reactive mesothelial cells in body cavity fluids. Diagn. Cytopathol. 2005, 32, 151–155. [Google Scholar] [CrossRef]
- Lozano, M.D.; Panizo, A.; Toledo, G.R.; Sola, J.J.; Pardo-Mindán, J. Immunocytochemistry in the differential diagnosis of serous effusions: A comparative evaluation of eight monoclonal antibodies in Papanicolaou stained smears. Cancer 2001, 93, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Athanassiadou, P.; Gonidi, M.; Liossi, A.; Petrakakou, E.; Nakopoulou, L.; Zerva, C.; Athanassiades, P. Moc-31, fibronectin and CEA in the differential diagnosis of malignant effusions: An immunocytochemical study. Pathol. Oncol. Res. 2000, 6, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Delahaye, M.; van der Ham, F.; van der Kwast, T.H. Complementary value of five carcinoma markers for the diagnosis of malignant mesothelioma, adenocarcinoma metastasis, and reactive mesothelium in serous effusions. Diagn. Cytopathol. 1997, 17, 115–120. [Google Scholar] [CrossRef]
- Kuenen-Boumeester, V.; van Loenen, P.; de Bruijn, E.M.; Henzen-Logmans, S.C. Quality control of immunocytochemical staining of effusions using a standardized method of cell processing. Acta Cytol. 1996, 40, 475–479. [Google Scholar] [CrossRef]
- Delahaye, M.; Hoogsteden, H.C.; Van der Kwast, T.H. Immunocytochemistry of malignant mesothelioma: OV632 as a marker of malignant mesothelioma. J. Pathol. 1991, 165, 137–143. [Google Scholar] [CrossRef]
- Jovanovic, D. Etiopathogenesis of malignant pleural effusion. AME Med. J. 2020, 6, 28. [Google Scholar] [CrossRef]
- Li, J.J.X.; Chan, W.C.; Chau, H.H.L.; Wu, C.; Tse, G.M. Cytologic diagnosis of metastatic malignant phyllodes tumor of the breast in pleural effusion. Diagn. Cytopathol. 2019, 47, 599–602. [Google Scholar] [CrossRef]
- Ng, J.K.M.; Chow, C.; Chan, R.C.K.; Chan, K.P.; Li, J.J.X.; Li, M.S.C.; To, K.-F. EGFR testing in paraffin-embedded cell block cytology material is reliable with increased detection for effusion fluid. Lung Cancer 2022, 174, 97–103. [Google Scholar] [CrossRef]
- Wei, S.; Talarchek, J.N.; Huang, M.; Gong, Y.; Du, F.; Ehya, H.; Flieder, D.B.; Patchefsky, A.S.; Wasik, M.A.; Pei, J. Cell block-based RNA next generation sequencing for detection of gene fusions in lung adenocarcinoma: An institutional experience. Cytopathology 2023, 34, 28–34. [Google Scholar] [CrossRef]
- Tang, F.H.; Wong, H.Y.T.; Tsang, P.S.W.; Yau, M.; Tam, S.Y.; Law, L.; Yau, K.; Wong, J.; Farah, F.H.M.; Wong, J. Recent advancements in lung cancer research: A narrative review. Transl. Lung Cancer Res. 2025, 14, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.H.; Ngai, A.M.Y.; Tang, C.Y.; Lee, J.H.S.; Lee, A.L.H.; Li, J.J.X.; Ip, P.P.C. Malignant ascites in ovarian cancer is compatible with long-term (10 year) survival with associations to clinicopathological features. J. Ovarian Res. 2025, 18, 98. [Google Scholar] [CrossRef] [PubMed]
- Kleinaki, M.; Vey, J.A.; Awounvo, S.; Ishak, A.; Arnaouti, M.; Ryu, H.S.; Nikas, I.P. The Diagnostic Accuracy of Claudin-4 Immunochemistry in Differentiating Metastatic Carcinomas From Mesothelial Processes in Serous Effusion Cytology: A Systematic Review and Meta-analysis. Arch. Pathol. Lab. Med. 2025, 149, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.P.H.; Lum, R.T.W.; Chan, J.W.Y.; Lau, R.W.H.; Ng, C.S.H.; Li, J.J.X. Neuroendocrine Lesions Arising From Mediastinal Teratoma—A Case Report and Literature Review. Int. J. Surg. Pathol. 2025, 33, 466–471. [Google Scholar] [CrossRef] [PubMed]
| Risk of Bias | Applicability Concerns | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Size | Year | Location | Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard | |
| Sridakhun 2023 [11] | 152 | 2023 | Thailand | L | L | H | L | L | L | L |
| Najjar 2023 [12] | 220 | 2023 | USA | L | L | H | L | L | L | L |
| Sahu 2021 [13] | 64 | 2021 | India | L | U | H | L | L | L | L |
| Subbarayan 2019 [14] | 84 | 2023 | India | L | L | H | L | L | L | L |
| Carneiro 2019 [15] | 55 | 2019 | Brazil | U | L | L | U | L | L | L |
| Sadullahoglu 2017 [16] | 142 | 2017 | Turkey | L | L | H | L | L | H | L |
| Oda 2016 [17] | 265 | 2016 | Japan | L | L | L | L | L | L | L |
| Lv 2015 [18] | 162 | 2015 | China | L | L | H | L | L | L | L |
| Knoepp 2013 [19] | 61 | 2013 | USA | H | H | H | H | L | H | L |
| Hyun 2012 [20] | 45 | 2012 | USA | H | L | L | L | L | L | L |
| Su 2011 [21] | 71 | 2011 | China | L | L | L | L | L | L | L |
| Kundu 2011 [22] | 113 | 2011 | USA | U | L | L | U | L | L | L |
| Ensani 2011 [23] | 211 | 2011 | Iran | L | L | L | L | L | L | L |
| Saleh 2009 [24] | 84 | 2009 | USA | L | L | L | L | L | L | L |
| Sun 2009 [25] | 118 | 2009 | China | L | L | L | L | L | L | L |
| Kim 2009 [26] | 293 | 2009 | Korea | L | L | H | L | L | L | L |
| Pu 2008 [27] | 43 | 2008 | USA | H | L | L | U | L | L | L |
| Lyons-Boudreaux 2008 [28] | 71 | 2008 | USA | L | L | U | L | L | L | L |
| Hecht 2006 [29] | 103 | 2006 | USA | H | L | L | U | L | L | L |
| Politi 2005 [30] | 134 | 2005 | Greece | H | L | L | U | L | L | L |
| Lozano 2001 [31] | 44 | 2001 | Spain | H | L | H | U | L | L | L |
| Athanassiadou 2000 [32] | 137 | 2000 | Greece | H | L | H | U | L | L | L |
| Delahaye 1997 [33] | 154 | 1997 | The Netherlands | L | L | L | U | L | L | L |
| Kuenen-Boumeester 1996 [34] | 108 | 1996 | The Netherlands | H | L | L | U | L | L | L |
| Delahaye 1991 [35] | 75 | 1991 | The Netherlands | H | L | H | U | L | H | L |
| Preparation | Source | Dilution | Intensity | Percentage | Pattern | |
|---|---|---|---|---|---|---|
| Sridakhun 2023 [11] | Cell block | Novocastra | 1:25 | NS * | NS | Membranous |
| Najjar 2023 [12] | Cell block | Dako | 1:50 | NS | NS | Membranous and/or cytoplasmic |
| Sahu 2021 [13] | Cell block | NS | NS | At least weak | NS | Membranous and/or cytoplasmic |
| Subbarayan 2019 [14] | Smear | Bio SB | NS | NS | >20% | Membranous and cytoplasmic |
| Carneiro 2019 [15] | Cell block | Dako | 1:200 | At least weak | >0% | Membranous |
| Sadullahoglu 2017 [16] | Smear | Eurodiagnostica | 1:40 | At least moderate | NS | NS |
| Oda 2016 [17] | Cell block | Dako | 1:50 | At least weak | >0% | Membranous |
| Lv 2015 [18] | Smear | Eurodiagnostica | 1:10 | NS | NS | Membranous and cytoplasmic |
| Knoepp 2013 [19] | Smear | Dako | 1:100 | NS | NS | NS |
| Hyun 2012 [20] | Cell block | Dako | 1:160 | NS | >10% | Membranous and/or cytoplasmic |
| Su 2011 [21] | Smear | Eurodiagnostica | 1:20 | NS | NS | Membranous |
| Kundu 2011 [22] | Cell block | Maixin Bio | Prediluted | At least weak | >10% | Membranous and/or cytoplasmic |
| Ensani 2011 [23] | Cell block | Novocastra | 1:50 | NS | NS | Membranous |
| Saleh 2009 [24] | Cell block | Dako | NS | NS | NS | Membranous |
| Sun 2009 [25] | Cell block | Maixin Bio | Prediluted | At least weak | >10% | Membranous and/or cytoplasmic |
| Kim 2009 [26] | Cell block | Dako | 1:100 | NS | >5% | Membrane and cytoplasmic |
| Pu 2008 [27] | Cell block | Dako | 1:30 | NS | >10% | Membranous or cytoplasmic |
| Lyons-Boudreaux 2008 [28] | Cell block | Dako | 1:10 | At least weak | NS | Membranous |
| Hecht 2006 [29] | Cell block | Biogenic | Prediluted | NS | >5% | Membranous, cytoplasmic or nuclear |
| Politi 2005 [30] | Cell block | Dako | 1:60 | At least weak | NS | Membranous |
| Lozano 2001 [31] | Cytospin | Dako | 1:200 | NS | NS | Membranous and/or cytoplasmic |
| Athanassiadou 2000 [32] | Smear | Biogenex | Prediluted | At least weak | >10% | Membranous, cytoplasmic, or nuclear |
| Delahaye 1997 [33] | Cell block | Dako | 1:200 | Any intensity | >0% | Membranous |
| Kuenen-Boumeester 1996 [34] | Cell block | Dako | 1:100 | NS | NS | Membranous and/or cytoplasmic |
| Delahaye 1991 [35] | Smear | Organon | 1:10 | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, A.H.; Hsu, M.; Ng, J.K.M.; Farahani, S.J.; Meçani, R.; Nano, J.; Li, J.J.X.; Vielh, P.; Muka, T. MOC31 for the Diagnosis of Metastatic Carcinoma and Mesothelial Lesions in Effusion Fluid—A Systematic Review and Meta-Analysis. Diagnostics 2025, 15, 2675. https://doi.org/10.3390/diagnostics15212675
Lin AH, Hsu M, Ng JKM, Farahani SJ, Meçani R, Nano J, Li JJX, Vielh P, Muka T. MOC31 for the Diagnosis of Metastatic Carcinoma and Mesothelial Lesions in Effusion Fluid—A Systematic Review and Meta-Analysis. Diagnostics. 2025; 15(21):2675. https://doi.org/10.3390/diagnostics15212675
Chicago/Turabian StyleLin, Alex H., Matthew Hsu, Joanna K. M. Ng, Sahar J. Farahani, Renald Meçani, Jana Nano, Joshua J. X. Li, Philippe Vielh, and Taulant Muka. 2025. "MOC31 for the Diagnosis of Metastatic Carcinoma and Mesothelial Lesions in Effusion Fluid—A Systematic Review and Meta-Analysis" Diagnostics 15, no. 21: 2675. https://doi.org/10.3390/diagnostics15212675
APA StyleLin, A. H., Hsu, M., Ng, J. K. M., Farahani, S. J., Meçani, R., Nano, J., Li, J. J. X., Vielh, P., & Muka, T. (2025). MOC31 for the Diagnosis of Metastatic Carcinoma and Mesothelial Lesions in Effusion Fluid—A Systematic Review and Meta-Analysis. Diagnostics, 15(21), 2675. https://doi.org/10.3390/diagnostics15212675








