Plasma Cystine as a Marker of Acute Stroke Severity
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
- Men and women aged up to 60 years inclusive;
- A time from the onset of stroke symptoms to the inclusion in this study of no more than 72 h;
- Patients who suffered their first stroke, verified using magnetic resonance imaging (MRI)/computer tomography (CT) scan of the brain;
- The patient or a disinterested witness signing and dating an informed consent form (if the patient was unable to sign due to physical limitations).
- A time from the onset of acute stroke symptoms to the inclusion in this study of more than 72 h;
- Patients with contraindications to CT/MRI (installed pacemaker/neurostimulator/pacemaker; inner ear prosthesis, ferromagnetic or electronic middle ear implants, hemostatic clips, prosthetic heart valves, and any other metal-containing structures or ferromagnetic fragments; and insulin pumps) or inability to undergo the CT/MRI procedure (pronounced claustrophobia, etc.);
- The presence of any neuroimaging (CT/MRI) signs of a brain tumor, arteriovenous malformation, brain abscess, cerebral vascular aneurysm, or edema of the infarct zone, leading to the dislocation of brain structures (malignant course of cerebral infarction);
- Repeated ischemic stroke, hemorrhagic stroke, or a history of unspecified stroke;
- Traumatic brain injury within the past 6 months before screening;
- Patients with a history of surgical intervention on the brain or spinal cord;
- Patients with a history of epilepsy or severe cognitive impairment.
- Positive blood tests for HIV, syphilis, or hepatitis B and/or C detected at the start of this study;
- The appearance of any diseases or conditions during this study that worsened the patient’s prognosis and made it impossible for the patient to continue participating in the clinical trial;
- Violation of the study protocol, such as incorrect inclusion of patients who did not meet the inclusion criteria, use of prohibited therapy, or other significant protocol violations according to the investigator’s opinion;
- A patient’s refusal to continue participating in this study.
2.2. Laboratory Studies
2.3. Data Processing
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | Apparent diffusion coefficient |
| aPTT | Activated partial thromboplastin time |
| ASCT1 | Neutral amino acid transporter A |
| AT | Atherothrombotic stroke |
| BBB | Blood–brain barrier |
| CAD | Coronary artery disease |
| CE | Cardioembolic stroke |
| CG | Cysteinylglycine |
| CRP | C-reactive protein |
| CSF | Cerebrospinal fluid |
| CT | Computer tomography |
| Cys | Cysteine |
| CysS | Cystine |
| CβS | Cystathionine beta-synthase |
| DM2 | Type 2 diabetes mellitus |
| DWI | Diffusion-weighted imaging |
| EAAT | Excitatory amino acid transporter |
| ER | Endoplasmic reticulum |
| EAAC1 | Excitatory amino acid carrier 1 |
| GLT-1 | Glutamate transporter-1 |
| Glu | Glutamate |
| GSH | Glutathione |
| Hcy | Homocysteine |
| HDL-C | High-density lipoprotein cholesterol |
| HHcy | Hyperhomocysteinemia |
| HS | Hemorrhagic stroke |
| ICH | Intracerebral hemorrhage |
| IL-1β | Interleukin 1β |
| IL-6 | Interleukin 6 |
| IS | Ischemic stroke |
| Lac | Lacunar stroke |
| LAT-1 | Large neutral amino acid transporter 1 |
| LDL-C | Low-density lipoprotein cholesterol |
| LMWTs | Low-molecular-weight aminothiols |
| MCAO | Middle cerebral artery occlusion |
| MRI | Magnetic resonance imaging |
| mRs | Modified Rankin Scale |
| NF-κB | Nuclear factor kappa b |
| NIHSS | National Institutes of Health Stroke Scale |
| NMDA | N-methyl-D-aspartate |
| OGD | Oxygen and glucose deprivation |
| OR | Odds ratio |
| OS | Oxidative stress |
| PLT | Platelets |
| QSM | Quantitative susceptibility mapping |
| rCys | Reduced cysteine |
| ROC | Receiver operating characteristic |
| ROS | Reactive oxygen species |
| RR | Relative risk ratio |
| SAH | Subarachnoid hemorrhage |
| T1 | First tertile |
| T3 | Third tertile |
| tCG | Total cysteinylglycine |
| tCys | Total cysteine |
| TGs | Triglycerides |
| tGSH | Total glutathione |
| tHcy | Total homocysteine |
| TNFα | Tumor necrosis factor-alpha |
| TOAST | Trial of ORG 10172 in Acute Stroke Treatment |
| WBCs | White blood cells |
| xc− | Cystine/glutamate transporter |
References
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Zafar, A.; Al-Khamis, F.A.; Al-Bakr, A.I.; Alsulaiman, A.A.; Msmar, A.H. Risk factors and subtypes of acute ischemic stroke. A study at King Fahd Hospital of the University. Neurosciences 2016, 21, 246–251. [Google Scholar] [CrossRef]
- Salaudeen, M.A.; Bello, N.; Danraka, R.N.; Ammani, M.L. Understanding the Pathophysiology of Ischemic Stroke: The Basis of Current Therapies and Opportunity for New Ones. Biomolecules 2024, 14, 305. [Google Scholar] [CrossRef]
- Vinknes, K.J.; Refsum, H.; Turner, C.; Khaw, K.T.; Wareham, N.J.; Forouhi, N.G.; Imamura, F. Plasma Sulfur Amino Acids and Risk of Cerebrovascular Diseases: A Nested Case-Control Study in the EPIC-Norfolk Cohort. Stroke 2021, 52, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Holmen, M.; Hvas, A.M.; Arendt, J.F.H. Hyperhomocysteinemia and Ischemic Stroke: A Potential Dose-Response Association-A Systematic Review and Meta-analysis. TH Open 2021, 5, e420–e437. [Google Scholar] [CrossRef]
- Lehotsky, J.; Kovalska, M.; Baranovicova, E.; Hnilicova, P.; Kalenska, D.; Kaplan, P. Ischemic Brain Injury in Hyperhomocysteinemia. In Cerebral Ischemia [Internet]; Pluta, R., Ed.; Exon Publications: Brisbane, Australia, 2021; Chapter 5. [Google Scholar]
- Harris, S.; Rasyid, A.; Kurniawan, M.; Mesiano, T.; Hidayat, R. Association of high blood homocysteine and risk of increased severity of ischemic stroke events. Int. J. Angiol. 2019, 28, 34–38. [Google Scholar] [CrossRef]
- Markišić, M.; Pavlović, A.M.; Pavlović, D.M. The Impact of homocysteine, vitamin B12, and vitamin D levels on functional outcome after firstever ischaemic stroke. BioMed Res. Int. 2017, 2017, 5489057. [Google Scholar] [CrossRef]
- Li, L.; Ma, X.; Zeng, L.; Pandey, S.; Wan, R.; Shen, R.; Zhang, Q. Impact of homocysteine levels on clinical outcome in patients with acute ischemic stroke receiving intravenous thrombolysis therapy. PeerJ 2020, 8, e9474. [Google Scholar] [CrossRef] [PubMed]
- Kahl, A.; Stepanova, A.; Konrad, C.; Anderson, C.; Manfredi, G.; Zhou, P.; Iadecola, C.; Galkin, A. Critical Role of Flavin and Glutathione in Complex I-Mediated Bioenergetic Failure in Brain Ischemia/Reperfusion Injury. Stroke 2018, 49, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef]
- Iskusnykh, I.Y.; Zakharova, A.A.; Pathak, D. Glutathione in Brain Disorders and Aging. Molecules 2022, 27, 324. [Google Scholar] [CrossRef]
- Paterson, P.G.; Lyon, A.W.; Kamencic, H.; Andersen, L.B.; Juurlink, B.H. Sulfur amino acid deficiency depresses brain glutathione concentration. Nutr. Neurosci. 2001, 4, 213–222. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Patterson, R.A.; Lamb, D.J.; Leake, D.S. Mechanisms by which cysteine can inhibit or promote the oxidation of low density lipoprotein by copper. Atherosclerosis 2003, 169, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Pfanzagl, B.; Tribl, F.; Koller, E.; Möslinger, T. Homocysteine strongly enhances metal-catalyzed LDL oxidation in the presence of cystine and cysteine. Atherosclerosis 2003, 168, 39–48. [Google Scholar] [CrossRef]
- Chan, S.J.; Chai, C.; Lim, T.W.; Yamamoto, M.; Lo, E.H.; Lai, M.K.; Wong, P.T. Cystathionine β-synthase inhibition is a potential therapeutic approach to treatment of ischemic injury. ASN Neuro. 2015, 7, 1759091415578711. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Lee, S.W.; Bian, J.S.; Low, C.M.; Wong, P.T. Hydrogen sulfide: Neurochemistry and neurobiology. Neurochem. Int. 2008, 52, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Wang, Z.; Chen, G. The role of hydrogen sulfide in stroke. Med. Gas Res. 2016, 6, 79–84. [Google Scholar] [CrossRef]
- Janáky, R.; Varga, V.; Hermann, A.; Saransaari, P.; Oja, S.S. Mechanisms of L-cysteine neurotoxicity. Neurochem. Res. 2000, 25, 1397–1405. [Google Scholar] [CrossRef]
- Olney, J.W.; Zorumski, C.; Price, M.T.; Labruyere, J. L-cysteine, a bicarbonate-sensitive endogenous excitotoxin. Science 1990, 248, 596–599. [Google Scholar] [CrossRef]
- Schurr, A.; West, C.A.; Heine, M.F.; Rigor, B.M. The neurotoxicity of sulfur-containing amino acids in energy-deprived rat hippocampal slices. Brain Res. 1993, 601, 317–320. [Google Scholar] [CrossRef]
- Wang, X.F.; Cynader, M.S. Pyruvate released by astrocytes protects neurons from copper-catalyzed cysteine neurotoxicity. J. Neurosci. 2001, 21, 3322–3331. [Google Scholar] [CrossRef] [PubMed]
- Stelmashook, E.V.; Budagova, T.Y.; Genrikhs, E.E.; Isaev, N.K. Extracellular Acidosis, Cysteine, and Glutathione Enhance the Toxic Effect of Copper Ions in Cultures of Cerebellar Granule Neurons. Bull. Exp. Biol. Med. 2024, 177, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Isaksson, A.; Brattström, L.; Hultberg, B. Homocysteine and other thiols determined in plasma by HPLC and thiol-specific postcolumn derivatization. Clin. Chem. 1993, 39, 1590–1597. [Google Scholar] [CrossRef]
- Williams, R.H.; Maggiore, J.A.; Reynolds, R.D.; Helgason, C.M. Novel approach for the determination of the redox status of homocysteine and other aminothiols in plasma from healthy subjects and patients with ischemic stroke. Clin. Chem. 2001, 47, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Carru, C.; Deiana, L.; Sotgia, S.; Pes, G.M.; Zinellu, A. Plasma thiols redox status by laser-induced fluorescence capillary electrophoresis. Electrophoresis 2004, 25, 882–889. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Popov, M.A.; Aleksandrin, V.; Pudova, P.A.; Galdobina, M.P.; Metelkin, A.A.; Kruglova, M.P.; Maslennikov, R.A.; Silina, E.V.; Stupin, V.A.; et al. Simultaneous determination of cysteine and other free aminothiols in blood plasma using capillary electrophoresis with pH-mediated stacking. Electrophoresis 2024, 45, 411–419. [Google Scholar] [CrossRef]
- Wong, P.T.; Qu, K.; Chimon, G.N.; Seah, A.B.; Chang, H.M.; Wong, M.C.; Ng, Y.K.; Rumpel, H.; Halliwell, B.; Chen, C.P. High plasma cyst(e)ine level may indicate poor clinical outcome in patients with acute stroke: Possible involvement of hydrogen sulfide. J. Neuropathol. Exp. Neurol. 2006, 65, 109–115. [Google Scholar] [CrossRef]
- Larsson, S.C.; Håkansson, N.; Wolk, A. Dietary cysteine and other amino acids and stroke incidence in women. Stroke 2015, 46, 922–926. [Google Scholar] [CrossRef]
- De Chiara, B.; Sedda, V.; Parolini, M.; Campolo, J.; De Maria, R.; Caruso, R.; Pizzi, G.; Disoteo, O.; Dellanoce, C.; Corno, A.R.; et al. Plasma total cysteine and cardiovascular risk burden: Action and interaction. Sci. World J. 2012, 2012, 303654. [Google Scholar] [CrossRef]
- Elkafrawy, H.; Mehanna, R.; Ali, F.; Barghash, A.; Dessouky, I.; Jernerén, F.; Turner, C.; Refsum, H.; Elshorbagy, A. Extracellular ysteine influences human preadipocyte differentiation and correlates with fat mass in healthy adults. Amino Acids 2021, 53, 1623–1634. [Google Scholar] [CrossRef]
- Ottosson, F.; Engström, G.; Orho-Melander, M.; Melander, O.; Nilsson, P.M.; Johansson, M. Plasma Metabolome Predicts Aortic Stiffness and Future Risk of Coronary Artery Disease and Mortality After 23 Years of Follow-Up in the General Population. J. Am. Heart Assoc. 2024, 13, e033442. [Google Scholar] [CrossRef]
- Ozkan, Y.; Ozkan, E.; Simşek, B. Plasma total homocysteine and cysteine levels as cardiovascular risk factors in coronary heart disease. Int. J. Cardiol. 2002, 82, 269–277. [Google Scholar] [CrossRef]
- Oda, M.; Fujibayashi, K.; Wakasa, M.; Takano, S.; Fujita, W.; Kitayama, M.; Nakanishi, H.; Saito, K.; Kawai, Y.; Kajinami, K. Increased plasma glutamate in non-smokers with vasospastic angina pectoris is associated with plasma cystine and antioxidant capacity. Scand. Cardiovasc. J. 2022, 56, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Salemi, G.; Gueli, M.C.; D’Amelio, M.; Saia, V.; Mangiapane, P.; Aridon, P.; Ragonese, P.; Lupo, I. Blood levels of homocysteine, cysteine, glutathione, folic acid, and vitamin B12 in the acute phase of atherothrombotic stroke. Neurol. Sci. 2009, 30, 361–364. [Google Scholar] [CrossRef]
- Goulart, V.A.M.; Sena, M.M.; Mendes, T.O.; Menezes, H.C.; Cardeal, Z.L.; Paiva, M.J.N.; Sandrim, V.C.; Pinto, M.C.X.; Resende, R.R. Amino Acid Biosignature in Plasma among Ischemic Stroke Subtypes. Biomed. Res. Int. 2019, 2019, 8480468. [Google Scholar] [CrossRef]
- Dong, W.C.; Guo, J.L.; Xu, L.; Jiang, X.H.; Chang, C.H.; Jiang, Y.; Zhang, Y.Z. Impact of homocysteine on acute ischemic stroke severity: Possible role of aminothiols redox status. BMC Neurol. 2024, 24, 175. [Google Scholar] [CrossRef] [PubMed]
- Maksimova, M.Y.; Ivanov, A.V.; Virus, E.D.; Nikiforova, K.A.; Ochtova, F.R.; Suanova, E.T.; Kruglova, M.P.; Piradov, M.A.; Kubatiev, A.A. Impact of glutathione on acute ischemic stroke severity and outcome: Possible role of aminothiols redox status. Redox Rep. Commun. Free Radic. Res. 2021, 26, 117–123. [Google Scholar] [CrossRef]
- Aoyama, K.; Nakaki, T. Impaired glutathione synthesis in neurodegeneration. Int. J. Mol. Sci. 2013, 14, 21021–21044. [Google Scholar] [CrossRef] [PubMed]
- Brott, T.; Adams, H.P., Jr.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef]
- Sulter, G.; Steen, C.; De Keyser, J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999, 30, 1538–1541. [Google Scholar] [CrossRef]
- Kaneva, A.M.; Potolitsyna, N.N.; Bojko, E.R. Range of values for lipid accumulation product (LAP) in healthy residents of the European north of Russia. Obes. Metab. 2020, 17, 179–186. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Popov, M.A.; Metelkin, A.A.; Aleksandrin, V.V.; Agafonov, E.G.; Kruglova, M.P.; Silina, E.V.; Stupin, V.A.; Maslennikov, R.A.; Kubatiev, A.A. Influence of Coronary Artery Bypass Grafts on Blood Aminothiols in Patients with Coronary Artery Disease. Metabolites 2023, 13, 743. [Google Scholar] [CrossRef]
- Krzyżanowska, W.; Pomierny, B.; Filip, M.; Pera, J. Glutamate transporters in brain ischemia: To modulate or not? Acta Pharmacol. Sin. 2014, 35, 444–462. [Google Scholar] [CrossRef]
- Massie, A.; Boillée, S.; Hewett, S.; Knackstedt, L.; Lewerenz, J. Main path and byways: Non-vesicular glutamate release by system xc− as an important modifier of glutamatergic neurotransmission. J. Neurochem. 2015, 135, 1062–1079. [Google Scholar] [CrossRef]
- Burdo, J.; Dargusch, R.; Schubert, D. Distribution of the cystine/glutamate antiporter system xc− in the brain, kidney, and duodenum. J. Histochem. Cytochem. 2006, 54, 549–557. [Google Scholar] [CrossRef]
- Lee, M.; Ko, D.G.; Hong, D.K.; Lim, M.S.; Choi, B.Y.; Suh, S.W. Role of Excitatory Amino Acid Carrier 1 (EAAC1) in Neuronal Death and Neurogenesis After Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 5676. [Google Scholar] [CrossRef]
- Kinoshita, C.; Aoyama, K. The Role of Non-Coding RNAs in the Neuroprotective Effects of Glutathione. Int. J. Mol. Sci. 2021, 22, 4245. [Google Scholar] [CrossRef] [PubMed]
- De Bundel, D.; Schallier, A.; Loyens, E.; Fernando, R.; Miyashita, H.; Van Liefferinge, J.; Vermoesen, K.; Bannai, S.; Sato, H.; Michotte, Y.; et al. Loss of system xI- does not induce oxidative stress but decreases extracellular glutamate in hippocampus and influences spatial working memory and limbic seizure susceptibility. J. Neurosci. 2011, 31, 5792–5803. [Google Scholar] [CrossRef] [PubMed]
- Sears, S.M.S.; Hewett, J.A.; Hewett, S.J. Decreased epileptogenesis in mice lacking the System xc− transporter occurs in association with a reduction in AMPA receptor subunit GluA1. Epilepsia Open 2019, 4, 133–143. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hewett, S.J. The Cystine/Glutamate Antiporter, System xc−, Contributes to Cortical Infarction After Moderate but Not Severe Focal Cerebral Ischemia in Mice. Front. Cell Neurosci. 2022, 16, 821036. [Google Scholar] [CrossRef]
- Lee, B.J.; Jun, H.O.; Kim, J.H.; Kim, J.H. Astrocytic cystine/glutamate antiporter is a key regulator of erythropoietin expression in the ischemic retina. FASEB J. 2019, 33, 6045–6054. [Google Scholar] [CrossRef]
- Liu, T.; Cui, Y.; Dong, S.; Kong, X.; Xu, X.; Wang, Y.; Wan, Q.; Wang, Q. Treadmill Training Reduces Cerebral Ischemia-Reperfusion Injury by Inhibiting Ferroptosis through Activation of SLC7A11/GPX4. Oxid. Med. Cell. Longev. 2022, 2022, 8693664. [Google Scholar] [CrossRef]
- Heit, B.S.; Chu, A.; McRay, A.; Richmond, J.E.; Heckman, C.J.; Larson, J. Interference with glutamate antiporter system xc− enables post-hypoxic long-term potentiation in hippocampus. Exp. Physiol. 2024, 109, 1572–1592. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Lin, Y.J.; Chen, W.L.; Huang, Y.C.; Chang, C.W.; Cheng, F.C.; Liu, R.S.; Shyu, W.C. HIF-1α triggers long-lasting glutamate excitotoxicity via system xc− in cerebral ischaemia-reperfusion. J. Pathol. 2017, 241, 337–349. [Google Scholar] [CrossRef]
- Soria, F.N.; Pérez-Samartín, A.; Martin, A.; Gona, K.B.; Llop, J.; Szczupak, B.; Chara, J.C.; Matute, C.; Domercq, M. Extrasynaptic glutamate release through cystine/glutamate antiporter contributes to ischemic damage. J. Clin. Investig. 2014, 124, 3645–3655. [Google Scholar] [CrossRef] [PubMed]
- Fogal, B.; Li, J.; Lobner, D.; McCullough, L.D.; Hewett, S.J. System xc− activity and astrocytes are necessary for interleukin-1β-mediated hypoxic neuronal injury. J. Neurosci. 2007, 27, 10094–10105. [Google Scholar] [CrossRef] [PubMed]
- Krzyżanowska, W.; Pomierny, B.; Bystrowska, B.; Pomierny-Chamioło, L.; Filip, M.; Budziszewska, B.; Pera, J. Ceftriaxone- and N-acetylcysteine-induced brain tolerance to ischemia: Influence on glutamate levels in focal cerebral ischemia. PLoS ONE 2017, 12, e0186243. [Google Scholar] [CrossRef]
- Li, X.; Wallin, C.; Weber, S.G.; Sandberg, M. Net efflux of cysteine, glutathione and related metabolites from rat hippocampal slices during oxygen/glucose deprivation: Dependence on γ-glutamyl transpeptidase. Brain Res. 1999, 815, 81–88. [Google Scholar] [CrossRef]
- Slivka, A.; Cohen, G. Brain ischemia markedly elevates levels of the neurotoxic amino acid, cysteine. Brain Res. 1993, 608, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, T.W.; Paul, B.D.; Parker, G.M.; Hester, L.D.; Snowman, A.M.; Taniguchi, Y.; Kamiya, A.; Snyder, S.H.; Sawa, A. The glutathione cycle shapes synaptic glutamate activity. Proc. Natl. Acad. Sci. USA 2019, 116, 2701–2706. [Google Scholar] [CrossRef] [PubMed]
- Omorou, M.; Liu, N.; Huang, Y.; Al-Ward, H.; Gao, M.; Mu, C.; Zhang, L.; Hui, X. Cystathionine beta-Synthase in hypoxia and ischemia/reperfusion: A current overview. Arch. Biochem. Biophys. 2022, 718, 109149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, X.; Xu, Y.; He, M.; Yang, J.; Li, J.; Li, Y.; Ao, G.; Cheng, J.; Jia, J. The cystathionine β-synthase/hydrogen sulfide pathway contributes to microglia-mediated neuroinflammation following cerebral ischemia. Brain Behav. Immun. 2017, 66, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and macrophage polarization—New prospects for brain repair. Nat. Rev. Neurol. 2015, 11, 56–64. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Hu, Q.; Liu, S.; Bai, X.; Xie, Y.; Zhang, T.; Bo, S.; Gao, X.; Wu, S.; et al. Neuroprotective Roles of l-Cysteine in Attenuating Early Brain Injury and Improving Synaptic Density via the CBS/H2S Pathway Following Subarachnoid Hemorrhage in Rats. Front. Neurol. 2017, 8, 176. [Google Scholar] [CrossRef]
- McCune, C.D.; Chan, S.J.; Beio, M.L.; Shen, W.; Chung, W.J.; Szczesniak, L.M.; Chai, C.; Koh, S.Q.; Wong, P.T.; Berkowitz, D.B. “Zipped Synthesis” by Cross-Metathesis Provides a Cystathionine β-Synthase Inhibitor that Attenuates Cellular H2S Levels and Reduces Neuronal Infarction in a Rat Ischemic Stroke Model. ACS Cent. Sci. 2016, 2, 242–252. [Google Scholar] [CrossRef]
- Cheung, N.S.; Peng, Z.F.; Chen, M.J.; Moore, P.K.; Whiteman, M. Hydrogen sulfide induced neuronal death occurs via glutamate receptor and is associated with calpain activation and lysosomal rupture in mouse primary cortical neurons. Neuropharmacology 2007, 53, 505–514. [Google Scholar] [CrossRef]
- Han, M.; Liu, D.; Qiu, J.; Yuan, H.; Hu, Q.; Xue, H.; Li, T.; Ma, W.; Zhang, Q.; Li, G.; et al. Evaluation of H2S-producing enzymes in cerebrospinal fluid and its relationship with interleukin-6 and neurologic deficits in subarachnoid hemorrhage. Biomed. Pharmacother. 2020, 123, 109722. [Google Scholar] [CrossRef]
- Lutsenko, S.; Roy, S.; Tsvetkov, P. Mammalian copper homeostasis: Physiological roles and molecular mechanisms. Physiol. Rev. 2025, 105, 441–491. [Google Scholar] [CrossRef]
- Peng, G.; Huang, Y.; Xie, G.; Tang, J. Exploring Copper’s role in stroke: Progress and treatment approaches. Front. Pharmacol. 2024, 15, 1409317. [Google Scholar] [CrossRef]
- Bartnicka, J.J.; Al-Salemee, F.; Firth, G.; Blower, P.J. L-Cysteine-mediated modulation of copper trafficking in prostate cancer cells: An in vitro and in vivo investigation with 64Cu and 64Cu-PET. Metallomics 2020, 12, 1508–1520. [Google Scholar] [CrossRef]
- Ozcelik, D.; Uzun, H.; Nazıroglu, M. N-acetylcysteine attenuates copper overload-induced oxidative injury in brain of rat. Biol. Trace Elem. Res. 2012, 147, 292–298. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Hughes, C.E.; Coody, T.K.; Jeong, M.Y.; Berg, J.A.; Winge, D.R.; Hughes, A.L. Cysteine Toxicity Drives Age-Related Mitochondrial Decline by Altering Iron Homeostasis. Cell 2020, 180, 296–310. [Google Scholar] [CrossRef]
- Blagov, A.; Postnov, A.; Sukhorukov, V.; Popov, M.; Uzokov, J.; Orekhov, A. Significance of Mitochondrial Dysfunction in the Pathogenesis of Parkinson’s Disease. Front. Biosci. Landmark Ed. 2024, 29, 36. [Google Scholar] [CrossRef]
- Patel, R.S.; Ghasemzadeh, N.; Eapen, D.J.; Sher, S.; Arshad, S.; Ko, Y.A.; Veledar, E.; Samady, H.; Zafari, A.M.; Sperling, L.; et al. Novel Biomarker of Oxidative Stress Is Associated With Risk of Death in Patients With Coronary Artery Disease. Circulation 2016, 133, 361–369. [Google Scholar] [CrossRef]
- Refsum, H.; Smith, A.D.; Ueland, P.M.; Nexo, E.; Clarke, R.; McPartlin, J.; Johnston, C.; Engbaek, F.; Schneede, J.; McPartlin, C.; et al. Facts and recommendations about total homocysteine determinations: An expert opinion. Clin. Chem. 2004, 50, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Liu, C.; Suliburk, J.; Hsu, J.W.; Muthupillai, R.; Jahoor, F.; Minard, C.G.; Taffet, G.E.; Sekhar, R.V. Supplementing Glycine and N-Acetylcysteine (GlyNAC) in Older Adults Improves Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Inflammation, Physical Function, and Aging Hallmarks: A Randomized Clinical Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Aging-related changes in the thiol/disulfide redox state: Implications for the use of thiol antioxidants. Exp. Gerontol. 2002, 37, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Kan, H.; Kano, Y.; Onda, K.; Sakurai, K.; Takada, K.; Ueki, Y.; Matsukawa, N.; Hillis, A.E.; Oishi, K. Longitudinal Changes in Iron and Myelination Within Ischemic Lesions Associate With Neurological Outcomes: A Pilot Study. Stroke 2024, 55, 1041–1050. [Google Scholar] [CrossRef]
- Uchida, Y.; Kan, H.; Inoue, H.; Oomura, M.; Shibata, H.; Kano, Y.; Kuno, T.; Usami, T.; Takada, K.; Yamada, K.; et al. Penumbra Detection With Oxygen Extraction Fraction Using Magnetic Susceptibility in Patients With Acute Ischemic Stroke. Front. Neurol. 2022, 13, 752450. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.J.; Wang, Q.X.; Sun, Y.; Li, J.; Li, F.L. Inflammatory markers in acute ischemic stroke. Clin. Chim. Acta 2025, 569, 120185. [Google Scholar] [CrossRef] [PubMed]
- Pawluk, H.; Tafelska-Kaczmarek, A.; Sopońska, M.; Porzych, M.; Modrzejewska, M.; Pawluk, M.; Kurhaluk, N.; Tkaczenko, H.; Kołodziejska, R. The Influence of Oxidative Stress Markers in Patients with Ischemic Stroke. Biomolecules 2024, 14, 1130. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Kurihara, S. Cystine and Theanine as Stress-Reducing AminoAcids—Perioperative Use for Early Recovery after Surgical Stress. Nutrients 2022, 14, 129. [Google Scholar] [CrossRef]

| Characteristics | Total | IS | HS |
|---|---|---|---|
| Number of patients | 210 | 138 | 72 |
| Stroke type and subtype | AT—74 (53.6%) CE—37 (26.8%) Lac—10 (7.2%) Other—17 (12.3%) | ICH—13 (18.1%) SAH—59 (81.9%) | |
| Age, years (Q1; Q3) | 55 (50; 57) | 55 (52; 57) | 55 (49; 57) |
| Male/female | 161/49 | 103/35 | 58/14 |
| NIHSS | 7.5 (6; 10) | 7 (6; 10) | 9 (7; 15) * |
| mRs | 3 (2; 3) | 3 (2; 3) | 3 (2; 3) |
| Risk factors | |||
| Hypertension, n (%) | 113 (53.8) | 73 (52.9) | 40 (55.6) |
| DM2, n (%) | 66 (31.4) | 40 (29.0) | 26 (36.1) |
| Dyslipidemia, n (%) | 148 (70.5) | 95 (68.8) | 53 (73.6) |
| HHcy: tHcy > 15 μM (%) | 44 (21.7) | 31 (23.0) | 13 (19.1) |
| CAD, n (%) | 110 (52.4) | 73 (52.9) | 37 (51.4) |
| Atrial fibrillation, n (%) | 132 (62.9) | 91 (65.5) | 41 (57.7) |
| Current cigarette smoking, n (%) | 179 (85.2) | 115 (82.7) | 64 (90.1) |
| Alcohol drinking, n (%) | 104 (49.5) | 66 (47.8) | 38 (52.8) |
| Body mass index | 27.6 (27.2; 28.0) | 28 (27.2; 28) | 27.6 (27.2; 28.6) |
| Body mass index > 25 kg/m2, n (%) | 198 (94.3) | 130 (93.5) | 68 (95.8) |
| Laboratory findings | |||
| Total cholesterol, mM | 3.5 (1.7; 4.0) | 3.5 (1.7; 4.0) | 3.5 (1.7; 3.9) |
| TGs, mM | 2.1 (1.5; 2.7) | 2.1 (1.5; 2.7) | 2.1 (1.7; 2.7) |
| HDL-C, mM | 1.2 (1.0; 1.4) | 1.2 (1.0; 1.4) | 1.2 (1.0; 1.3) |
| LDL-C, mM | 2.4 (2.2; 3.4) | 2.4 (2.2; 3.2) | 2.4 (2.2; 3.4) |
| High atherogenic coefficient (%) | 116 (55.2) | 77 (55.4) | 39 (54.9) |
| Glucose, mM | 4.9 (4.2; 5.1) | 4.9 (4.7; 6.1) | 4.8 (4.7; 5.1) |
| aPTT, s | 33 (27; 35) | 33 (27; 35) | 33 (27; 35) |
| Fibrinogen, g/L | 3.8 (3.7; 3.9) | 3.8 (3.6; 4.0) | 3.8 (3.7; 3.9) |
| WBCs, 109/L | 7.0 (5.25–8.0) | 7.0 (5.0–8.0) | 7.0 (6.0–8.0) |
| PLT, 109/L | 278 (234; 312) | 289 (234; 312) | 278 (234; 311) |
| CRP, mg/L | 4 (3;6) | 4 (3; 6) | 4 (3; 7) |
| IL-6, pg/mL | 4 (3;6) | 4 (3; 6) | 4 (3; 6) |
| Ferritin, μg/L | 75 (45; 90) | 75 (45; 90) | 75 (45; 90) |
| LMWTs | |||
| tCys, μM | 211 (173; 253) | 213 (171; 251) | 211 (179; 255) |
| tGSH, μM | 2.9 (2.3; 3.8) | 3.17 (2.48; 3.89) | 2.70 (2.09; 3.34) * |
| tHcy, μM | 11.3 (8.2; 14.7) | 11.5 (8.6; 14.8) | 10.0 (7.8; 14.4) |
| tCG, μM | 20.0 (16.5; 25.0) | 20.0 (16.5; 24.8) | 20.1 (16.5; 26.1) |
| rCys, μM | 13.7 (9.7; 19.1) | 12.9 (9.9; 19.1) | 16.6 (9.3; 18.9) |
| CysS, μM | 49.8 (40.1; 58.3) | 49.3 (39.6; 57.4) | 51.1 (40.9; 61.1) |
| LMWTs | tCys | CysS | rCys | tCG | tHcy | tGSH |
|---|---|---|---|---|---|---|
| tCys | - | 0.393 *** | −0.178 | 0.551 *** | 0.645 *** | 0.160 |
| CysS | - | −0.041 | 0.041 | 0.260 ** | 0.229 * | |
| rCys | - | −0.149 | −0.360 *** | −0.230 * | ||
| tCG | - | 0.450 *** | 0.221 * | |||
| tHcy | - | 0.371 *** | ||||
| Cholesterol | 0.173 | −0.162 | 0.077 | −0.027 | −0.193 | −0.208 * |
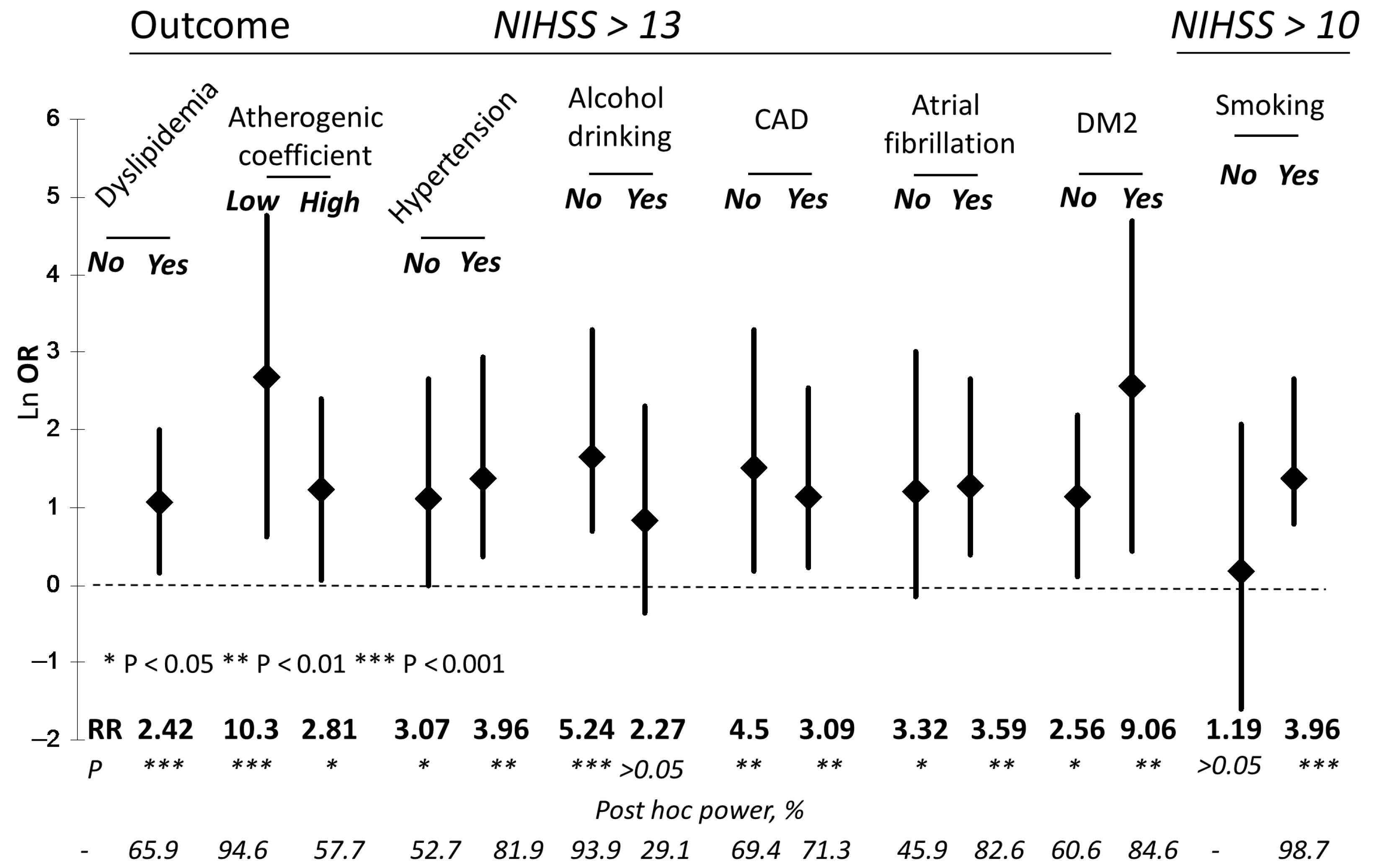
| Variable | NIHSS ≤ 13 (N = 168) | NIHSS > 13 (N = 42) | PMann-U |
|---|---|---|---|
| Age, years | 55 (50.3; 57) | 55 (49; 58) | 0.72 |
| tCys, μM | 216 (172; 254) | 204 (173; 252) | 0.604 |
| tGSH, μM | 2.89 (2.23; 3.85) | 3.11 (2.60; 3.52) | 0.394 |
| tCG, μM | 19.1 (16.4; 24.8) | 21.1 (18.3; 28.1) | 0.088 |
| tHcy, μM | 11.3 (8.0; 14.8) | 11.1 (9.0; 14.2) | 0.872 |
| CysS, μM | 51.1 (41.0; 59.4) | 44.7 (34.5; 53.4) | 0.0063 * |
| rCys, μM | 13.7 (8.9; 18.8) | 13.3 (10.0; 20.3) | 0.6 |
| CysS, μM | Proportion of Patients with NIHSS > 13 (%) | RR (p) | OR (95% CI) | Post Hoc Power, % |
|---|---|---|---|---|
| Whole cohort | ||||
| ≤54 | 34 out of 124 (27.4) | 3.56 (0.0003) | 4.53 (1.80–11.39) | 95.3 |
| >54 | 6 out of 78 (7.7) | |||
| IS | ||||
| ≤54 | 20 out of 87 (23) | 5.29 (0.003) | 6.57 (1.46–29.51) | 84.2 |
| >54 | 2 out of 46 (4.3) | |||
| HS | ||||
| ≤54 | 14 out of 37 (37.8) | 3.12 (0.0069) | 4.41 (1.28–15.23) | 70.2 |
| >54 | 4 out of 33 (12.1) | |||
| Factor | NNIHSS>13/ NCysS≤54 μM | NNIHSS>13/ NCysS>54 μM | RR (p) | OR (95% CI) | Post Hoc Power, % |
|---|---|---|---|---|---|
| T1 tGSH (0.64–2.48 μM) | 7/43 | 2/23 | 1.87 (>0.05) | 2.042 (0.39–10.75) | N/d |
| T3 tGSH (3.42–22.3 μM) | 10/34 | 2/30 | 4.41 (0.01) | 5.83 (1.16–29.27) | 65.2 |
| T1 tHcy (3.1–9.1 μM) | 9/50 | 1/16 | 2.88 (>0.05) | 3.29 (0.38–28.24) | N/d |
| T3 tHcy (13.3–30.9 μM) | 9/30 | 2/34 | 5.1 (0.005) | 6.86 (1.35–34.93) | 72.6 |
| T1 tCys (62.4–185 μM) | 15/54 | 1/12 | 3.33 (>0.05) | 4.23 (0.50–35.67) | N/d |
| T3 tCys (239–385 μM) | 11/30 | 2/34 | 6.23 (0.0013) | 9.26 (1.85–46.34) | 87.3 |
| T1 tCG (9.1–17.0 μM) | 6/38 | 3/28 | 1.47 (>0.05) | 1.56 (0.36–6.87) | N/d |
| T3 tCG (23.6–73.7 μM) | 9/44 | 5/20 | 0.82 (>0.05) | 0.77 (0.22–2.69) | N/d |
| T1 rCys (0.87–10.6 μM) | 10/34 | 3/28 | 2.75 (0.036) | 3.47 (0.85–14.2) | 42 |
| T3 rCys (17.3–51.6 μM) | 14/40 | 2/22 | 3.85 (0.013) | 5.39 (1.1–26.46) | 62.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, A.V.; Popov, M.A.; Pudova, P.A.; Maslennikov, R.A.; Aleksandrin, V.V.; Galdobina, M.P.; Kruglova, M.P.; Silina, E.V.; Stupin, V.A.; Maksimova, M.Y.; et al. Plasma Cystine as a Marker of Acute Stroke Severity. Diagnostics 2025, 15, 2662. https://doi.org/10.3390/diagnostics15202662
Ivanov AV, Popov MA, Pudova PA, Maslennikov RA, Aleksandrin VV, Galdobina MP, Kruglova MP, Silina EV, Stupin VA, Maksimova MY, et al. Plasma Cystine as a Marker of Acute Stroke Severity. Diagnostics. 2025; 15(20):2662. https://doi.org/10.3390/diagnostics15202662
Chicago/Turabian StyleIvanov, Alexander Vladimirovich, Mikhail Aleksandrovich Popov, Polina Alexandrovna Pudova, Ruslan Andreevich Maslennikov, Valery Vasil’evich Aleksandrin, Maria Pavlovna Galdobina, Maria Petrovna Kruglova, Ekaterina Vladimirovna Silina, Victor Alexandrovich Stupin, Marina Yurievna Maksimova, and et al. 2025. "Plasma Cystine as a Marker of Acute Stroke Severity" Diagnostics 15, no. 20: 2662. https://doi.org/10.3390/diagnostics15202662
APA StyleIvanov, A. V., Popov, M. A., Pudova, P. A., Maslennikov, R. A., Aleksandrin, V. V., Galdobina, M. P., Kruglova, M. P., Silina, E. V., Stupin, V. A., Maksimova, M. Y., & Kubatiev, A. A. (2025). Plasma Cystine as a Marker of Acute Stroke Severity. Diagnostics, 15(20), 2662. https://doi.org/10.3390/diagnostics15202662






