Advancing Aortic Dissection Imaging: First Clinical Experience of Photon-Counting CT with Ultra-Fast Spectral Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Scanning Protocol
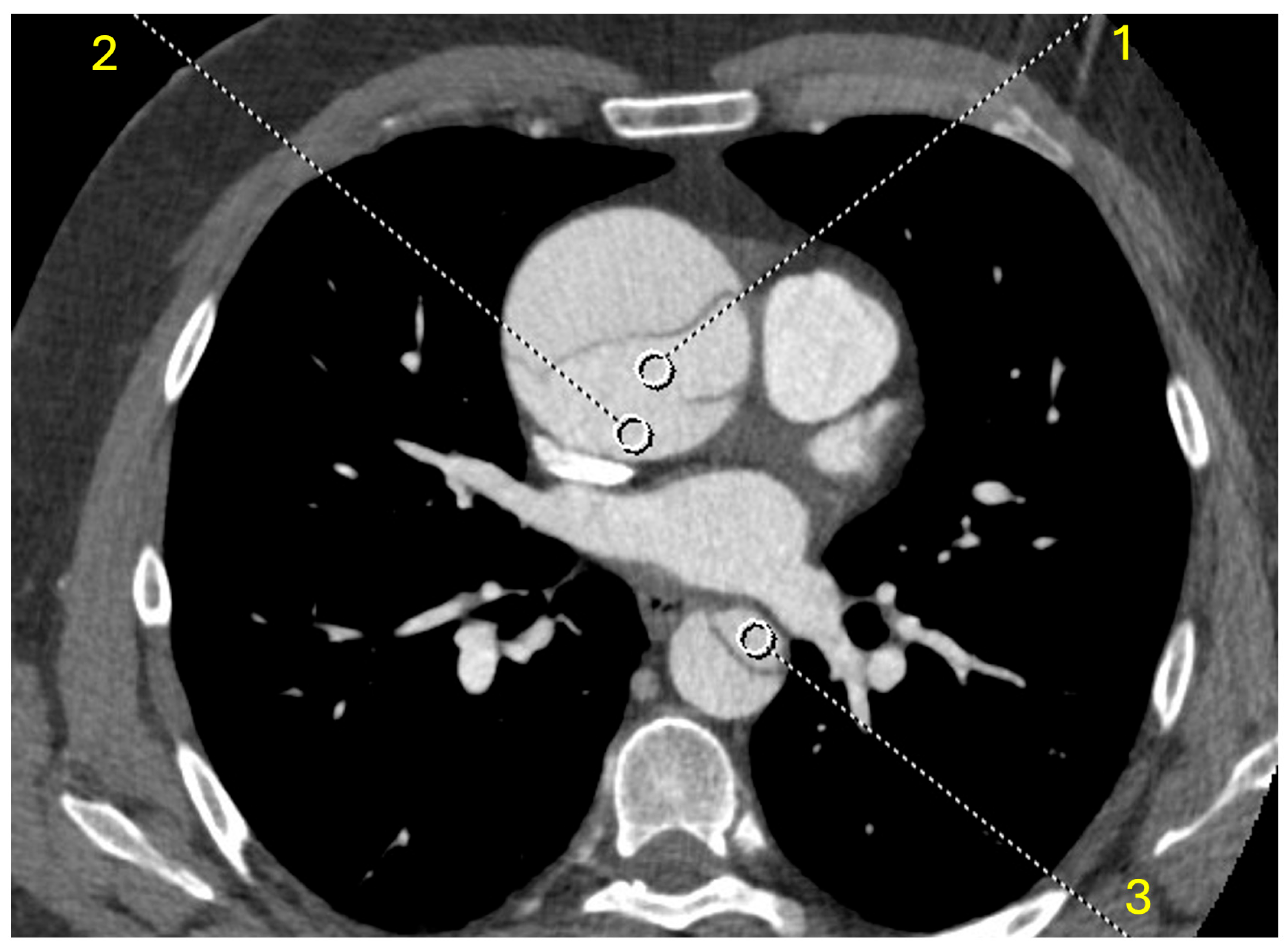
2.3. Objective Analysis
2.4. Subjective Analysis
2.5. Statistical Analysis
3. Results
3.1. Baseline Study Characteristics
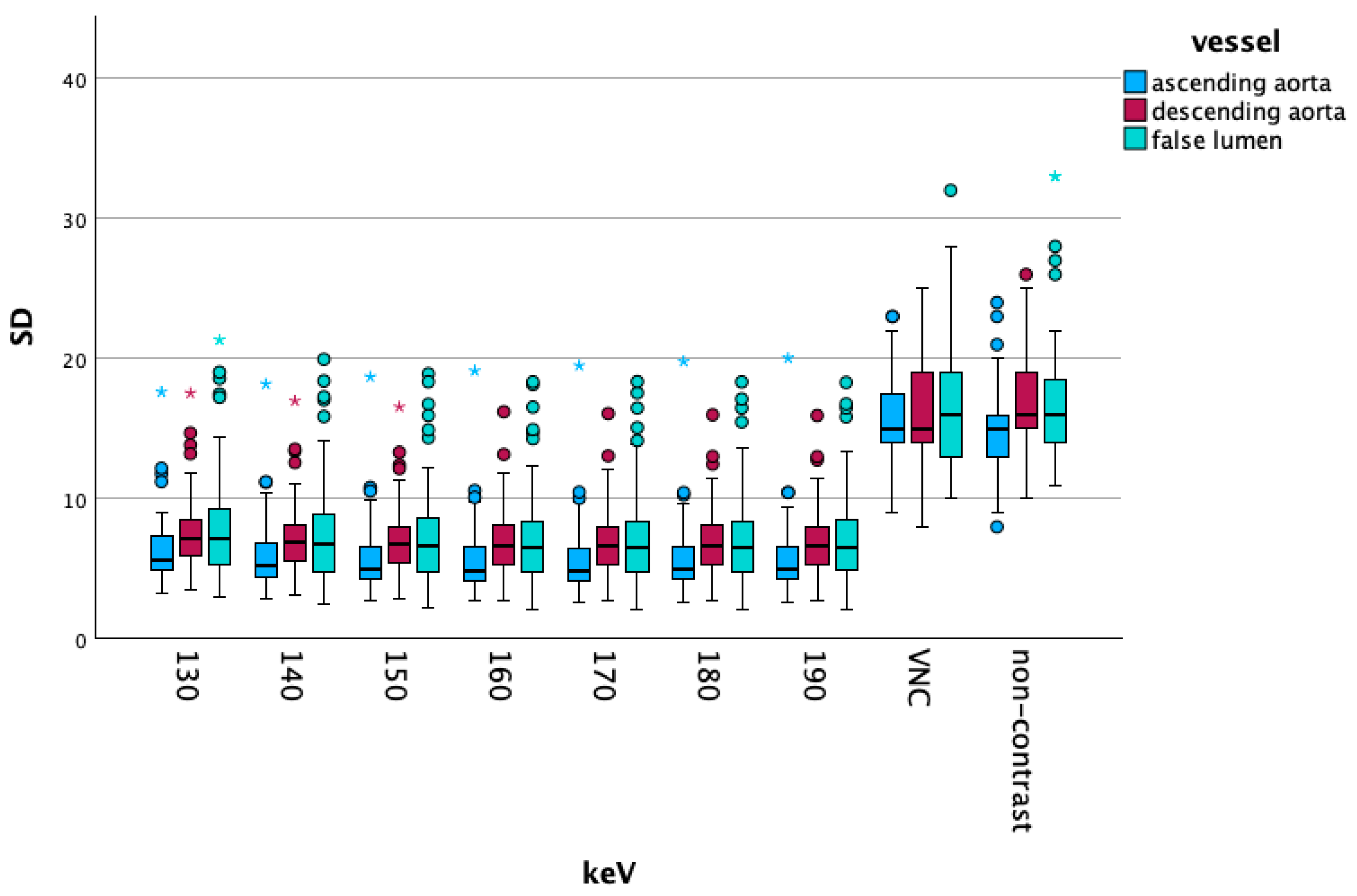
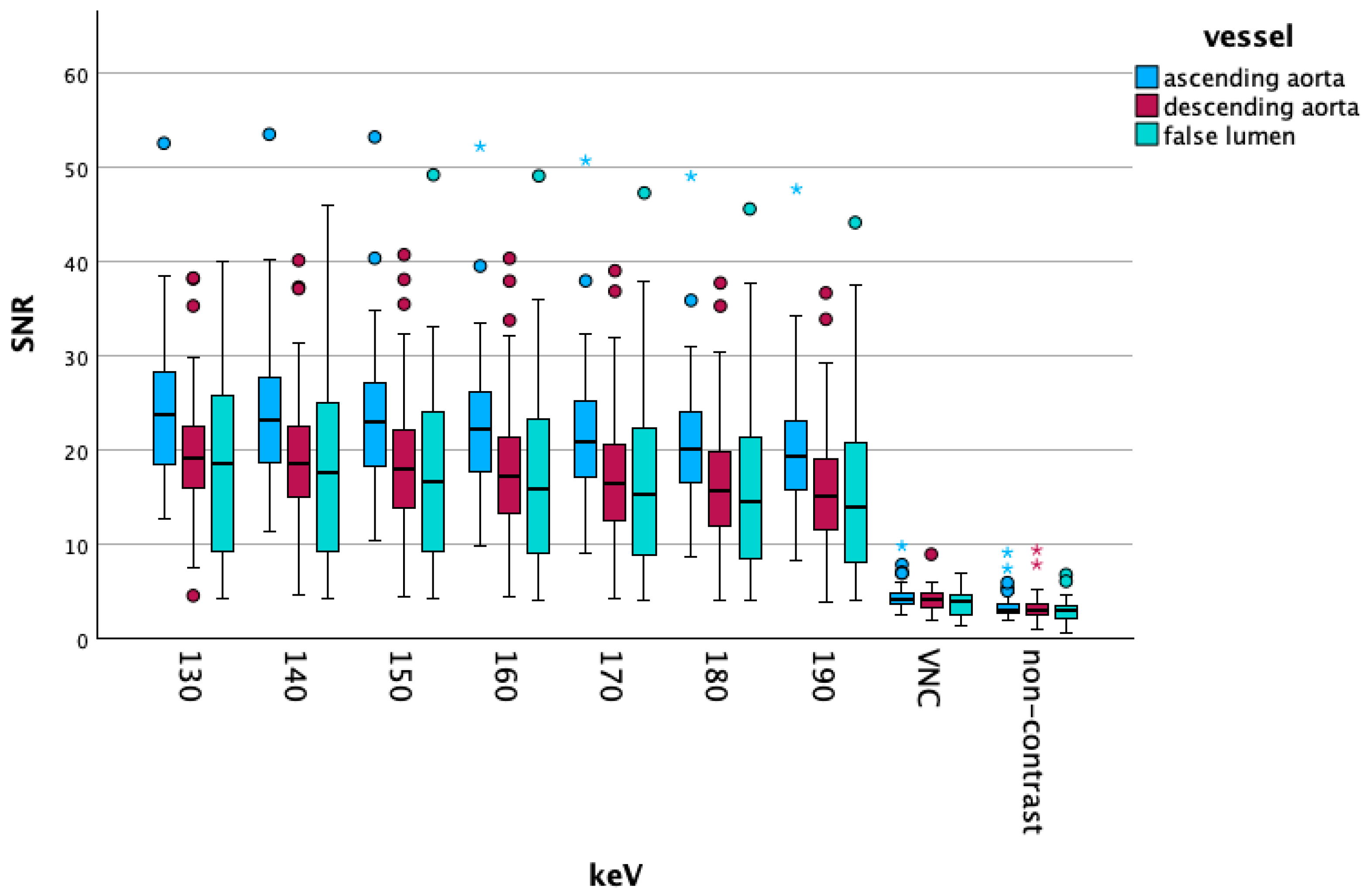
3.2. Objective Results
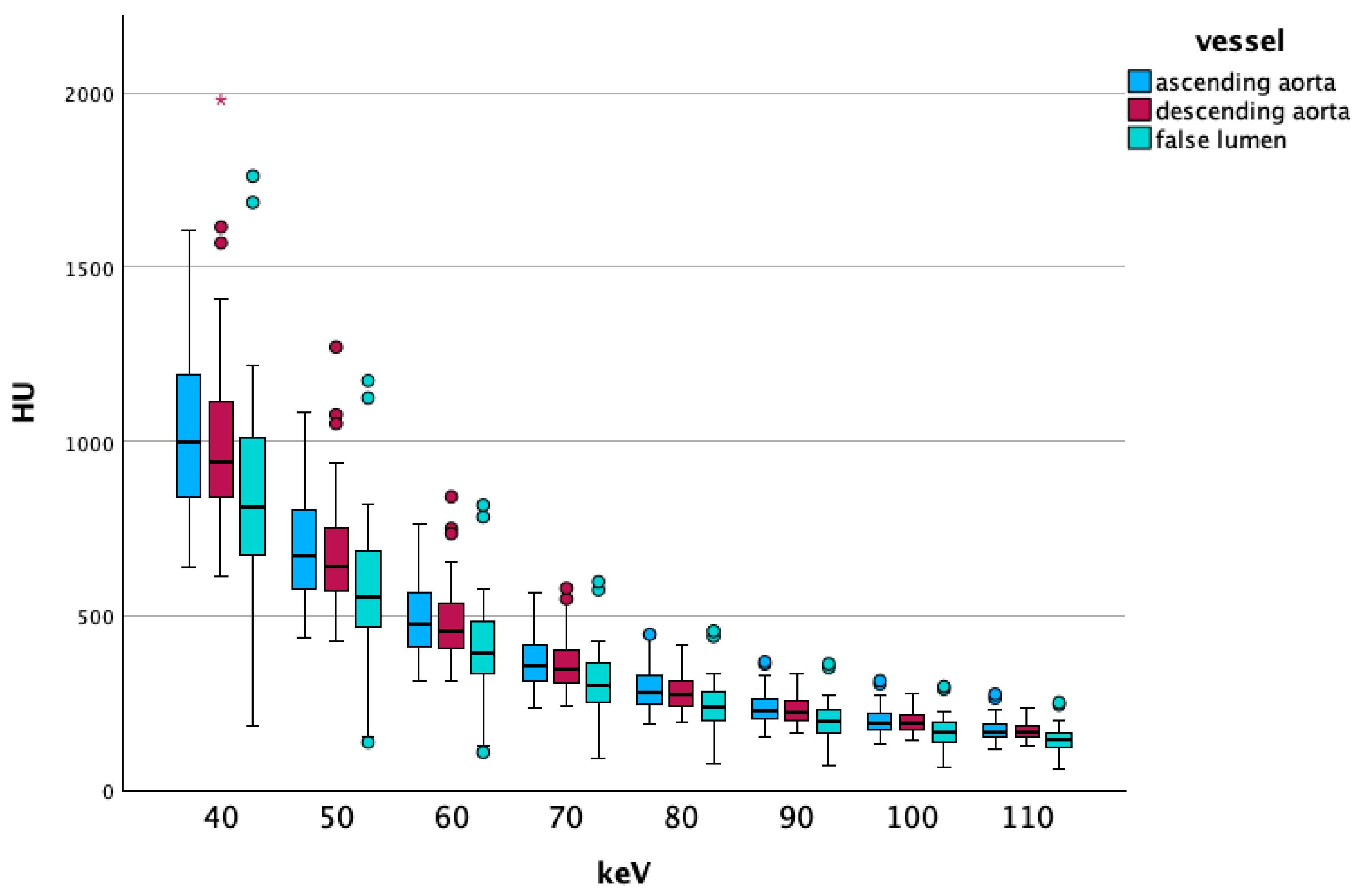
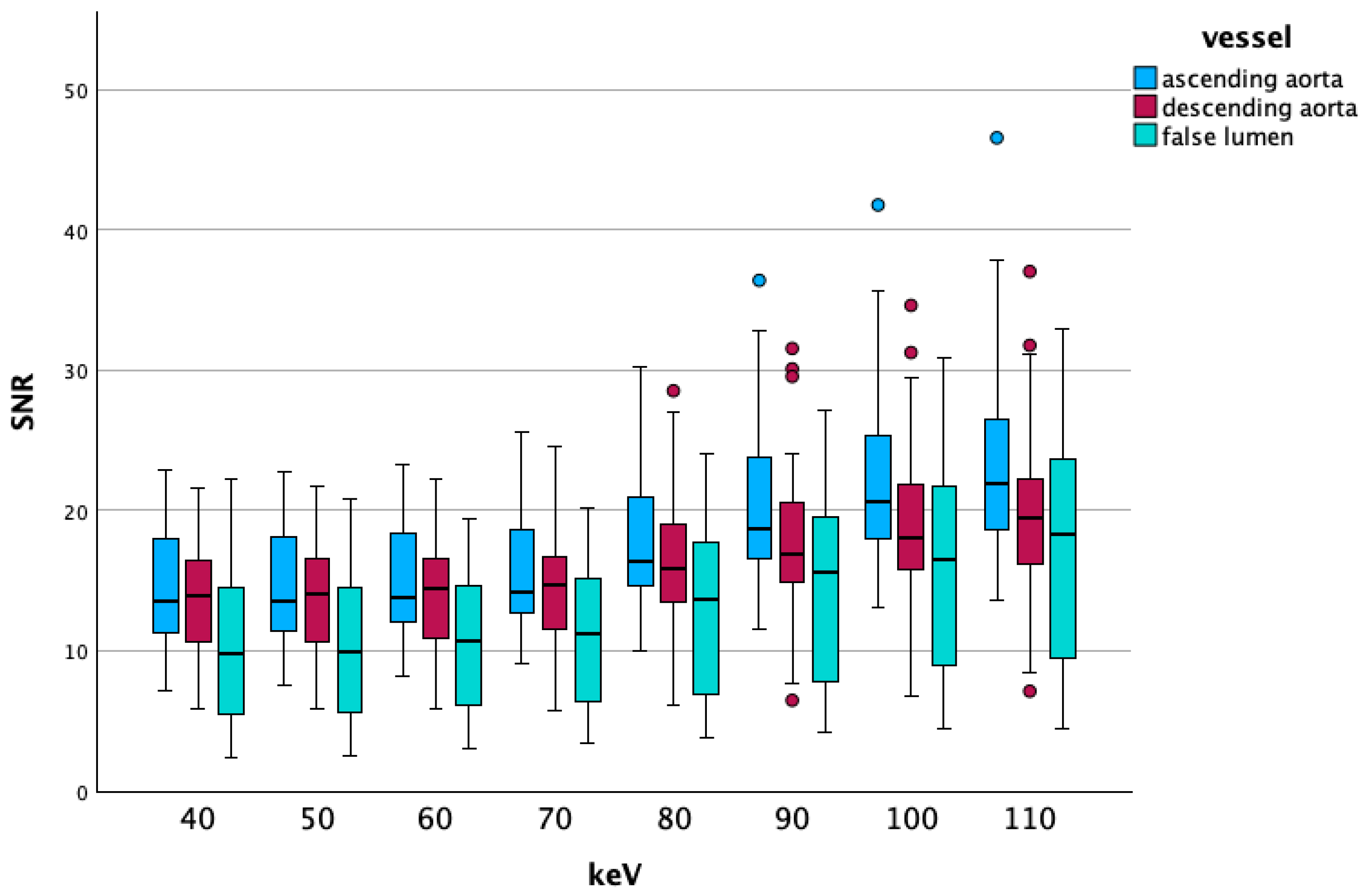
3.2.1. Low-Energy Comparisons
Overall Data
Vascular Segment-Specific Data
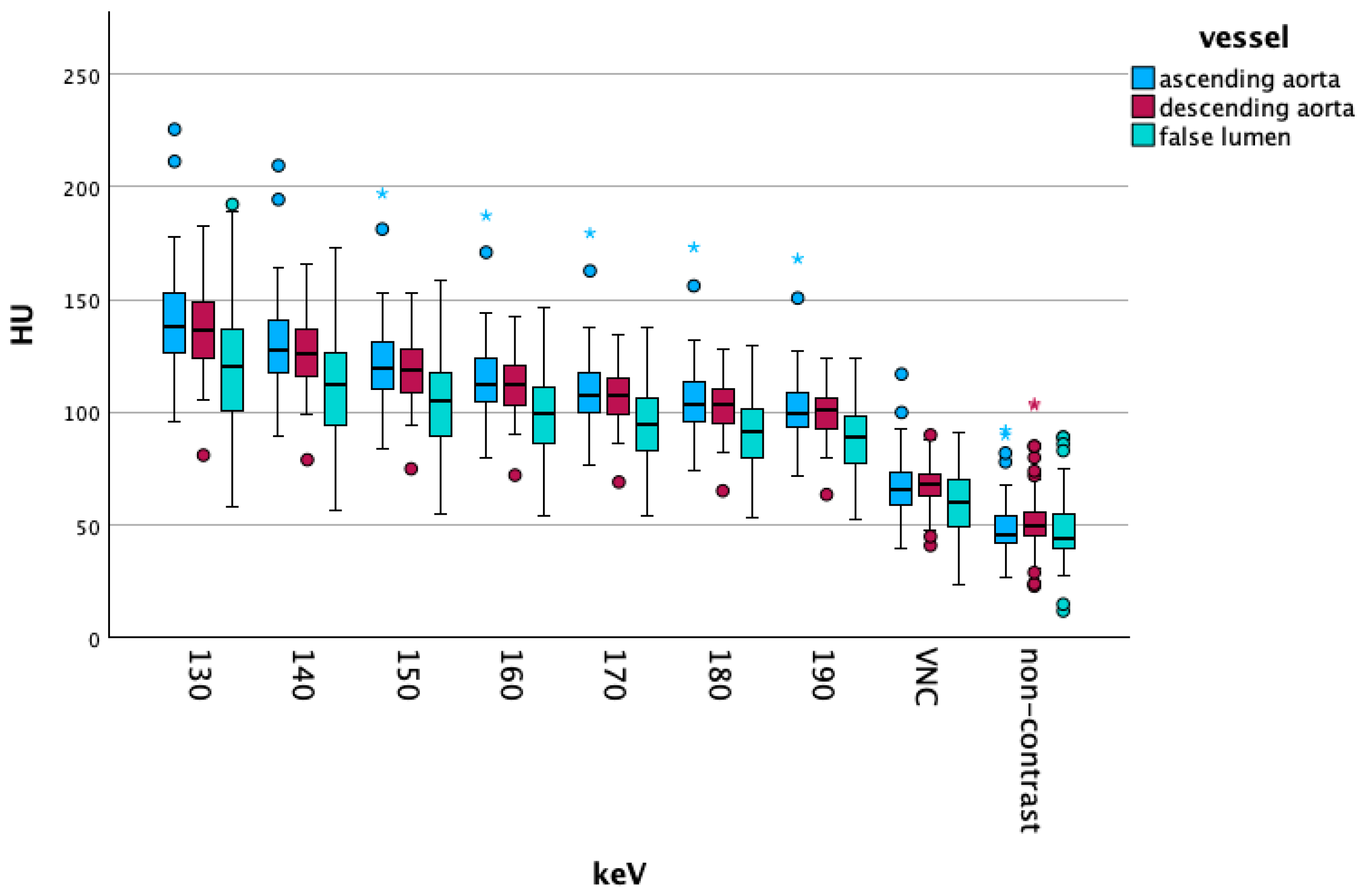
3.2.2. High-Energy/VNC/NC Comparisons
Overall Data
Vascular Segment-Specific Data
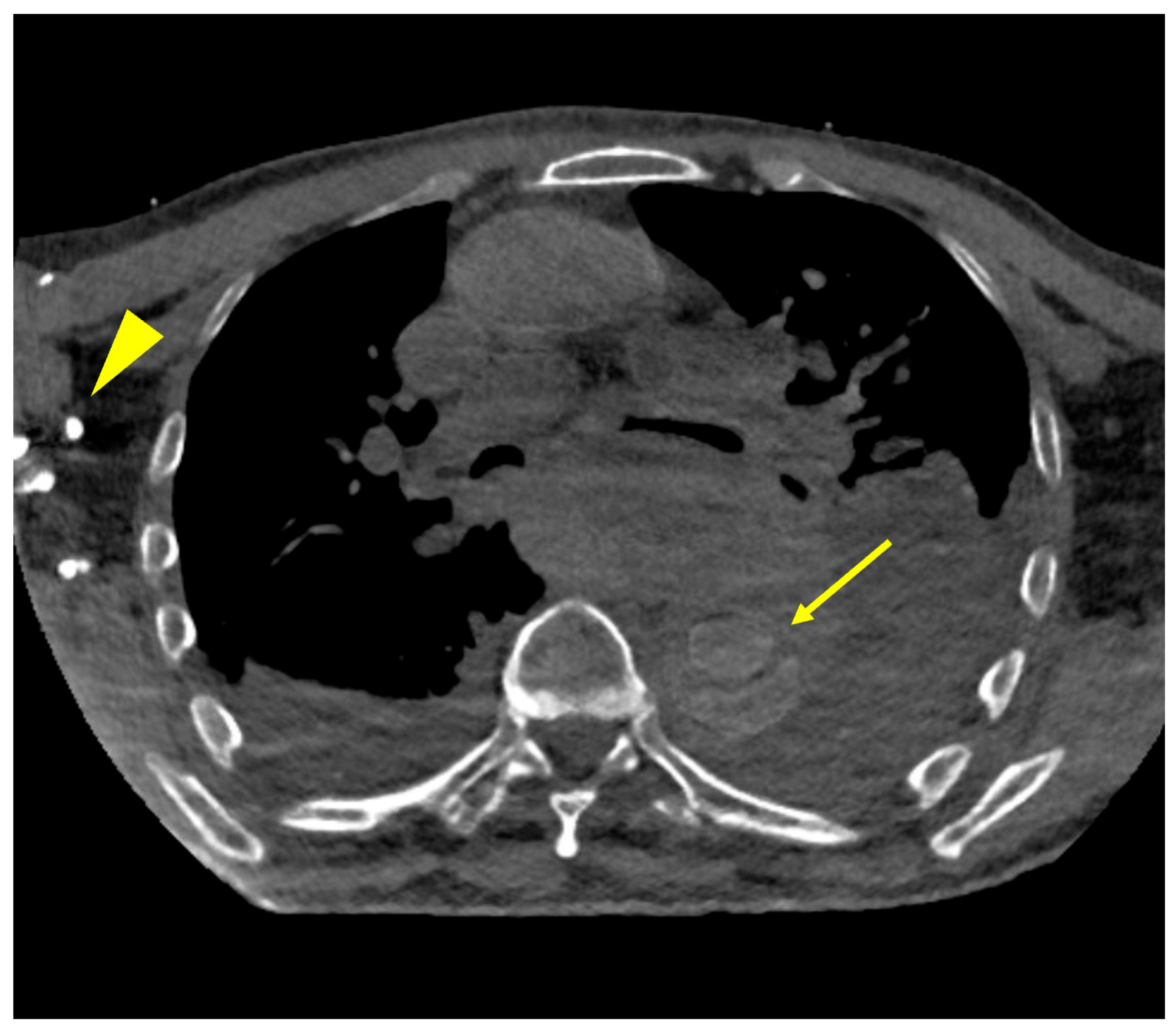
3.3. Subjective Results
4. Discussion
4.1. Low keV Data
4.2. High keV and NC/VNC Scans
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| AD | Aortic dissection |
| PCDCT | Photon-counting detector CT |
| VME | Virtual monoenergetic |
| VNC | Virtual non-contrast |
| NC | Non-contrast |
| SNR | Signal-to-noise ratio |
| CTDI | CT dose index |
| DLP | Dose length product |
| ROI | Region of interest |
References
- Kurz, S.D.; Falk, V.; Kempfert, J.; Gieb, M.; Ruschinski, T.M.; Kukucka, M.; Tsokos, M.; Grubitzsch, H.; Herbst, H.; Semmler, J.; et al. Insight into the incidence of acute aortic dissection in the German region of Berlin and Brandenburg. Int. J. Cardiol. 2017, 241, 326–329. [Google Scholar] [CrossRef]
- Sayed, A.; Munir, M.; Bahbah, E.I. Aortic Dissection: A Review of the Pathophysiology, Management and Prospective Advances. Curr. Cardiol. Rev. 2021, 17, e230421186875. [Google Scholar] [CrossRef]
- Sievers, H.H.; Rylski, B.; Czerny, M.; Baier, A.L.M.; Kreibich, M.; Siepe, M.; Beyersdorf, F. Aortic dissection reconsidered: Type, entry site, malperfusion classification adding clarity and enabling outcome prediction. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 451–457. [Google Scholar] [CrossRef]
- Czerny, M.; Grabenwoger, M.; Berger, T.; Aboyans, V.; Della Corte, A.; Chen, E.P.; Desai, N.D.; Dumfarth, J.; Elefteriades, J.A.; Etz, C.D.; et al. EACTS/STS Guidelines for diagnosing and treating acute and chronic syndromes of the aortic organ. Eur. J. Cardiothorac. Surg. 2024, 65, ezad426. [Google Scholar] [CrossRef]
- Carroll, B.J.; Schermerhorn, M.L.; Manning, W.J. Imaging for acute aortic syndromes. Heart 2020, 106, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Vargo, P.R.; Reich, H.; Roselli, E.E. Computed Tomography Imaging of Aortic Dissections with Endovascular Treatment Considerations. Curr. Cardiol. Rep. 2021, 23, 113. [Google Scholar] [CrossRef]
- Becker, B.V.; Kaatsch, H.L.; Nestler, K.; Overhoff, D.; Schneider, J.; Dillinger, D.; Piechotka, J.; Brockmann, M.A.; Ullmann, R.; Port, M.; et al. Initial experience on abdominal photon-counting computed tomography in clinical routine: General image quality and dose exposure. Eur. Radiol. 2023, 33, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Rau, A.; Straehle, J.; Stein, T.; Diallo, T.; Rau, S.; Faby, S.; Nikolaou, K.; Schoenberg, S.O.; Overhoff, D.; Beck, J.; et al. Photon-Counting Computed Tomography (PC-CT) of the spine: Impact on diagnostic confidence and radiation dose. Eur. Radiol. 2023, 33, 5578–5586. [Google Scholar] [CrossRef]
- Stein, T.; Rau, A.; Russe, M.F.; Arnold, P.; Faby, S.; Ulzheimer, S.; Weis, M.; Froelich, M.F.; Overhoff, D.; Horger, M.; et al. Photon-Counting Computed Tomography—Basic Principles, Potenzial Benefits, and Initial Clinical Experience. Rofo 2023, 195, 691–698. [Google Scholar] [CrossRef]
- Dillinger, D.; Overhoff, D.; Ayx, I.; Kaatsch, H.L.; Hagen, A.; Schonberg, S.O.; Waldeck, S. Optimizing Arterial Vessel Contrast in Portal Venous Phase with Virtual Monoenergetic Images from Photon-Counting Detector CT Scans of the Abdomen-First Clinical Experiences. Diagnostics 2024, 14, 627. [Google Scholar] [CrossRef] [PubMed]
- Dillinger, D.; Overhoff, D.; Booz, C.; Kaatsch, H.L.; Piechotka, J.; Hagen, A.; Froelich, M.F.; Vogl, T.J.; Waldeck, S. Impact of CT Photon-Counting Virtual Monoenergetic Imaging on Visualization of Abdominal Arterial Vessels. Diagnostics 2023, 13, 938. [Google Scholar] [CrossRef]
- Dillinger, D.; Overhoff, D.; Froelich, M.F.; Kaatsch, H.L.; Booz, C.; Hagen, A.; Vogl, T.J.; Schonberg, S.O.; Waldeck, S. Photon-Counting Detector CT Virtual Monoenergetic Images in Cervical Trauma Imaging-Optimization of Dental Metal Artifacts and Image Quality. Diagnostics 2024, 14, 626. [Google Scholar] [CrossRef]
- Kaatsch, H.L.; Vollmecke, M.F.; Becker, B.V.; Dillinger, D.; Kubitscheck, L.; Wohler, A.; Schaaf, S.; Piechotka, J.; Schreyer, C.; Schwab, R.; et al. Improved Discriminability of Severe Lung Injury and Atelectasis in Thoracic Trauma at Low keV Virtual Monoenergetic Images from Photon-Counting Detector CT. Diagnostics 2024, 14, 2231. [Google Scholar] [CrossRef]
- Risch, F.; Bette, S.; Sinzinger, A.; Rippel, K.; Scheurig-Muenkler, C.; Kroencke, T.; Decker, J.A. Multiphase photon counting detector CT data sets—Which combination of contrast phase and virtual non-contrast algorithm is best suited to replace true non-contrast series in the assessment of active bleeding? Eur. J. Radiol. 2023, 168, 111125. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Wichmann, J.L.; Scholtz, J.E.; Leithner, D.; D’Angelo, T.; Weyer, H.; Booz, C.; Lenga, L.; Vogl, T.J.; Albrecht, M.H. Noise-Optimized Virtual Monoenergetic Dual-Energy CT Improves Diagnostic Accuracy for the Detection of Active Arterial Bleeding of the Abdomen. J. Vasc. Interv. Radiol. 2017, 28, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Maturen, K.E.; Kaza, R.K.; Liu, P.S.; Quint, L.E.; Khalatbari, S.H.; Platt, J.F. “Sweet spot” for endoleak detection: Optimizing contrast to noise using low keV reconstructions from fast-switch kVp dual-energy CT. J. Comput. Assist. Tomogr. 2012, 36, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Niwa, T.; Nomura, T.; Yoshida, R.; Koyanagi, K.; Hashimoto, J. Low-keV Virtual Monoenergetic Imaging for Bronchial Artery Visualization on Photon-Counting Detector Computed Tomography. Diagnostics 2025, 15, 1354. [Google Scholar] [CrossRef]
- Strassl, A.; Lauriero, F.; Rueda, M.A.; Wassipaul, C.; Weber, M.; Loewe, C.; Beitzke, D.; Beer, L. High-pitch photon-counting detector computed tomography angiography of the coronary arteries: Qualitative and quantitative evaluation of monoenergetic image reconstructions. Eur. J. Radiol. Open 2025, 15, 100666. [Google Scholar] [CrossRef]
- Chu, S.; Kilinc, O.; Pradella, M.; Weiss, E.; Baraboo, J.; Maroun, A.; Jarvis, K.; Mehta, C.K.; Malaisrie, S.C.; Hoel, A.W.; et al. Baseline 4D Flow-Derived in vivo Hemodynamic Parameters Stratify Descending Aortic Dissection Patients with Enlarging Aortas. Front. Cardiovasc. Med. 2022, 9, 905718. [Google Scholar] [CrossRef]
- Kilinc, O.; Chu, S.; Baraboo, J.; Weiss, E.K.; Engel, J.; Maroun, A.; Giese, D.; Jin, N.; Chow, K.; Bi, X.; et al. Hemodynamic Evaluation of Type B Aortic Dissection Using Compressed Sensing Accelerated 4D Flow MRI. J. Magn. Reson. Imaging 2023, 57, 1752–1763. [Google Scholar] [CrossRef]
- Turrion Gomollon, A.M.; Mergen, V.; Sartoretti, T.; Polacin, M.; Nakhostin, D.; Puippe, G.; Alkadhi, H.; Euler, A. Photon-Counting Detector CT Angiography for Endoleak Detection After Endovascular Aortic Repair: Triphasic CT with True Noncontrast Versus Biphasic CT with Virtual Noniodine Imaging. Investig. Radiol. 2023, 58, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Mergen, V.; Racine, D.; Jungblut, L.; Sartoretti, T.; Bickel, S.; Monnin, P.; Higashigaito, K.; Martini, K.; Alkadhi, H.; Euler, A. Virtual Noncontrast Abdominal Imaging with Photon-counting Detector CT. Radiology 2022, 305, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Sartoretti, T.; Landsmann, A.; Nakhostin, D.; Eberhard, M.; Roeren, C.; Mergen, V.; Higashigaito, K.; Raupach, R.; Alkadhi, H.; Euler, A. Quantum Iterative Reconstruction for Abdominal Photon-counting Detector CT Improves Image Quality. Radiology 2022, 303, 339–348. [Google Scholar] [CrossRef] [PubMed]
| Rating | Dissection Assessability | Subjective Non-Contrast Imaging |
|---|---|---|
| 1 | no dissection membrane/details visible | complete contrast media effect, vascular wall calcification might be obscured |
| 2 | slight visibility of the dissection membrane and other vascular details, no diagnostic quality | major contrast medium visibility, limited access to vascular wall structures including calcifications |
| 3 | clearly visible dissection membrane and other vascular details, major pathologies of the intima can be seen | average contrast media aspect, vessel walls and structures can be judged with an appropriate diagnostic quality |
| 4 | dissection membrane/details visible with minor impairment of diagnostic quality | minor contrast media effects, vascular wall and calcifications still fully visible |
| 5 | all dissection aspects and details, including entry can be clearly seen | no contrast media visible, vessel wall including calcification fully assessable |
| Overall | Female | Male | |
|---|---|---|---|
| Age | 66 [52; 77] | 67 [63; 78] | 63 [49; 72] |
| CTDi non enhanced | 7.23 ± 2.29 | 6.23 ± 2.05 | 7.69 ± 2.29 |
| DLP non enhanced | 480.46 ± 201.24 | 399.88 ± 149.35 | 512.70 ± 213.31 |
| CTDi contrast | 5.02 ± 1.77 | 4.49 ± 1.75 | 5.24 ± 1.78 |
| DLP contrast | 386.71 ± 144.97 | 338.88 ± 121.59 | 405.85 ± 151.87 |
| Overall Data [HU] | Ascending Aorta [HU] | Descending Aorta [HU] | False Lumen [HU] | |
|---|---|---|---|---|
| 40 keV | 922 [780; 1127] | 997 [838; 1192] | 940 [841; 1116] | 810 [674; 1015] |
| 50 keV | 626 [536; 759] | 674 [574; 802] | 639 [572; 754] | 552 [466; 686] |
| 60 keV | 445 [390; 536] | 478 [412; 565] | 457 [406; 536] | 394 [334; 486] |
| 70 keV | 332 [297; 401] | 357 [310; 419] | 346 [305; 401] | 298 [248; 363] |
| 80 keV | 261 [239; 313] | 279 [246; 326] | 273 [242; 314] | 240 [197; 285] |
| 90 keV | 215 [196; 254] | 227 [204; 264] | 224 [201; 255] | 196 [162; 230] |
| 100 keV | 184 [167; 212] | 191 [174; 220] | 191 [172; 214] | 168 [138; 192] |
| 110 keV | 162 [145; 184] | 167 [154; 190] | 167 [151; 186] | 145 [121; 166] |
| 120 keV | 146 [131; 163] | 152 [137; 170] | 149 [136; 165] | 131 [111; 148] |
| 130 keV | 133 [120; 147] | 138 [126; 155] | 136 [124; 149] | 121 [100; 137] |
| 140 keV | 123 [112; 135] | 127 [117; 143] | 126 [116; 137] | 113 [94; 127] |
| 150 keV | 116 [105; 126] | 120 [110; 133] | 119 [108; 128] | 105 [89; 119] |
| 160 keV | 110 [99; 113] | 113 [105; 124] | 112 [103; 121] | 99 [85; 112] |
| 170 keV | 105 [95; 113] | 108 [99; 118] | 108 [99; 115] | 95 [82; 106] |
| 180 keV | 100 [92; 109] | 103 [96; 114] | 104 [95; 110] | 91 [79; 102] |
| 190 keV | 97 [89; 105] | 100 [93; 109] | 101 [92; 107] | 89 [77; 99] |
| VNC | 66 [57; 73] | 66 [59; 74] | 68 [63; 73] | 61 [49; 71] |
| NC | 48 [42; 55] | 46 [42; 55] | 50 [45; 57] | 44 [39; 55] |
| Dissection Assessability | Subjective Non-Contrast Imaging | |
|---|---|---|
| 40 keV | 420 (5.00 ± 0.00) | 195 (2.33 ± 0.54) |
| 70 keV | 416 (4.95 ± 0.21) | 192 (2.29 ± 0.77) |
| 110 keV | 251 (2.99 ± 0.59) | 262 (3.12 ± 0.82) |
| 150 keV | 179 (2.13 ± 0.46) | 317 (3.77 ± 0.53) |
| 190 keV | 161 (1.92 ± 0.42) | 346 (4.12 ± 0.43) |
| VNC | 129 (1.54 ± 0.50) | 400 (4.76 ± 0.43) |
| NC | 92 (1.10 ± 0.30) | 420 (5.00 ± 0.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dillinger, D.; Weiss, M.; Kaatsch, H.L.; Bauer, C.; Hagen, A.; Froelich, M.F.; Waldeck, S.; Overhoff, D. Advancing Aortic Dissection Imaging: First Clinical Experience of Photon-Counting CT with Ultra-Fast Spectral Imaging. Diagnostics 2025, 15, 2655. https://doi.org/10.3390/diagnostics15202655
Dillinger D, Weiss M, Kaatsch HL, Bauer C, Hagen A, Froelich MF, Waldeck S, Overhoff D. Advancing Aortic Dissection Imaging: First Clinical Experience of Photon-Counting CT with Ultra-Fast Spectral Imaging. Diagnostics. 2025; 15(20):2655. https://doi.org/10.3390/diagnostics15202655
Chicago/Turabian StyleDillinger, Daniel, Maria Weiss, Hanns L. Kaatsch, Christian Bauer, Achim Hagen, Matthias F. Froelich, Stephan Waldeck, and Daniel Overhoff. 2025. "Advancing Aortic Dissection Imaging: First Clinical Experience of Photon-Counting CT with Ultra-Fast Spectral Imaging" Diagnostics 15, no. 20: 2655. https://doi.org/10.3390/diagnostics15202655
APA StyleDillinger, D., Weiss, M., Kaatsch, H. L., Bauer, C., Hagen, A., Froelich, M. F., Waldeck, S., & Overhoff, D. (2025). Advancing Aortic Dissection Imaging: First Clinical Experience of Photon-Counting CT with Ultra-Fast Spectral Imaging. Diagnostics, 15(20), 2655. https://doi.org/10.3390/diagnostics15202655







