A Rare Combination: Cold Agglutinin Disease Followed by Waldenström Macroglobulinemia—A Case of Early Treatment Response
Abstract
1. Introduction
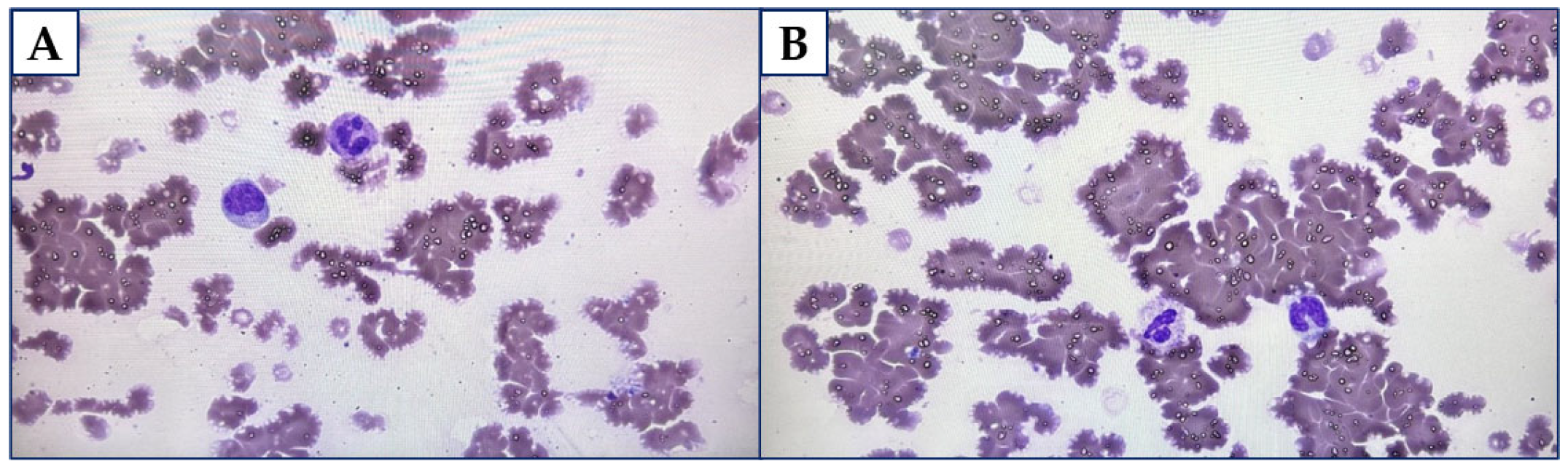
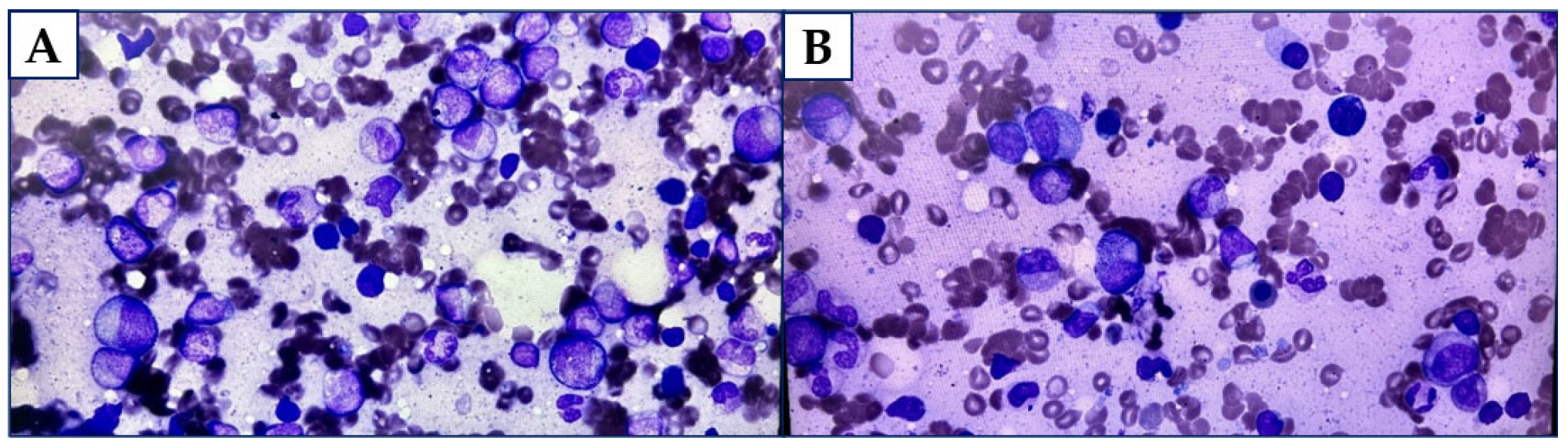
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WM | Waldenström macroglobulinemia |
| IgM | Immunoglobulin M |
| CAD | Cold agglutinin disease |
| Hb | Haemoglobin |
| DAT | Direct antiglobulin test |
| AIHA | Autoimmune haemolytic anaemia |
| NCCN | National Comprehensive Cancer Network |
| BR | Bendamustine/rituximab |
| LDH | Lactate dehydrogenase |
| IgG | Immunoglobulin G |
| IgA | Immunoglobulin A |
| ANA | Antinuclear antibodies |
| HIV | Human immunodeficiency virus |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| CMV | Cytomegalovirus |
| EBV | Epstein–Barr virus |
| RBC | Red blood cells |
| WBC | White blood cells |
| FLC | Free light chain |
| SPEP | Serum protein electrophoresis |
| ESMO | European Society For Medical Oncology |
| ISSWM | International prognostic scoring system for Waldenström macroglobulinemia |
| DRC | Dexamethasone/rituximab/cyclophosphamide |
| PFS | Progression-free survival |
| IR | Ibrutinib/rituximab |
| FILO | French Innovative Leukaemia Organisation |
Appendix A
Appendix A.1. SPEP Results During Diagnostics
| Peak | Result [%] | Result [g/dL] |
|---|---|---|
| 1 | 18.4 | 1.31 |
| 2 | 0.6 | 0.04 |
| 3 | 0.6 | 0.04 |
| 4 | 0.3 | 0.02 |
Appendix A.2. Complete Flow Cytometry Examination
| Immunophenotype | Result | Additional Remarks |
|---|---|---|
| CD5 | - | |
| CD23 | - | |
| CD20 | + (bright) | |
| CD79b | + ~83% | |
| CD22 | + | |
| CD10 | - | |
| CD38 | −/+ ~47% (part pos) | |
| CD200 | +/− ~74% (het) | |
| CD103 | - | |
| CD71 | −/+ ~43% | |
| κ | + ~85% | Interpretation of this result was interrupted due to nonspecific reactions of the antibodies. |
| λ | ~1% | |
| CD34 | - | |
| jTdT | - | |
| CD138 | −/+ ~22% | |
| CD3 | - | |
| CD7 | - | |
| CD8 | - | |
| CD4 | - | |
| CD56/CD16 | - | |
| CD117 | - |
- -
- blast cells from myeloid and monoidal lines SSCmed CD45dim/med CD34−/+part pos CD117+ HLA-DR+ CD33+het CD64−/+ CD66c− CD14− (in total 2.2% of all cells, among them blast cells CD34+ were calculated as 0.3%),
- -
- cells from monoidal line SSCmed CD45med/bridght CD34− CD117− HLA−DR+het CD64+bright CD33+bridght (3.6% of all cells, the majority of them (2.2% of all cells) were mature/maturing CD14+),
- -
- cells from granulocyte line SSChigh CD45med CD34− CD117− CD66c+ CD33+ (73.0% of all cells, the mature cells CD10+ accounted for 20% of cells from this line),
- -
- promyelocytes SSChigh CD45med CD34− CD117+ CD66c+ CD33+ (1.5% of all cells),
- -
- plasma cells line SSCmed CD45med/bright CD117− CD138+bright CD38+bright CD19+/− CD20- (ratio cytκ/cytλ = 57.6%/42.5% = 1.4; <0.1% of all cells, the cells did not show clearly monoclonal features in the assessment based on FLC κ and λ),
- -
- T lymphocytes with dominant immunophenotype SSClow CD45bright CD3+ CD7+ CD5+ (5.3% of all cells):T lymphocytes SSClow CD45bright CD3+ CD4+ (2.0% of all cells),T lymphocytes SSClow CD45bright CD3+ CD8+ (2.8% of all cells),CD3+CD4+/CD3+CD8+ = 0.7natural killers T (NKT) cells SSClow CD45bright CD3+ CD5616+ (2.1% of all cells),
- -
- natural killers (NK) cells SSClow CD45bright CD3− CD7+ CD56/CD16+ (0.9% of all cells).
- ❖
- − or negative—<10% of positive cells in the population,
- ❖
- −/+—10–50% of positive cells in the population,
- ❖
- +/−—50–80% of positive cells in the population,
- ❖
- + or positive—>80% of positive cells in the population,
- ❖
- dim—the population is partly or entirely displaced towards the positive cells’ side, their expression is weaker in comparison with the positive cells,
- ❖
- bright—homogenous population with only positive cells, which do not coincide with negative cells, their expression is the same or stronger in comparison with the referential positive cells,
- ❖
- het—heterogeneous expression and >50z5 positive cells,
- ❖
- part pos—there are two populations of the cells detected, only one of them is positive (≥10% positive cells),
- ❖
- low, med, high—the characteristics of parameters of FSC and SSC scattering,
- ❖
- neg, dim, med, bright—the characteristics of CD45 expression.
Appendix A.3. SPEP Results During the Treatment
| Protein Fraction | Result [%] | Reference [%] | Result [g/dL] | Reference [g/dL] |
|---|---|---|---|---|
| Albumin | 62.3 | 54.3–65.5 | 3.92 | 3.80–4.60 |
| α1 | 3.9 | 1.2–3.3 | 0.25 | 0.08–0.23 |
| α2 | 14.0 | 8.3–15.0 | 0.88 | 0.58–1.10 |
| β1 | 7.8 | 6.5–11.5 | 0.49 | 0.46–0.81 |
| β2 | 4.2 | 2.5–7.2 | 0.26 | 0.18–0.50 |
| γ | 7.8 | 7.1–19.5 | 0.49 | 0.50–1.40 |
References
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. (Eds.) World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues; IARC Press: Lyon, France, 2008. [Google Scholar]
- Finder, K.A.; McCollough, M.L.; Dixon, S.L.; Majka, A.J.; Jaremko, W. Hypergammaglobulinemic Purpura of Waldenström. J. Am. Acad. Dermatol. 1990, 23, 669–676. [Google Scholar] [CrossRef]
- Sekhar, J.; Sanfilippo, K.; Zhang, Q.; Trinkaus, K.; Vij, R.; Morgensztern, D. Waldenström Macroglobulinemia: A Surveillance, Epidemiology, and End Results Database Review from 1988 to 2005. Leuk. Lymphoma 2012, 53, 1625–1626. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, B.; Masthan, S.S.; Hameed, M.; Akhtar, H.H.; Khalid, A.; Ghafoor, S.; Allah, H.M.; Arshad, M.M.; Iqbal, I.; Iftikhar, A.; et al. Waldenström Macroglobulinemia: A Review of Pathogenesis, Current Treatment, and Future Prospects. Ann. Hematol. 2024, 103, 1859–1876. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A. Waldenström Macroglobulinemia: 2025 Update on Diagnosis, Risk Stratification, and Management. Am. J. Hematol. 2025, 100, 1061–1073. [Google Scholar] [CrossRef]
- Berentsen, S.; Röth, A.; Randen, U.; Jilma, B.; Tjønnfjord, G.E. Cold Agglutinin Disease: Current Challenges and Future Prospects. J. Blood Med. 2019, 10, 93–103. [Google Scholar] [CrossRef]
- Bozzi, S.; Umarje, S.; Hawaldar, K.; Tyma, J.; Schinkel, J.; Agatep, B.; Pulungan, Z.; Petruski-Ivleva, N. Prevalence and Incidence of Primary Autoimmune Hemolytic Anemia and Cold Agglutinin Disease in the United States, 2016-2022: A Retrospective Study in Administrative Claims. Blood 2023, 142, 5202. [Google Scholar] [CrossRef]
- Ulvestad, E.; Berentsen, S.; Bø, K.; Shammas, F.V. Clinical Immunology of Chronic Cold Agglutinin Disease. Eur. J. Haematol. 1999, 63, 259–266. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ®) Waldenström Macroglobulinemia/Lymphoplasmacytic Lymphoma. Version 1.2025—13 September 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/waldenstroms.pdf (accessed on 10 December 2024).
- Suzuki, M.; Noda, T.; Kodama, H.; Sahashi, K.; Wakita, A. A case of Waldenström’s macroglobulinemia with cold agglutinin disease. Rinsho Ketsueki 1986, 27, 376–380. [Google Scholar]
- Hattori, N.; Ishii, N.; Ariizumi, H.; Adachi, D.; Matsuda, I.; Nakamaki, T.; Tomoyasu, S. Improvement of the Thermal Amplitude after Rituximab Treatment for Cold Agglutinin Disease with Waldenström’s Macroglobulinemia. Ann. Hematol. 2010, 89, 103–104. [Google Scholar] [CrossRef]
- Caballero, J.C.; Askari, E.; Carrasco, N.; Piris, M.A.; Perez de Camino, B.; Pardo, L.; Cornago, J.; Lopez-Lorenzo, J.L.; Llamas, P.; Solan, L. Invasive Cutaneous Candidiasis, Autoimmune Hemolytic Anemia and Pancytopenia: A Challenging Scenario for Waldenström Macroglobulinemia in an Elderly Patient. Biomedicines 2023, 11, 1007. [Google Scholar] [CrossRef] [PubMed]
- Theisen, E.; Lee, D.E.; Pei, S.; Schauder, D.M.; Chiu, Y.E.; Brandling-Bennett, H.; Curran, M.L.; Klein-Gitelman, M.; Co, D.O.; Arkin, L.M. Hypergammaglobulinemic Purpura of Waldenström in Children. Pediatr. Dermatol. 2020, 37, 467–475. [Google Scholar] [CrossRef]
- Kristinsson, S.Y.; Koshiol, J.; Björkholm, M.; Goldin, L.R.; McMaster, M.L.; Turesson, I.; Landgren, O. Immune-Related and Inflammatory Conditions and Risk of Lymphoplasmacytic Lymphoma or Waldenstrom Macroglobulinemia. J. Natl. Cancer Inst. 2010, 102, 557–567. [Google Scholar] [CrossRef][Green Version]
- Manasanch, E.E.; Kristinsson, S.Y.; Landgren, O. Etiology of Waldenström Macroglobulinemia: Genetic Factors and Immune-Related Conditions. Clin. Lymphoma Myeloma Leuk. 2013, 13, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, S.; Schejbel, L.; Breinholt, M.F.; Pedersen, M.Ø.; Hammer, T.; Munksgaard, L.; Nørgaard, P.; Høgdall, E.; Gjerdrum, L.M.R.; Nielsen, T.H. Mutational Landscape in Waldenström Macroglobulinemia Evaluated Using a Next-Generation Sequencing Lymphoma Panel in Routine Clinical Practice. Leuk. Lymphoma 2024, 65, 758–767. [Google Scholar] [CrossRef]
- de Tute, R.; Rawstron, A.; Evans, P.; Owen, R. Cold Agglutinin Disease Is a Phenotypically Distinct Clonal B-Cell Disorder. Clin. Lymphoma Myeloma Leuk. 2015, 15, e184. [Google Scholar] [CrossRef]
- Cao, X.X.; Meng, Q.; Cai, H.; He, T.H.; Zhang, C.L.; Su, W.; Sun, J.; Li, Y.; Xu, W.; Zhou, D.; et al. Detection of MYD88 L265P and WHIM-like CXCR4 Mutation in Patients with IgM Monoclonal Gammopathy Related Disease. Ann. Hematol. 2017, 96, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, J.; Vos, J.M.I.; Pluimers, T.E.; Japzon, N.; Patel, A.; Salter, S.; Kwakernaak, A.J.; Gupta, R.; Rismani, A.; Kyriakou, C.; et al. Clinical and Clonal Characteristics of Monoclonal Immunoglobulin M-Associated Type I Cryoglobulinaemia. Br. J. Haematol. 2024, 204, 177–185. [Google Scholar] [CrossRef]
- Kastritis, E.; Leblond, V.; Dimopoulos, M.A.; Kimby, E.; Staber, P.; Kersten, M.J.; Tedeschi, A.; Buske, C. Waldenström’s macroglobulinaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv41–iv50. [Google Scholar] [CrossRef] [PubMed]
- Barcellini, W.; Fattizzo, B. The Changing Landscape of Autoimmune Hemolytic Anemia. Front. Immunol. 2020, 11, 946. [Google Scholar] [CrossRef]
- Joly, F.; Schmitt, L.A.; Watson, P.A.M.G.; Pain, E.; Testa, D. The Burden of Cold Agglutinin Disease on Patients’ Daily Life: Web-Based Cross-Sectional Survey of 50 American Patients. JMIR Form. Res. 2022, 6, e34248. [Google Scholar] [CrossRef]
- Swiecicki, P.L.; Hegerova, L.T.; Gertz, M.A. Cold Agglutinin Disease. Blood 2013, 122, 1114–1121. [Google Scholar] [CrossRef]
- Berentsen, S.; Fattizzo, B.; Barcellini, W. The Choice of New Treatments in Autoimmune Hemolytic Anemia: How to Pick from the Basket? Front. Immunol. 2023, 14, 1180509. [Google Scholar] [CrossRef] [PubMed]
- Barcellini, W.; Fattizzo, B. The Evolving Management Algorithm for the Patient with Newly Diagnosed Cold Agglutinin Disease. Expert. Rev. Hematol. 2024, 17, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Berentsen, S.; Barcellini, W.; D’Sa, S.; Randen, U.; Tvedt, T.H.A.; Fattizzo, B.; Haukås, E.; Kell, M.; Brudevold, R.; Dahm, A.E.A.; et al. Cold Agglutinin Disease Revisited: A Multinational, Observational Study of 232 Patients. Blood 2020, 136, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Berentsen, S. How I Treat Cold Agglutinin Disease. Blood 2021, 137, 1295–1303. [Google Scholar] [CrossRef]
- Nakasone, H.; Kako, S.; Endo, H.; Ito, A.; Sato, M.; Terasako, K.; Okuda, S.; Tanaka, Y.; Yamazaki, R.; Oshima, K.; et al. Diabetes Mellitus Is Associated with High Early-Mortality and Poor Prognosis in Patients with Autoimmune Hemolytic Anemia. Hematology 2009, 14, 361–365. [Google Scholar] [CrossRef]
- Paludo, J.; Abeykoon, J.P.; Shreders, A.; Ansell, S.M.; Kumar, S.; Ailawadhi, S.; King, R.L.; Koehler, A.B.; Reeder, C.B.; Buadi, F.K.; et al. Bendamustine and Rituximab (BR) versus Dexamethasone, Rituximab, and Cyclophosphamide (DRC) in Patients with Waldenström Macroglobulinemia. Ann. Hematol. 2018, 97, 1417–1425. [Google Scholar] [CrossRef]
- Arulogun, S.O.; Brian, D.; Goradia, H.; Cooney, A.; Menne, T.; Koo, R.; O’Neill, A.T.; Vos, J.M.I.; Pratt, G.; Turner, D.; et al. Bendamustine plus Rituximab for the Treatment of Waldenström Macroglobulinemia: Patient Outcomes and Impact of Bendamustine Dosing. Am. J. Hematol. 2023, 98, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Laribi, K.; Poulain, S.; Willems, L.; Merabet, F.; Le Calloch, R.; Eveillard, J.R.; Herbaux, C.; Roos-Weil, D.; Chaoui, D.; Roussel, X.; et al. Bendamustine plus Rituximab in Newly-Diagnosed Waldenström Macroglobulinaemia Patients. A Study on Behalf of the French Innovative Leukaemia Organization (FILO). Br. J. Haematol. 2019, 186, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Laribi, K.; Poulain, S.; Willems, L.; Merabet, F.; Herbaux, C.; Roos-Weil, D.; Laribi de Materre, I.; Roussel, X.; Nudel, M.; Tricot, S.; et al. Long-Term Results of Waldenström Macroglobulinaemia Treatment by Bendamustine and Rituximab: A Study on Behalf of the French Innovative Leukemia Organization (FILO). Br. J. Haematol. 2024, 204, 2233–2236. [Google Scholar] [CrossRef]
- Chan, W.-L.; Chong, V.C.L.; Wee, I.J.Y.; Poon, L.M.; Chan, E.H.L.; Lee, J.; Chee, Y.-L.; Jeyasekharan, A.D.; Chng, W.-J.; Samuel, M.; et al. Efficacy and Safety of Front-Line Treatment Regimens for Waldenstrom Macroglobulinaemia: A Systematic Review and Meta-Analysis. Blood Cancer J. 2023, 13, 140. [Google Scholar] [CrossRef] [PubMed]

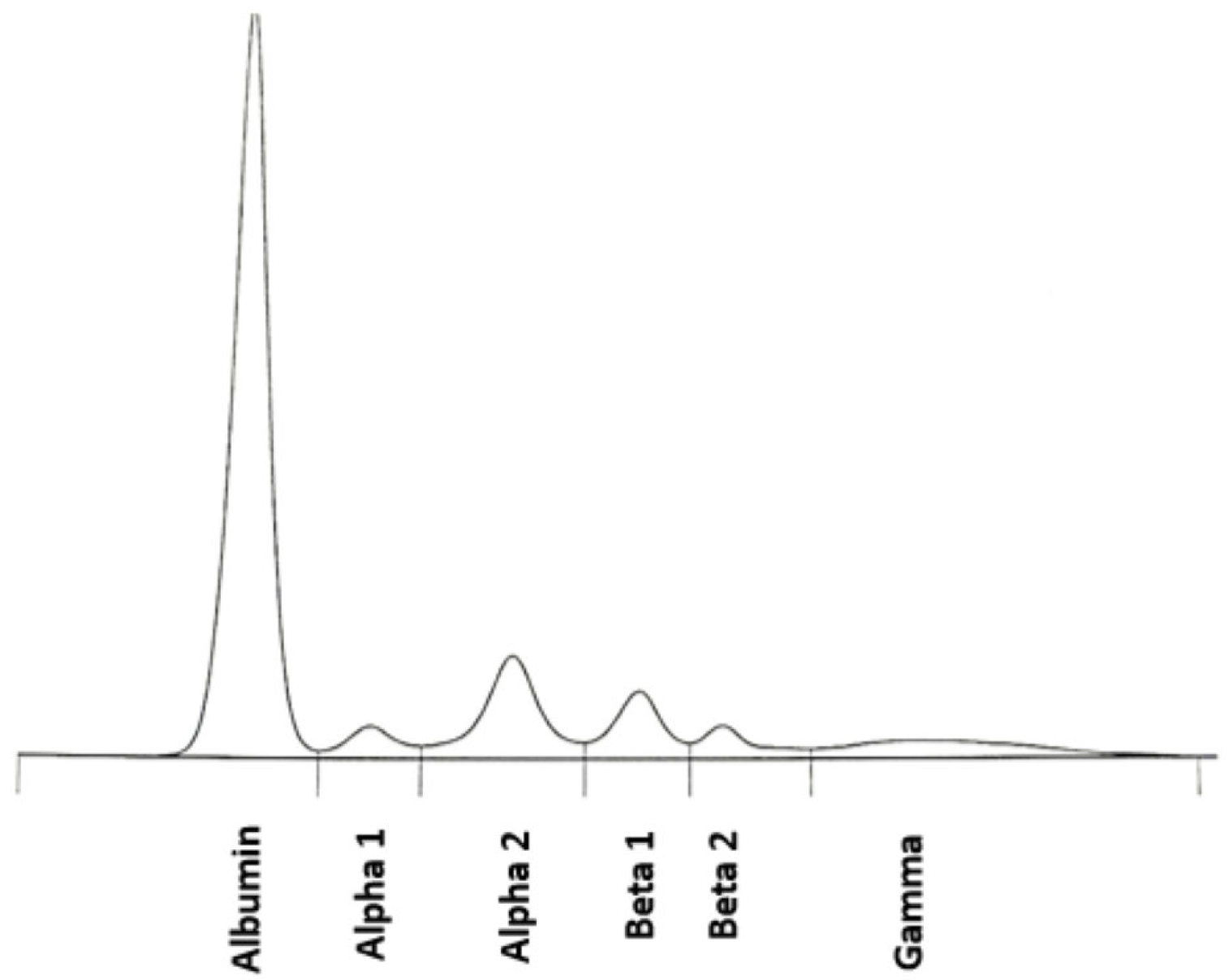
| Protein Fraction | Result [%] | Reference [%] | Result [g/dL] | Reference [g/dL] |
|---|---|---|---|---|
| Albumin | 48.4 | 54.3–65.5 | 3.44 | 3.80–4.60 |
| α1 | 2.9 | 1.2–3.3 | 0.21 | 0.08–0.23 |
| α2 | 9.7 | 8.3–15.0 | 0.69 | 0.58–1.10 |
| β1 | 5.9 | 6.5–11.5 | 0.42 | 0.46–0.81 |
| β2 | 1.5 | 2.5–7.2 | 0.11 | 0.18–0.50 |
| γ | 31.6 | 7.1–19.5 | 2.24 | 0.50–1.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozub, A.; Nasiek, A.; Bohun, N.; Bednarczyk, M.; Sędek, Ł.; Grosicki, S. A Rare Combination: Cold Agglutinin Disease Followed by Waldenström Macroglobulinemia—A Case of Early Treatment Response. Diagnostics 2025, 15, 2654. https://doi.org/10.3390/diagnostics15202654
Kozub A, Nasiek A, Bohun N, Bednarczyk M, Sędek Ł, Grosicki S. A Rare Combination: Cold Agglutinin Disease Followed by Waldenström Macroglobulinemia—A Case of Early Treatment Response. Diagnostics. 2025; 15(20):2654. https://doi.org/10.3390/diagnostics15202654
Chicago/Turabian StyleKozub, Anna, Aleksandra Nasiek, Natalia Bohun, Martyna Bednarczyk, Łukasz Sędek, and Sebastian Grosicki. 2025. "A Rare Combination: Cold Agglutinin Disease Followed by Waldenström Macroglobulinemia—A Case of Early Treatment Response" Diagnostics 15, no. 20: 2654. https://doi.org/10.3390/diagnostics15202654
APA StyleKozub, A., Nasiek, A., Bohun, N., Bednarczyk, M., Sędek, Ł., & Grosicki, S. (2025). A Rare Combination: Cold Agglutinin Disease Followed by Waldenström Macroglobulinemia—A Case of Early Treatment Response. Diagnostics, 15(20), 2654. https://doi.org/10.3390/diagnostics15202654






