Epidemiological and Histopathological Characterization of Endometrial Carcinoma: A Retrospective Cohort from Romania
Abstract
1. Introduction
2. Materials and Methods
3. Results
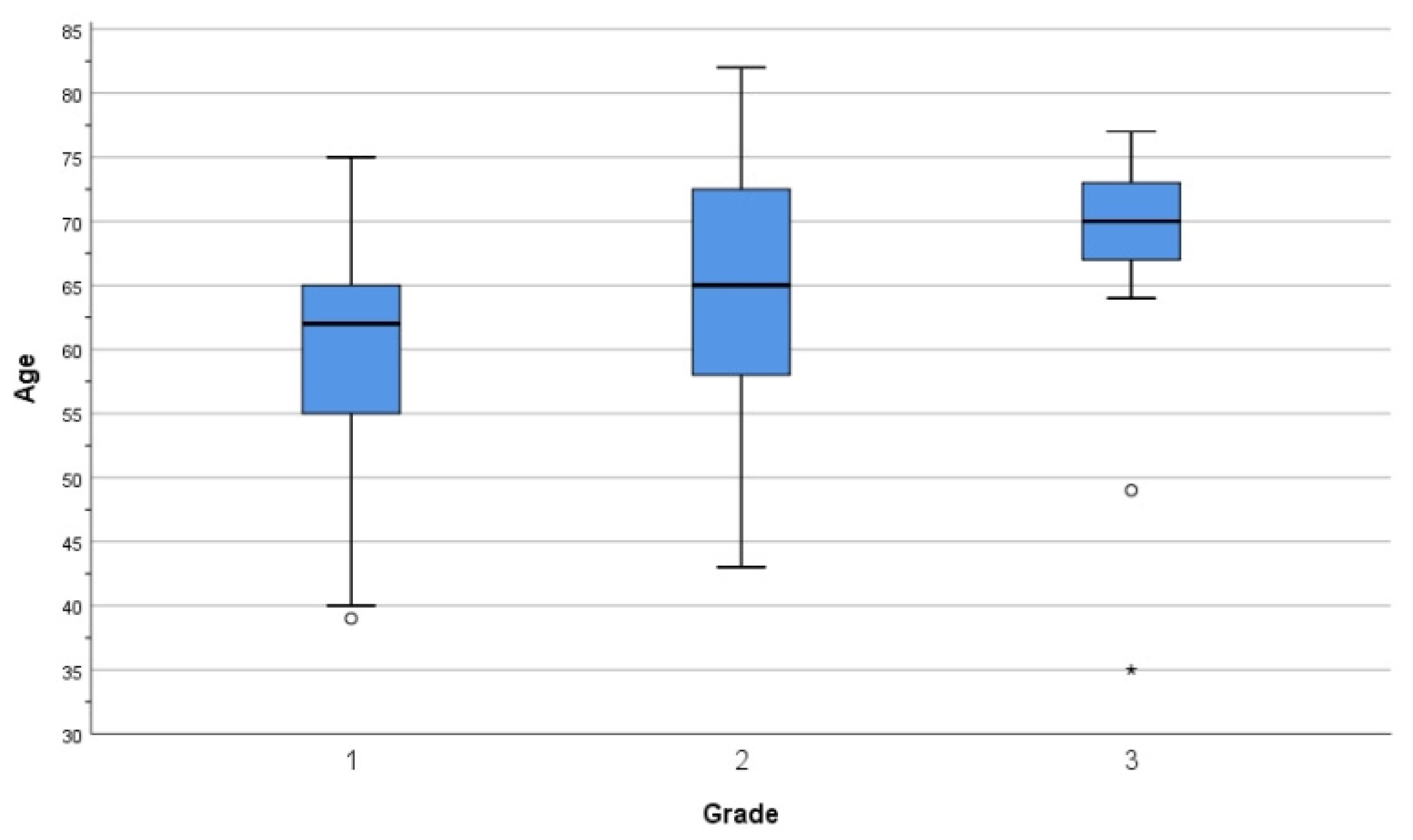
3.1. Epidemiological Data
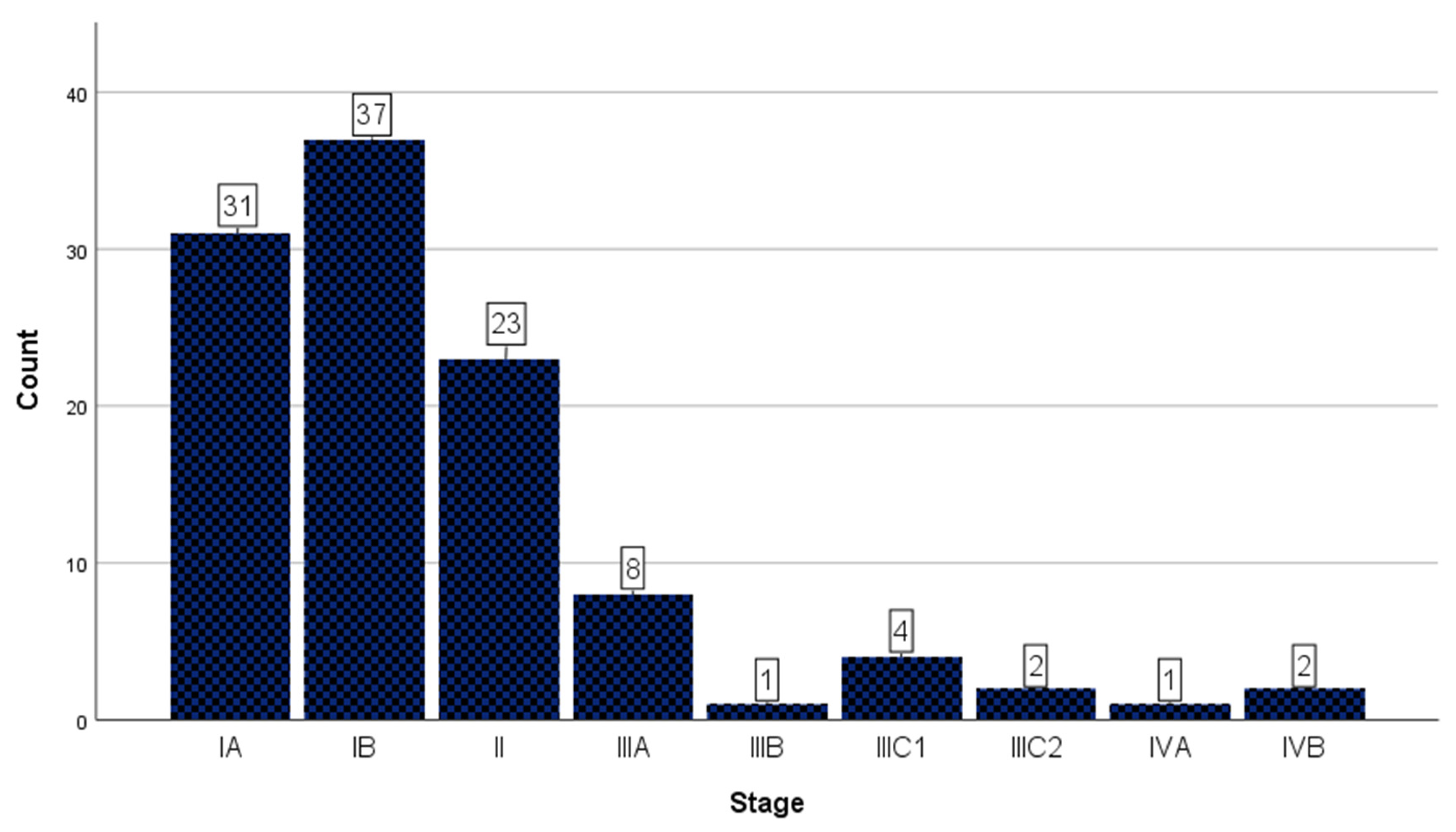
3.2. Staging Data
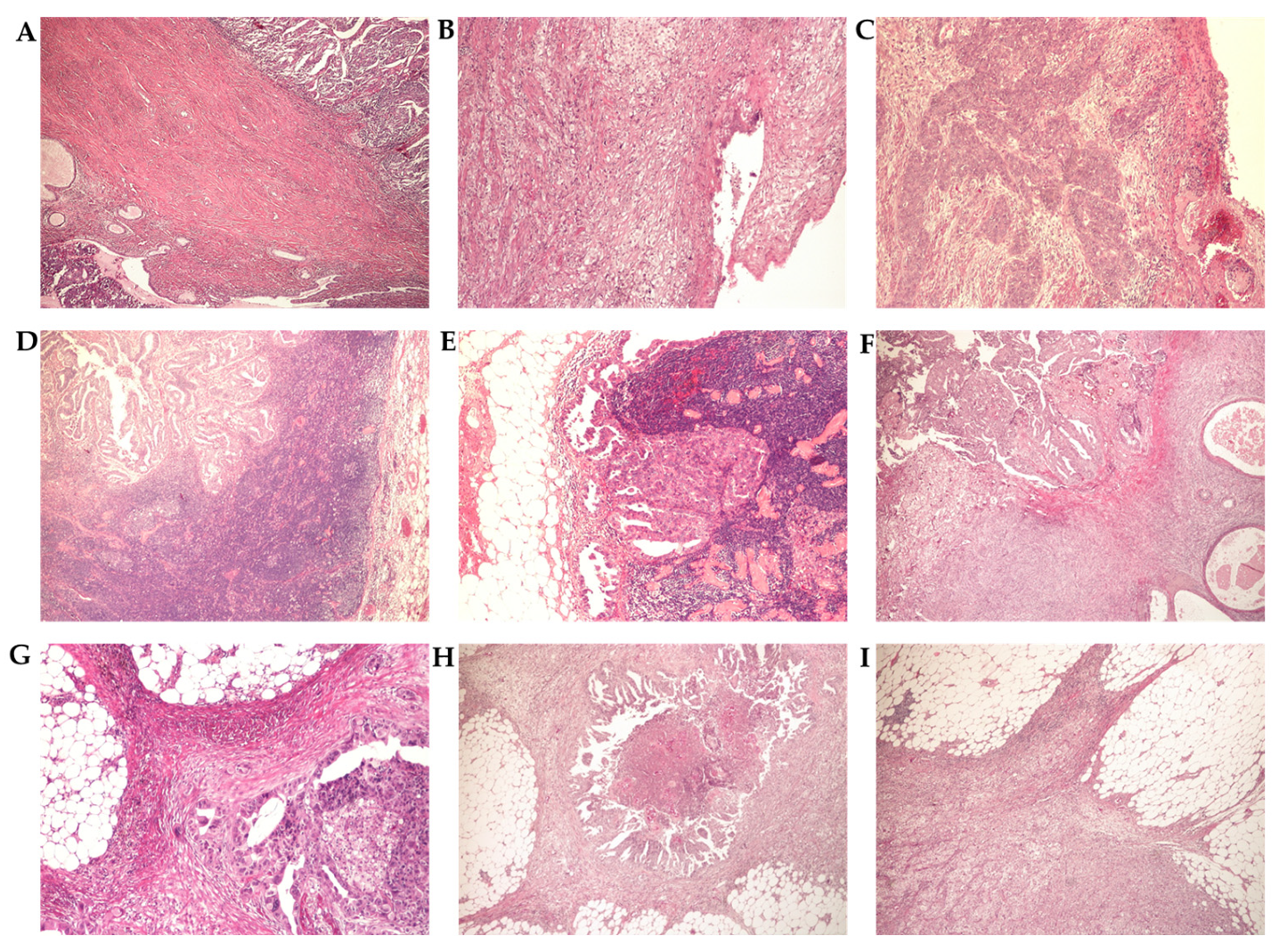
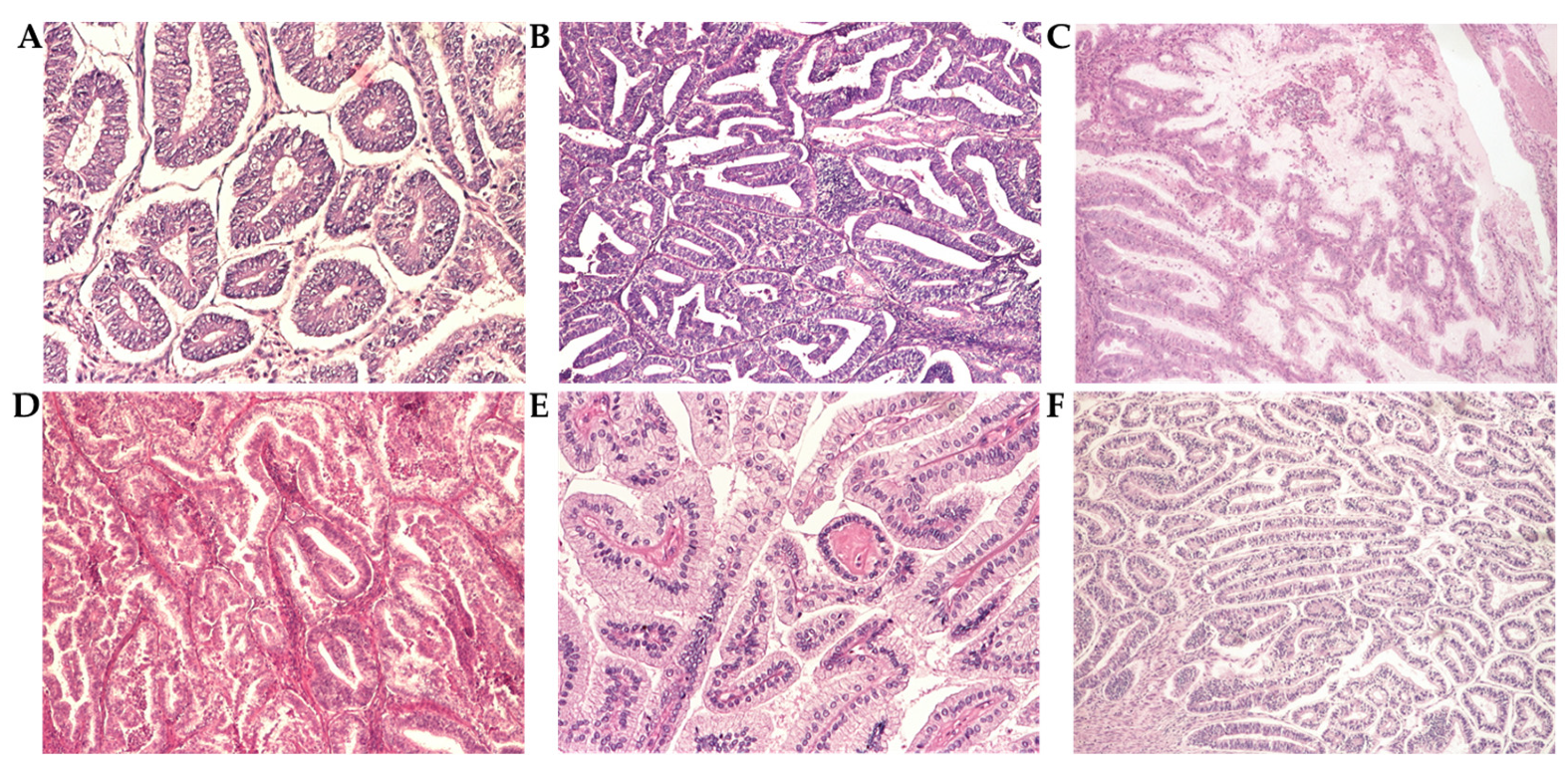
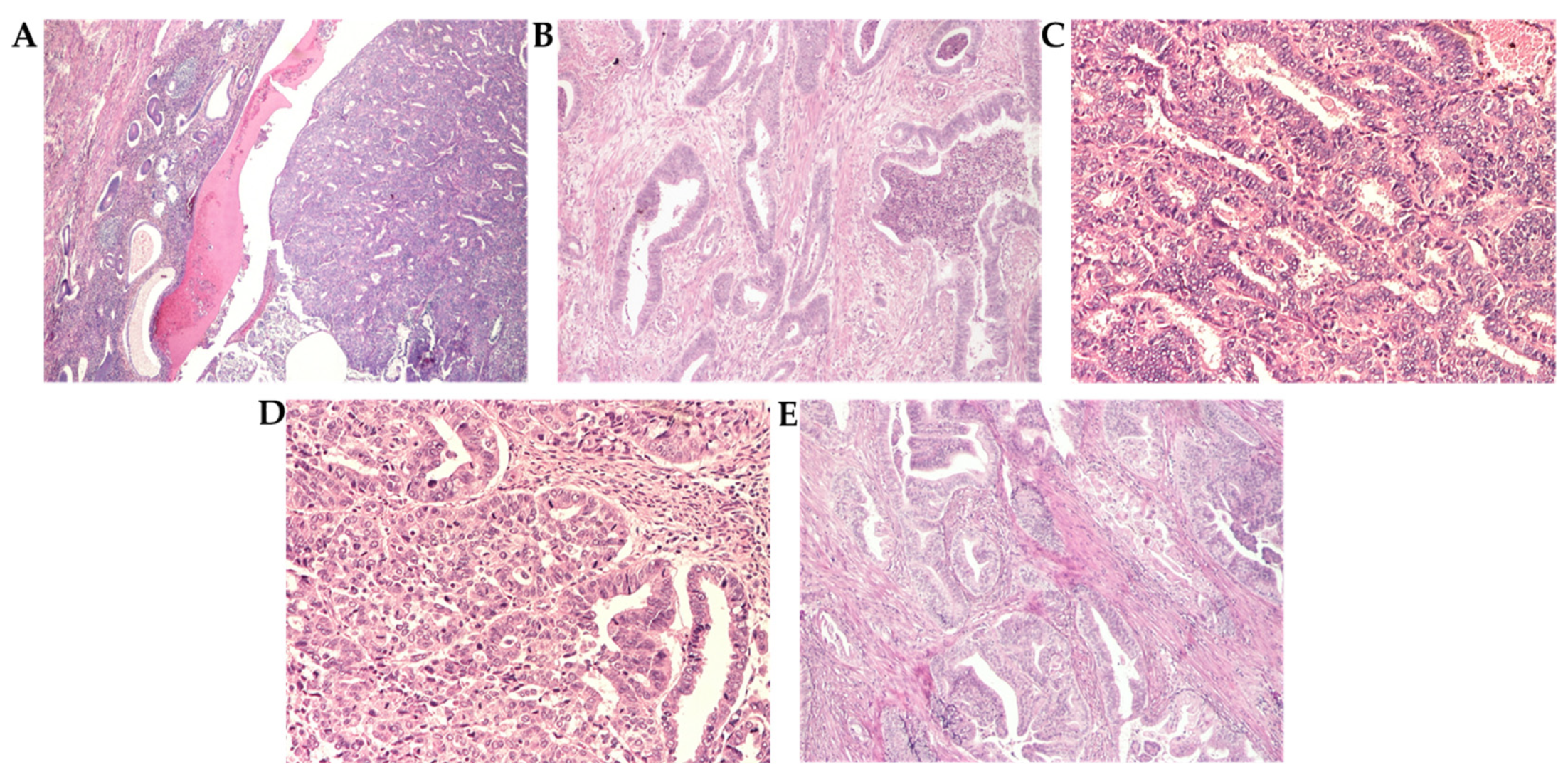
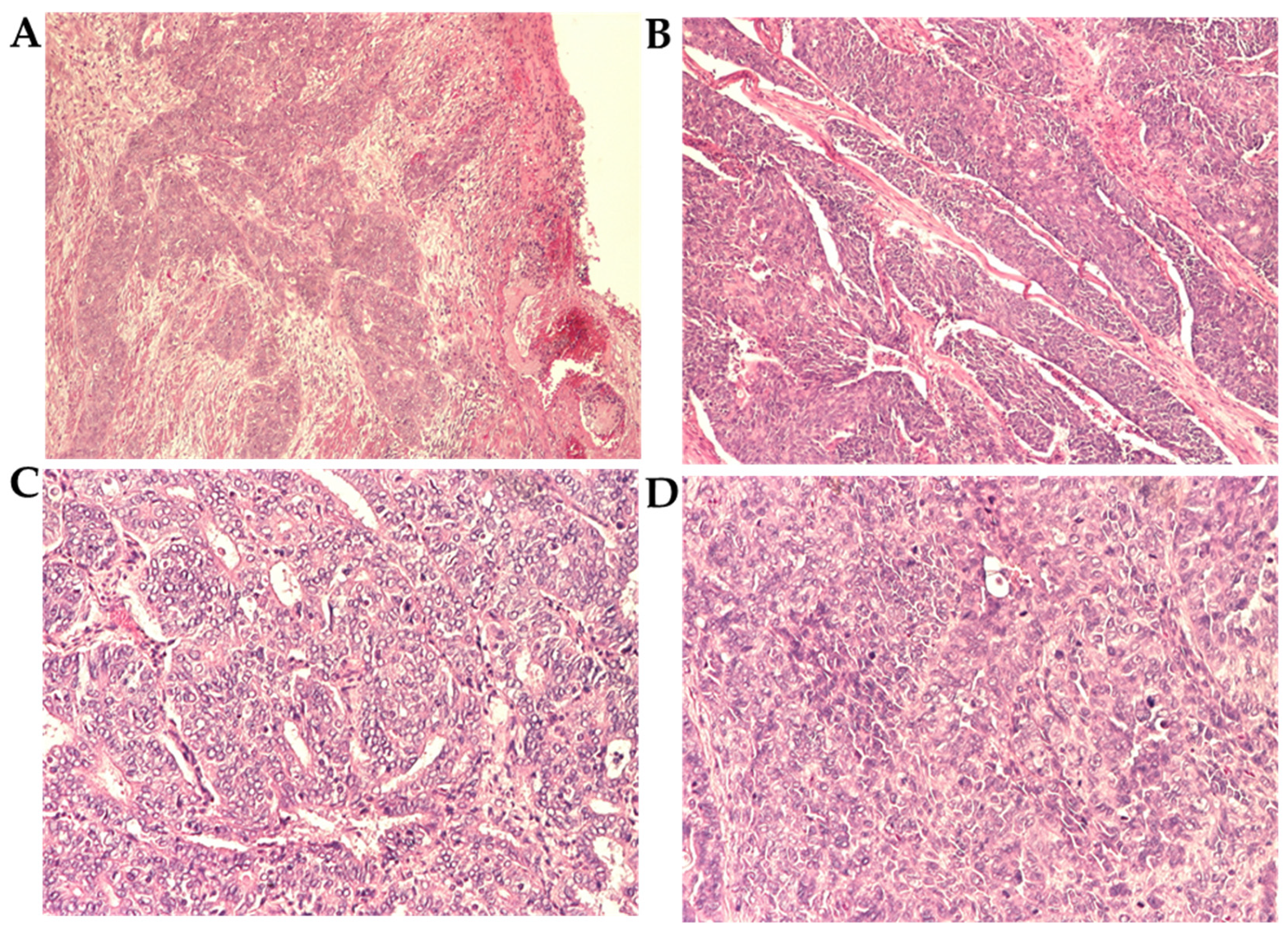
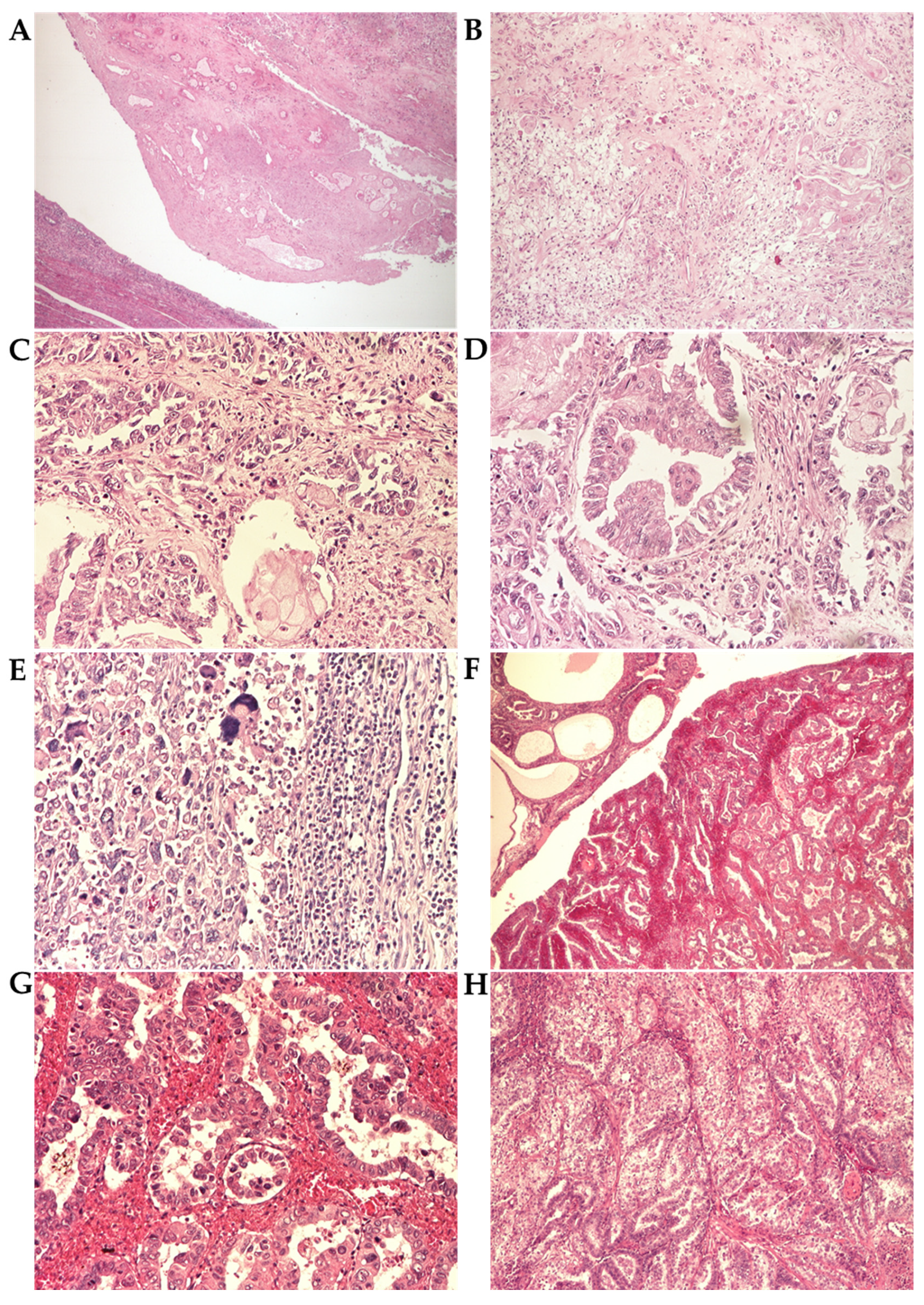
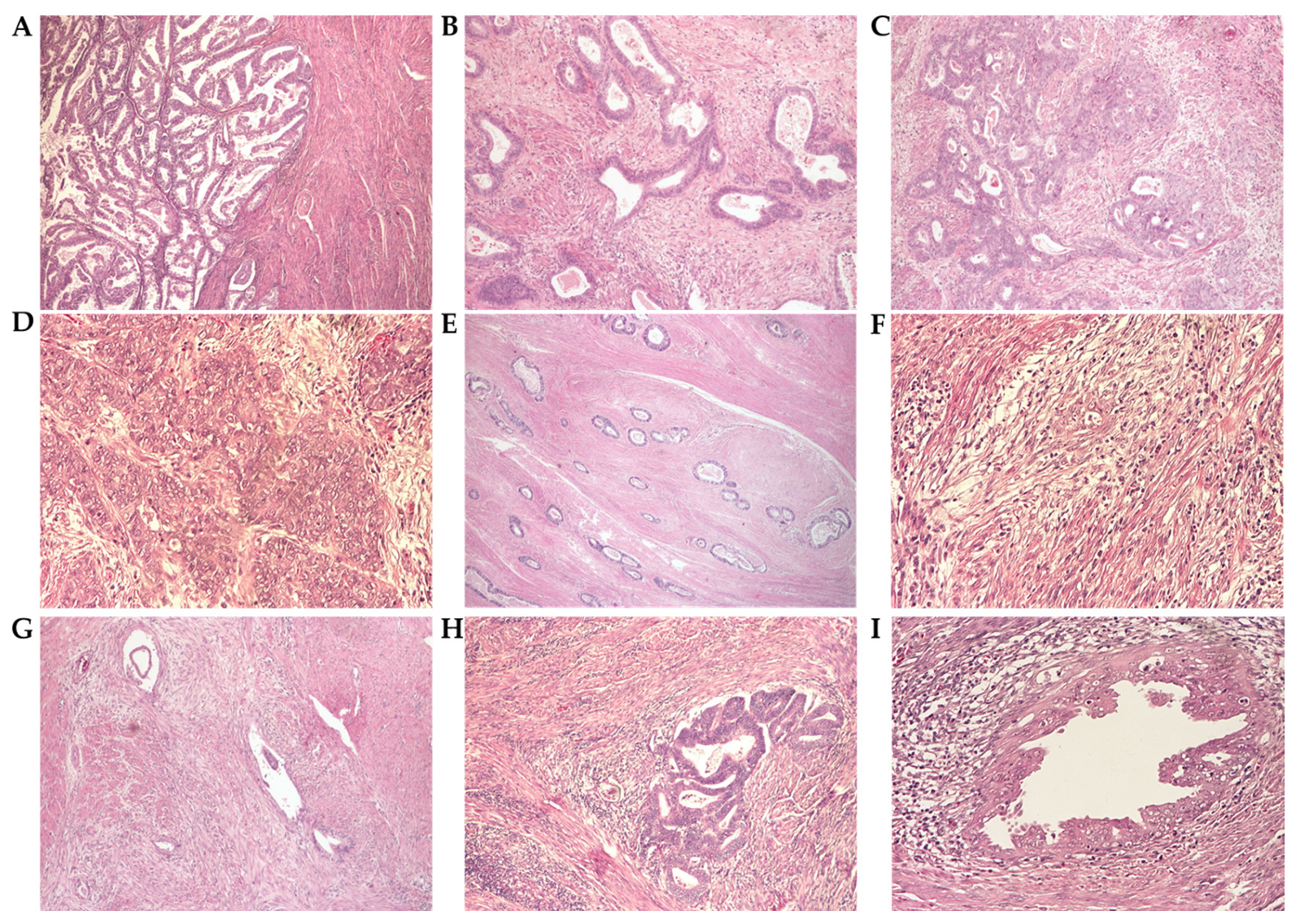
3.3. Histopathological Analysis
3.3.1. Grade and Histological Type
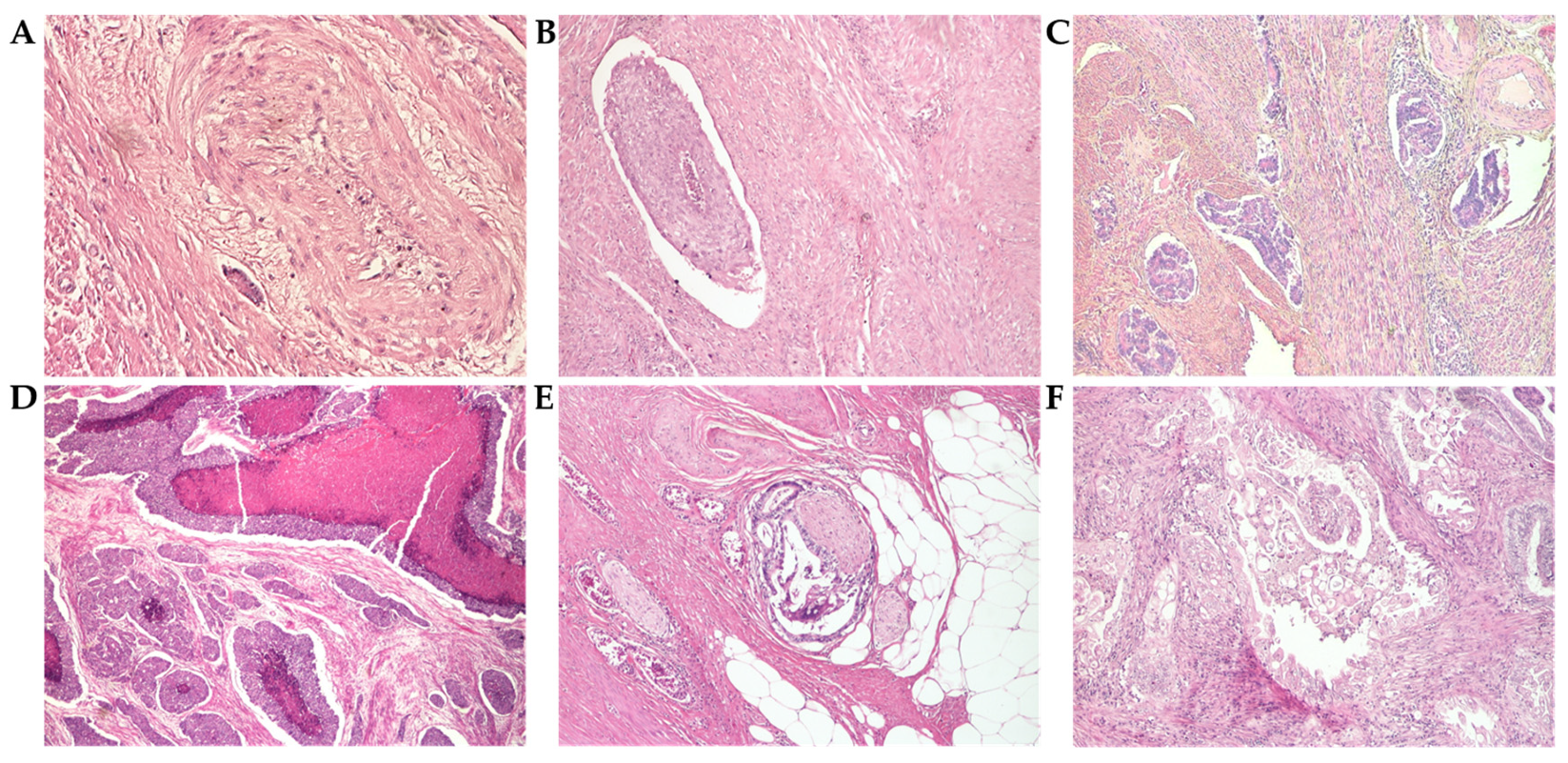
3.3.2. Histopathological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LVI | Lymphovascular invasion |
| MI | Myoinvasion |
| PNI | Perineural invasion |
| LNI | Lymph node |
| SEER | Surveillance, Epidemiology and End Results program |
| WHO | World Health Organization |
| FIGO | International Federation of Gynaecological Oncologists |
| EEC | Endometrioid endometrial carcinoma |
Appendix A
| Study and Cohort | Number of Cases | Age Mean (Years) and Interval or SD | FIGO Grade | FIGO Stage | Histological Types (Endometrioid vs. Non-Endometrioid) | LVI | MI | N |
|---|---|---|---|---|---|---|---|---|
| Present study | 109 | 62.72 35–82 SD: 9.49 | G1 53 (48.6%) G2 39 (35.7%) G3 17 (15.7%) | FIGO I–II 91 (83.5%) FIGO III–IV 18 (16.5%) | 102 endometrioid (93.5%) 7 non-endometrioid (6.5%) | 10 (9.2%) | Absent: 4 (3.7%) <1/2: 41 (37.6%) >1/2: 64 (58.7%) | At least N1: 7 (6.5%) N0: 35 (32.1%) Nx: 67 (61.4%) |
| Khatib et al., 2020 [15] | 776 * | 57 26–91 | G1 427 (55%) G2 283 (36.4%) G3 50 (6.4%) | FIGO I–II 675 (87%) FIGO III–IV 83 (10.7%) | n/a | 213 (27.4%) | <1/2: 502 (64.7%) >1/2: 219 (28.2%) | At least N1: 50 (6.4%) N0: 657 (84.6%) |
| Bilgi et al., 2022 [13] | 412 | 59.5 33–88 | G1 244 (59.2%) G2 95 (23.1%) G3 73 (17.7%) | FIGO I–II 331 (80.4%) FIGO III–IV 81 (19.6%) | 366 endometrioid (88.9%) 46 non-endometrioid (11.1%) | 69 (16.7%) | <1/2: 285 (69.2%) >1/2: 127 (30.8%) | n/a |
| Bing et al., 2024 [30] | 133 | 56.5 SD: 10.71 | G1 59 (44.3%) G2 49 (36.9%) G3 25 (18.8%) | FIGO I–II 111 (83.4%) FIGO III–IV 22 (16.6%) | 122 endometrioid (91.7%) 11 non-endometrioid (8.3%) | 35 (26.3%) | n/a | At least N1: 18 (13.5%) |
| Kim et al., 2022 [14] ** | 237 * | 63 59–72 | n/a | FIGO I–II 188 (79.3%) FIGO III–IV 49 (36.7%) | 194 endometrioid (81.8%) 43 non-endometrioid (18.2%) | 72 (30.3%) | <1/2: 161 (67.9%) >1/2: 76 (32.1%) | At least N1: 32 (13.5%) |
| Yang et al., 2023 [12] ** | 70 * | 60 38–83 SD: 1.41 | G1 24 (34.3%) G2 36 (51.4%) G3 9 (12.8%) | n/a | 64 endometrioid (91.4%) 6 non-endometrioid (8.6%) | 13 (18.5%) | <1/2: 29 (41.4%) >1/2: 17 (24.2%) | n/a |
| Montoya-González et al., 2021 [17] (population based study) | 210 * | 61.2 30–83 SD: 10.1 | G1 94 (44.7%) | FIGO I–II 96 (45.7%) FIGO III–IV 60 (28.5%) | 156 endometrioid (74.3%) 46 non-endometrioid (22%) | n/a | n/a | n/a |
| Agarwal et al., 2023 [9] | 86 | 54 | G1 38 (44.2%) G2 34 (39.5%) G3 14 (16.3%) | FIGO I–II 64 (74.4%) FIGO III–IV 22 (25.6%) | 72 endometrioid (83.7%) 14 non-endometrioid (16.3%) | n/a | <1/2: 40 (46.5%) >1/2: 46 (53.5%) | n/a |
| Olatunde et al., 2024 [21] | 44 * | 59 29–86 | G1 19 (43.2%) G2 10 (22.7%) G3 14 (31.8%) | n/a | 35 endometrioid (79.5%) 9 non endometrioid (20.5%) | n/a | n/a | n/a |
| Burney et al., 2024 [25] | 50 | 61 31–86 | G1 20 (40%) G2 17 (34%) G3 13 (26%) | FIGO I–II 38 (76%) FIGO III–IV 12 (24%) | 36 endometrioid (72%) 14 non-endometrioid (28%) | n/a | n/a | n/a |
| Sait et al., 2023 [16] | 200 | 58.6 SD: 11.2 | G1 78 (39%) G2 63 (31.5%) G3 59 (29.5%) | FIGO I–II 133 (66.5%) FIGO III–IV 67 (33.5%) | 157 endometrioid (78.5%) 43 non-endometrioid (21.5%) | 73 (36.5%) | Absent: 7 cases (3.5%) <1/2: 111 (55.5%) >1/2: 82 (41%) | n/a |
| Thompson et al., 2022 [10] | 1357 * | 64.9 SD: 11.3 | G1 and G2 954 (70.3%) G3 403 (29.7%) | FIGO I–II 1034 (79.5%) FIGOIII–IV 267 (20.5%) | 1098 endometrioid (80.9%) 260 non-endometrioid (19.1%) | 397 (29.2%) | n/a | n/a |
| Papathemelis et al., 2024 [32] ** (population based study) | 8891 * | n/a | G1 3585 (40.3%) G2 2793 (31.4%) G3 2322 (26.1%) | n/a | 7459 endometrioid (83.8%) 1432 non-endometrioid (16.2%) | 1630 (18.3%) | n/a | At least N1: 839 (9.4%) N0: 6988 (78.6) Nx: 1064 (12%) |
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today, International Agency for Research on Cancer: Lyon, France. 2022. Available online: https://gco.iarc.who.int/today (accessed on 2 August 2025).
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int. J. Cancer 2019, 145, 1719–1730. [Google Scholar] [CrossRef]
- Editorial Board WHOCOT. WHO Classification of Tumors Female Genital Tumors, 5th ed.; Herrington, C.S., Ed.; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Baker-Rand, H.; Kitson, S.J. Recent Advances in Endometrial Cancer Prevention, Early Diagnosis and Treatment. Cancers 2024, 16, 1028. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N.; Endometrial Cancer Staging Subcommittee; et al. FIGO staging of endometrial cancer: 2023. Int. J. Gynecol. Obs. 2023, 162, 383–394. [Google Scholar] [CrossRef]
- Kommoss, S.; McConechy, M.K.; Kommoss, F.; Leung, S.; Bunz, A.; Magrill, J.; Britton, H.; Kommoss, F.; Grevenkamp, F.; Karnezis, A.; et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann. Oncol. 2018, 29, 1180–1188. [Google Scholar] [CrossRef]
- Creasman, W. Revised FIGO staging for carcinoma of the endometrium. Int. J. Gynecol. Obstet. 2009, 105, 109. [Google Scholar] [CrossRef]
- Agarwal, S.; Melgandi, W.; Sonkar, D.R.; Ansari, F.A.; Arora, S.; Rathi, A.K.; Singh, K. Epidemiological characteristics of endometrial cancer patients treated at a tertiary health center in National Capital Territory of India. J. Cancer Res. Ther. 2023, 19, 452–456. [Google Scholar] [CrossRef]
- Thompson, E.F.; Huvila, J.; Jamieson, A.; Leung, S.; Lum, A.; Offman, S.; Lytwyn, A.; Sur, M.L.; Hoang, L.; Irving, J.; et al. Variability in endometrial carcinoma pathology practice: Opportunities for improvement with molecular classification. Mod. Pathol. Off. J. US Can. Acad. Pathol. 2022, 12, 1974–1982. [Google Scholar] [CrossRef]
- Hoang, L.N.; Kinloch, M.A.; Leo, J.M.; Grondin, K.; Lee, C.H.; Ewanowich, C.; Köbel, M.; Cheng, A.; Talhouk, A.; McConechy, M.; et al. Interobserver Agreement in Endometrial Carcinoma Histotype Diagnosis Varies Depending on the Cancer Genome Atlas (TCGA)-based Molecular Subgroup. Am. J. Surg. Pathol. 2017, 41, 245–252. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, X.; Liu, X.; Ma, K.; Meng, Y.-T.; Yin, H.-F.; Wen, J.; Yang, J.-H.; Zhen, Z.; Feng, Z.-H.; et al. Clinical characteristics and prognostic characterization of endometrial carcinoma: A comparative analysis of molecular typing protocols. BMC Cancer 2023, 23, 243. [Google Scholar] [CrossRef] [PubMed]
- Bilgi, A.; Guler, A.H.; Kulhan, M.; Cintesun, E.; Ates, M.; Celik, C. The struggle against endometrial cancer: Ten years of experience of a tertiary center. Ginekol. Pol. 2022, 93, 351–360. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, H.; Yeo, M.K.; Won, K.Y.; Kim, Y.S.; Han, G.H.; Kim, H.S.; Na, K. Mesonephric-like Adenocarcinoma of the Uterine Corpus: Comprehensive Analyses of Clinicopathological, Molecular, and Prognostic Characteristics with Retrospective Review of 237 Endometrial Carcinoma Cases. Cancer Genom. Proteom. 2022, 19, 526–539. [Google Scholar] [CrossRef]
- Khatib, G.; Gulec, U.K.; Guzel, A.B.; Bagir, E.; Paydas, S.; Vardar, M.A. Prognosis Trend of Grade 2 Endometrioid Endometrial Carcinoma: Toward Grade 1 or 3? Pathol. Oncol. Res. 2020, 26, 2351–2356. [Google Scholar] [CrossRef]
- Sait, K.H.; Anfinan, N.; Sait, H.; Shamrani, H.; Sait, M. Overall and progression-free survival in endometrial carcinoma: A single-center retrospective study of patients treated between 2000–2018. Ann. Saudi Med. 2023, 43, 315–328. [Google Scholar] [CrossRef]
- Montoya-González, M.C.; Arias-Ortiz, N.E.; Arboleda-Ruiz, W.A. Incidence, mortality and survival of endometrial cancer in Manizales, Colombia 2003–2017. Rev. Peru. Med. Exp. Salud. Publica 2021, 38, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Hussein, Y.; Bandyopadhyay, S.; Cote, M.; Hassan, O.; Abdulfatah, E.; Alosh, B.; Guan, H.; Soslow, R.A.; Ali-Fehmi, R. Interobserver Variability in the Diagnosis of Uterine High-Grade Endometrioid Carcinoma. Arch. Pathol. Lab. Med. 2016, 140, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Restaino, S.; Paglietti, C.; Arcieri, M.; Biasioli, A.; Della Martina, M.; Mariuzzi, L.; Andreetta, C.; Titone, F.; Bogani, G.; Raimondo, D.; et al. Management of Patients Diagnosed with Endometrial Cancer: Comparison of Guidelines. Cancers 2023, 15, 1091. [Google Scholar] [CrossRef]
- Abel, M.K.; Liao, C.-I.; Chan, C.; Lee, D.; Rohatgi, A.; Darcy, K.M.; Tian, C.; Mann, A.K.; Maxwell, G.L.; Kapp, D.S.; et al. Racial disparities in high-risk uterine cancer histologic subtypesA United States Cancer Statistics study. Gynecol. Oncol. 2021, 161, 470–476. [Google Scholar] [CrossRef]
- Olatunde, O.A.; Samaila, M.O.; Imam, M.I.; Uchime, K.E.; Dauda, S.E. Histopathological patterns of endometrial carcinoma in a tertiary hospital in North-West Nigeria. Ecancermedicalscience 2024, 18, 1651. [Google Scholar] [CrossRef] [PubMed]
- Saris, D.H.; Smith, A.J.B.; Brensinger, C.; Kim, S.H.; Haggerty, A.F.; Latif, N.; Cory, L.; Giuntoli, R.L.; Morgan, M.A.; Lin, L.L.; et al. Disparities in cancer-specific and overall survival in black women with endometrial cancer: A Medicare-SEER study. Gynecol. Oncol. Rep. 2022, 40, 100922. [Google Scholar] [CrossRef]
- Guttery, D.S.; Blighe, K.; Polymeros, K.; Symonds, R.P.; Macip, S.; Moss, E.L. Racial differences in endometrial cancer molecular portraits in the Cancer Genome Atlas. Oncotarget 2018, 9, 17093–17103. [Google Scholar] [CrossRef]
- Foley, K.G.; Adli, M.; Kim, J.J. Single-nuclei sequencing of uterine serous carcinoma reveals racial differences in immune signaling. Proc. Natl. Acad. Sci. USA 2024, 121, e2402998121. [Google Scholar] [CrossRef]
- Burney, I.A.; Al Ghafri, S.; Al Noumani, J.; Al Jabri, A.; Hasan, A.O.; Bella, S.; Al-Sayegh, H.; Al Ajmi, R.; Al Kalbani, M. Clinicopathological Features and Outcomes of Endometrial Cancer: A single institution experience. Sultan Qaboos Univ. Med. J. 2024, 24, 203–208. [Google Scholar] [CrossRef]
- Peters, E.E.M.; Nucci, M.R.; Gilks, C.B.; McCluggage, W.G.; Bosse, T. Practical guidance for assessing and reporting lymphovascular space invasion (LVSI) in endometrial carcinoma. Histopathology 2025, 86, 173–182. [Google Scholar] [CrossRef]
- Li, Z.; Peng, J.; Zhang, B.; Zhao, C.; Chen, Z.; Xiao, H.; Zhang, Z.; Ma, X.; Gao, F.; Xin, R.; et al. The prognostic and clinical significance of substantial lymphovascular space invasion in early-stage endometrial carcinoma. Eur. J. Cancer 2025, 218, 115258. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, S.; Pifer, P.M.; Bhargava, R.; Keller, A.; Musunuru, H.B.; Patel, A.K.; Sukumvanich, P.; Boisen, M.; Berger, J.L.; Taylor, S.; et al. Is Substantial Lymphovascular Space Invasion Prognostic for Clinical Outcomes in Type II Endometrial Cancer? Clin. Oncol. (R. Coll. Radiol.) 2022, 34, 452–458. [Google Scholar] [CrossRef]
- Pifer, P.M.; Jaishankar, S.; Bhargava, R.; Schad, M.D.; Keller, A.; Musunuru, H.B.; Cohen, M.; Sukumvanich, P.; Courtney-Brooks, M.; Boisen, M.; et al. Is Substantial Lymphovascular Space Invasion Prognostic in Patients with Pathologically Lymph Node-Negative Endometrial Cancer? Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, 148–153. [Google Scholar] [CrossRef]
- Bing, R.-S.; Ding, D.-C.; Hsu, C.-S. Prognostic factors and survival of endometrial cancer: An 11-year retrospective cohort study in southern Taiwan. Taiwan. J. Obstet. Gynecol. 2024, 63, 679–684. [Google Scholar] [CrossRef]
- Yarandi, F.; Shirali, E.; Akhavan, S.; Nili, F.; Ramhormozian, S. The impact of lymphovascular space invasion on survival in early stage low-grade endometrioid endometrial cancer. Eur. J. Med. Res. 2023, 28, 118. [Google Scholar] [CrossRef] [PubMed]
- Papathemelis, T.; Ortmann, O.; Kohl, C.; Neuser, P.; Tol, K.K.; Klinkhammer-Schalke, M.; Ugocsai, P.; Walter, C.B.; Rottmann, M.; Real, C.; et al. Treatment of endometrial cancer from 2000 to 2020 in Germany: A retrospective population based cohort study. J. Cancer Res. Clin. Oncol. 2024, 150, 279. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Gottumukkala, R.V.; Schieda, N.; Lavallée, L.; Adam, B.A.; Silverman, S.G. Perineural Invasion and Spread in Common Abdominopelvic Diseases: Imaging Diagnosis and Clinical Significance. Radiographics 2023, 43, e220148. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, D.; Yang, F.; Pan, W.; Zeng, F.; Wu, J.; Xie, H.; Li, J.; Yao, S. Perineural invasion in endometriotic lesions contributes to endometriosis-associated pain. J. Pain Res. 2018, 11, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Huang, T.; Gu, S.L.; Wang, J.; Liu, Y.; Sun, X.; Wang, Y.D. DRG Neurons Promote Perineural Invasion of Endometrial Cancer via GluR2. J. Cancer 2020, 11, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
| Age (Years) | Number of Cases | % |
|---|---|---|
| 35–39 | 2 | 1.8 |
| 40–49 | 11 | 10.1 |
| 50–59 | 22 | 20.2 |
| 60–69 | 48 | 44 |
| 70–79 | 25 | 22.9 |
| 80+ | 1 | 1 |
| Total | 109 | 100.00 |
| Age (Years) | Grade 1 | Grade 2 | Grade 3 | Total |
|---|---|---|---|---|
| 30–39 | 1 | 0 | 1 | 2 |
| 40–49 | 8 | 2 | 1 | 11 |
| 50–59 | 12 | 10 | 0 | 22 |
| 60–69 | 29 | 13 | 6 | 48 |
| 70–79 | 3 | 13 | 9 | 25 |
| >80 | 0 | 1 | 0 | 1 |
| Total | 53 | 39 | 17 | 109 |
| Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | ||
| Stage | IA | 31 | 7 | 3 | 6 | 4 | 4 | 4 | 3 |
| IB | 37 | 9 | 9 | 3 | 2 | 1 | 4 | 9 | |
| II | 23 | 4 | 4 | 1 | 5 | 1 | 1 | 7 | |
| IIIA | 8 | 0 | 2 | 0 | 1 | 2 | 2 | 1 | |
| IIIB | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| IIIC1 | 4 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | |
| IIIC2 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |
| IVA | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| IVB | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | |
| Total | 109 | 23 | 19 | 11 | 13 | 9 | 13 | 21 | |
| Figo 2009 Stage | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| IA | IB | II | IIIA | IIIB | IIIC1 | IIIC2 | IVA | IVB | |
| Type I carcinoma | 31 | 34 | 19 | 5 | 0 | 2 | 0 | 0 | 1 |
| Type II carcinoma | 0 | 3 | 4 | 3 | 1 | 2 | 2 | 1 | 1 |
| Total | 31 | 37 | 23 | 8 | 1 | 4 | 2 | 1 | 2 |
| Type | Number of Cases | % |
|---|---|---|
| G1 endometrioid carcinoma | 53 | 48.6% |
| G2 endometrioid carcinoma | 39 | 35.7% |
| G3 endometrioid carcinoma | 10 | 9.2% |
| Serous carcinoma | 2 | 1.9% |
| Carcinosarcoma | 3 | 2.8% |
| Clear cell carcinoma | 1 | 0.9% |
| Mixed carcinoma | 1 | 0.9% |
| Total endometrioid carcinoma cases | 102 | 93.5% |
| Total non-endometrioid carcinoma cases | 7 | 6.5% |
| Parameter | Count | % | |
|---|---|---|---|
| Myoinvasion | absent | 4 | 3.7 |
| <1/2 | 41 | 37.6 | |
| >1/2 | 64 | 58.7 | |
| Vascular invasion | absent | 99 | 90.8 |
| present | 10 | 9.2 | |
| Tumor necrosis | absent | 94 | 86.2 |
| present | 15 | 13.8 | |
| Perineural invasion | absent | 106 | 97.2 |
| present | 3 | 2.8 | |
| Squamous metaplasia | absent | 39 | 35.7 |
| present | 70 | 64.3 | |
| Lymph node involvement | x | 67 | 61.4 |
| 0 | 35 | 32.1 | |
| 1 | 7 | 6.5 |
| Significance of Correlation | Advanced FIGO Stage | High Tumor Grade |
|---|---|---|
| Myoinvasion | 0.013 | 0.0018 |
| Lymphovascular invasion | 0.0001 | 0.04 |
| Lymph node involvement | n/a | 0.0006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muraru, A.; Stepan, A.-E.; Margaritescu, C.; Florescu, M.M.; Badiu, A.-M.; Cretu, I.O.; Andreiana, B.C.; Ciurea, R.N. Epidemiological and Histopathological Characterization of Endometrial Carcinoma: A Retrospective Cohort from Romania. Diagnostics 2025, 15, 2645. https://doi.org/10.3390/diagnostics15202645
Muraru A, Stepan A-E, Margaritescu C, Florescu MM, Badiu A-M, Cretu IO, Andreiana BC, Ciurea RN. Epidemiological and Histopathological Characterization of Endometrial Carcinoma: A Retrospective Cohort from Romania. Diagnostics. 2025; 15(20):2645. https://doi.org/10.3390/diagnostics15202645
Chicago/Turabian StyleMuraru, Andrei, Alex-Emilian Stepan, Claudiu Margaritescu, Mirela Marinela Florescu, Anne-Marie Badiu, Iulia Oana Cretu, Bianca Catalina Andreiana, and Raluca Niculina Ciurea. 2025. "Epidemiological and Histopathological Characterization of Endometrial Carcinoma: A Retrospective Cohort from Romania" Diagnostics 15, no. 20: 2645. https://doi.org/10.3390/diagnostics15202645
APA StyleMuraru, A., Stepan, A.-E., Margaritescu, C., Florescu, M. M., Badiu, A.-M., Cretu, I. O., Andreiana, B. C., & Ciurea, R. N. (2025). Epidemiological and Histopathological Characterization of Endometrial Carcinoma: A Retrospective Cohort from Romania. Diagnostics, 15(20), 2645. https://doi.org/10.3390/diagnostics15202645






