Evaluation of the Risk of Urinary System Stone Recurrence Using Anthropometric Measurements and Lifestyle Behaviors in a Developed Artificial Intelligence Model
Abstract
1. Introduction
2. Materials and Methods
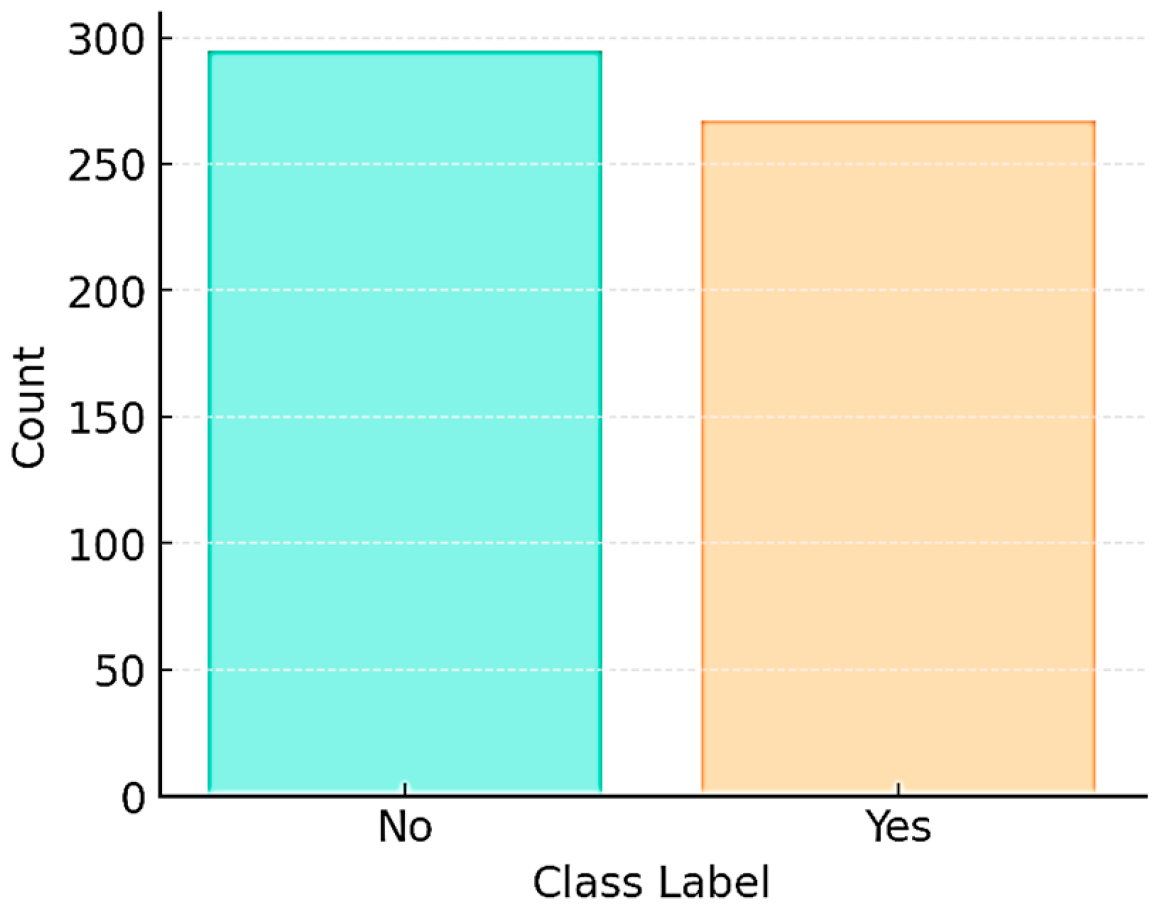
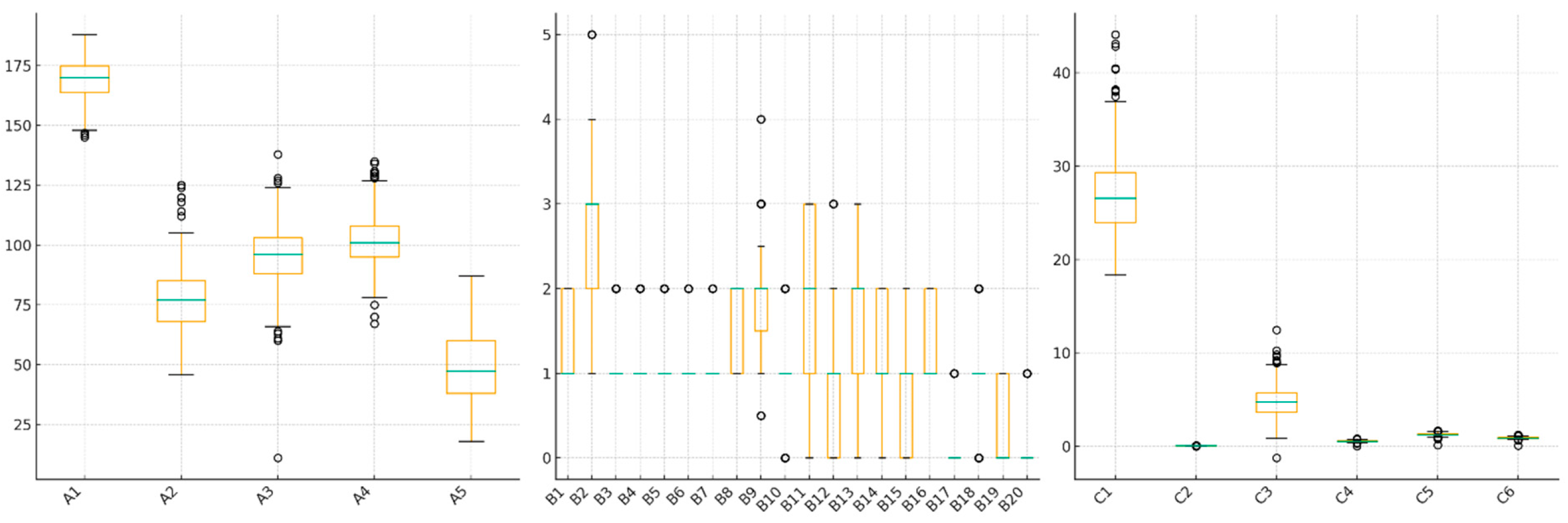
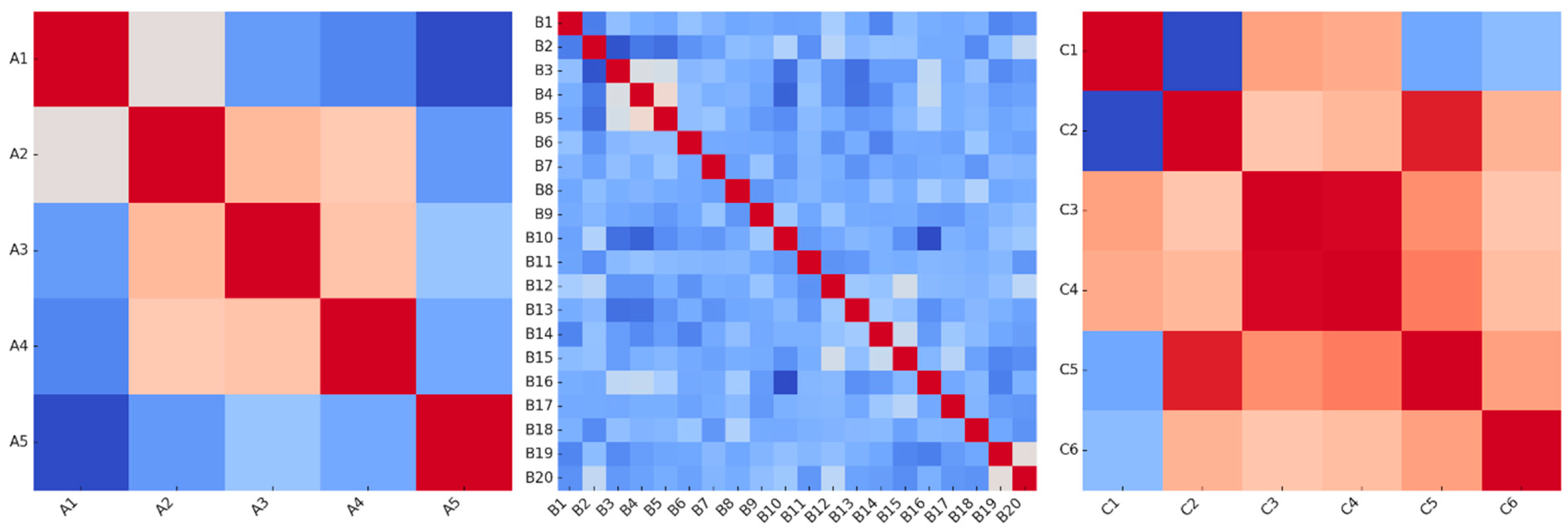
2.1. Dataset
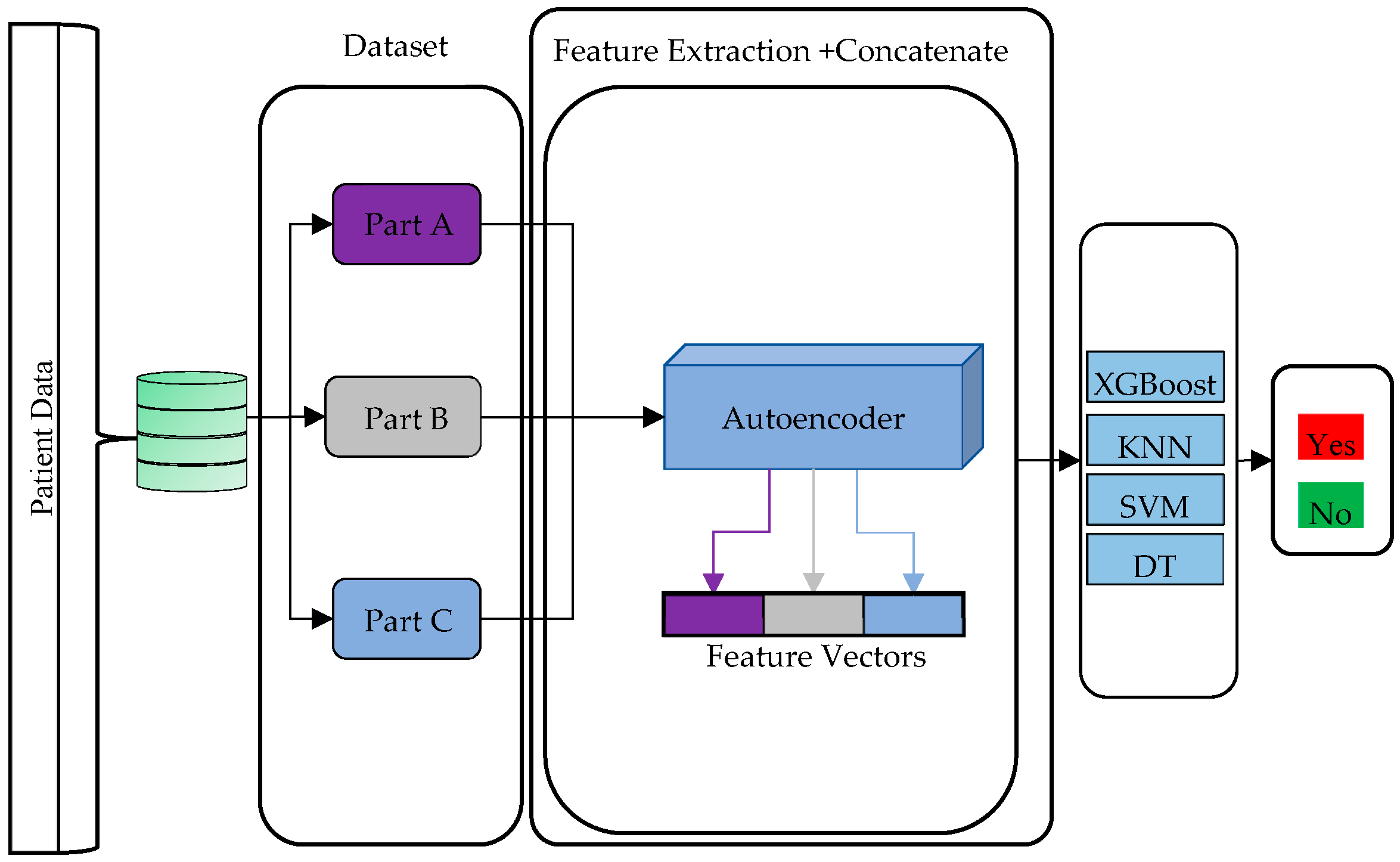
2.2. Autoencoder-Based Feature Extraction and Classifiers
2.3. Proposed Model
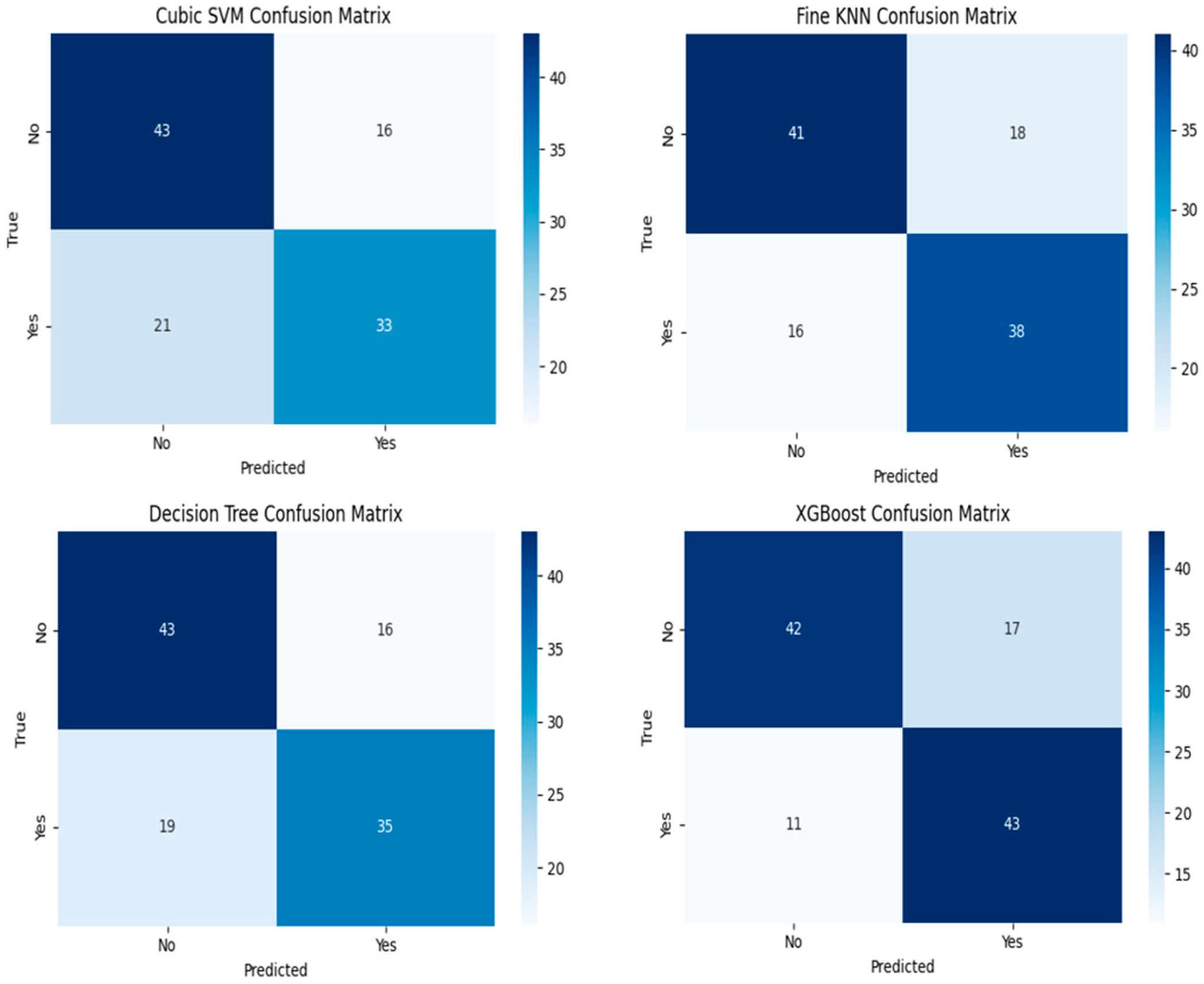
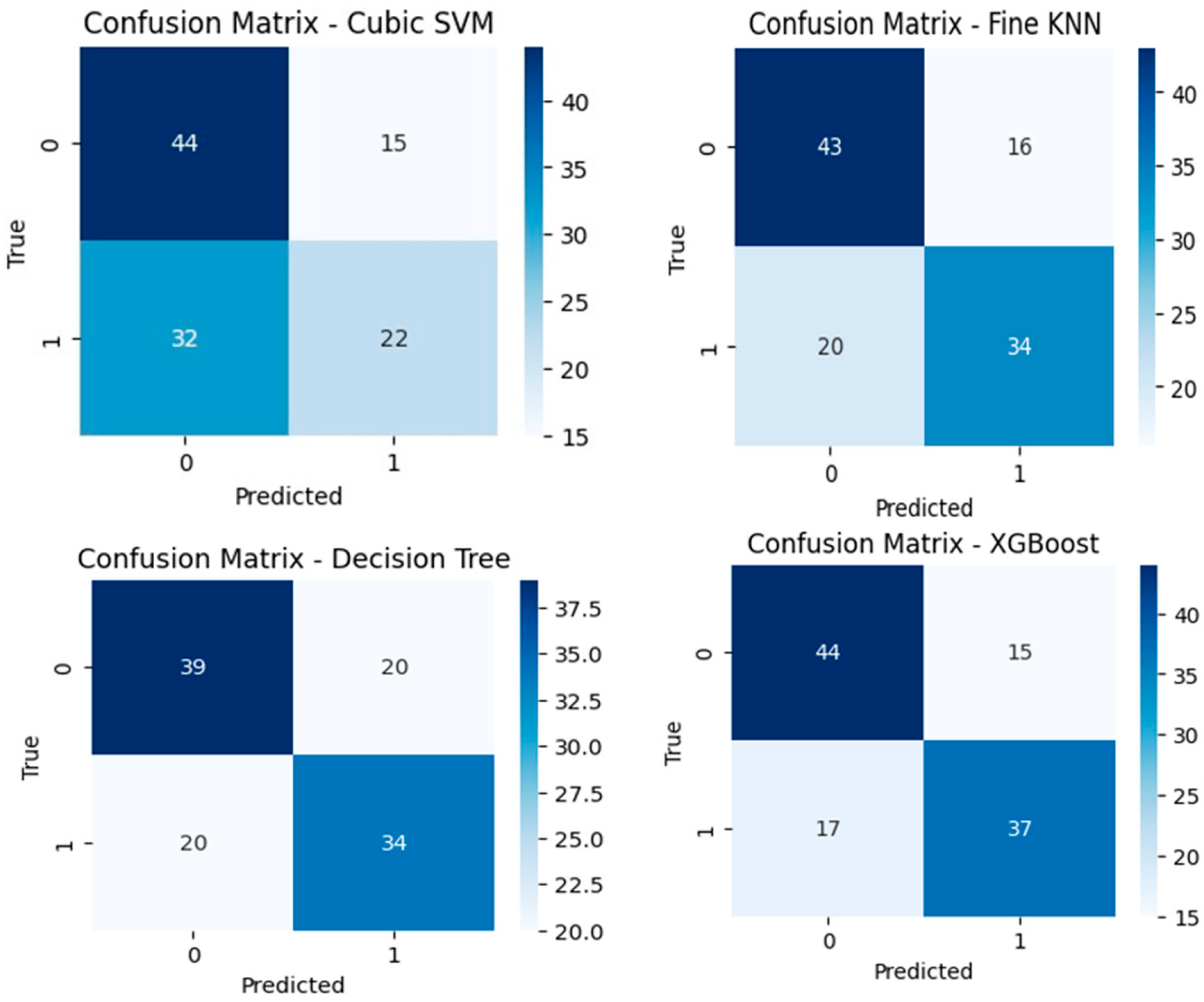
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abufaraj, M.; Xu, T.; Cao, C.; Waldhoer, T.; Seitz, C.; D’aNdrea, D.; Siyam, A.; Tarawneh, R.; Fajkovic, H.; Schernhammer, E.; et al. Prevalence and trends in kidney stone among adults in the USA: Analyses of national health and nutrition examination survey 2007–2018 data. Eur. Urol. Focus 2021, 7, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Giarrusso, P.; Raio, C.; Bhagavath, A.; Kalu, C.; Schwartz, A.; Klein, L. Do Emergency Department Observation Units Help Prevent Revisits for Patients with Renal Colic? Am. J. Emerg. Med. 2024, 89, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Pearle, M.S.; Goldfarb, D.S.; Assimos, D.G.; Curhan, G.; Denu-Ciocca, C.J.; Matlaga, B.R.; Monga, M.; Penniston, K.L.; Preminger, G.M.; Turk, T.M.; et al. Medical management of kidney stones: AUA guideline. J. Urol. 2014, 192, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Coe, F.L.; Evan, A.; Worcester, E. Kidney stone disease. J. Clin. Investig. 2005, 115, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Fink, H.A.; Wilt, T.J.; Eidman, K.E.; Garimella, P.S.; MacDonald, R.; Rutks, I.R.; Brasure, M.; Kane, R.L.; Ouellette, J.; Monga, M. Medical management to prevent recurrent nephrolithiasis in adults: A systematic review for an American College of Physicians Clinical Guideline. Ann. Intern. Med. 2013, 158, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, L.P.; Marins, M.A.; da Silva, E.A.; Lima Netto, S. Variational autoencoder. In Variational Methods for Machine Learning with Applications to Deep Networks; Springer: Berlin/Heidelberg, Germany, 2021; pp. 111–149. [Google Scholar]
- Chen, T.; He, T.; Benesty, M.; Khotilovich, V.; Tang, Y.; Cho, H.; Chen, K.; Mitchell, R.; Cano, I.; Zhou, T.; et al. Xgboost: Extreme Gradient Boosting, R Package Version 0.4-2; SCIRP Open Access: Irvine, CA, USA, 2015; Volume 1, pp. 1–4. [Google Scholar]
- Hearst, M.A.; Dumais, S.T.; Osuna, E.; Platt, J. Scholkopf, Support vector machines. IEEE Intell. Syst. Their Appl. 1998, 13, 18–28. [Google Scholar] [CrossRef]
- Guo, G.; Wang, H.; Bell, D.; Bi, Y.; Greer, K. KNN model-based approach in classification. In Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2003; Volume 2888, pp. 986–996. [Google Scholar] [CrossRef]
- Rokach, L.; Maimon, O. Decision Trees, Data Min. Knowl. Discov. Handb. 2006, 165–192. [Google Scholar] [CrossRef]
- Sorokin, I.; Mamoulakis, C.; Miyazawa, K.; Rodgers, A.; Talati, J.; Lotan, Y. Epidemiology of stone disease across the world. World J. Urol. 2017, 35, 1301–1320. [Google Scholar] [CrossRef]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine learning in medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef]
- Skolarikos, A.; Somani, B.; Neisius, A.; Jung, H.; Petřík, A.; Tailly, T.; Davis, N.; Tzelves, L.; Geraghty, R.; Lombardo, R.; et al. Metabolic evaluation and recurrence prevention for urinary stone patients: An EAU guidelines update. Eur. Urol. 2024, 86, 343–363. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, J.; Zhang, J.; Xu, C.; Wang, X.; Liu, C. Development and validation of a machine learning-based prediction model for urinary calculi recurrence. Urolithiasis 2025, 53, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, F.; Andishgar, A.; Mahmoudi, E.; Monsef, A.; Bazmi, S.; Tabrizi, R. Predicting symptomatic kidney stones using machine learning algorithms: Insights from the Fasa adults cohort study (FACS). BMC Res. Notes 2024, 17, 318. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Bae, H. Associations between multiple inflammatory biomarkers and the risk of developing kidney stones. BMC Urol. 2025, 25, 48. [Google Scholar] [CrossRef] [PubMed]
- Eyre, K.S.; Lewis, F.; Cui, H.; Grout, E.; Mihai, R.; Turney, B.W.; Howles, S.A. Utility of blood tests in screening for metabolic disorders in kidney stone disease. BJU Int. 2021, 127, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Pang, P.C.-I.; Chen, C.; Zheng, Q.; Zhang, C.; Li, J.; Guo, J.; Mao, C.; He, Y. Automatic kidney stone identification: An adaptive feature-weighted LSTM model based on urine and blood routine analysis. Urolithiasis 2024, 52, 145. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Chen, X.-Y.; Guo, H. Clinical characteristics and nomogram model for predicting the risk of recurrence of complicated urinary tract infection in pediatric patients. Sci. Rep. 2024, 14, 25393. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; Taylor, E.N.; Eisner, B.H.; Gambaro, G.; Rimm, E.B.; Mukamal, K.J.; Curhan, G.C. History of kidney stones and the risk of coronary heart disease. JAMA 2013, 310. [Google Scholar] [CrossRef] [PubMed]

| Part | Variable | Description | Unit |
|---|---|---|---|
| Part A | A1 | Height | cm |
| A2 | Weight | kg | |
| A3 | Waist Circumference | cm | |
| A4 | Hip Circumference | cm | |
| A5 | Age | years | |
| Part B | B1 | Gender | 1: Male, 2: Female |
| B2 | Education Level | 1: Literate, 2: Primary Education, 3: High School, 4: Bachelor’s Degree, 5: Master’s Degree | |
| B3 | Diabetes | 1: No, 2: Yes | |
| B4 | Hypertension | 1: No, 2: Yes | |
| B5 | Cardiovascular Disease | 1: No, 2: Yes | |
| B6 | Inflammatory Bowel Disease | 1: No, 2: Yes | |
| B7 | History of Bowel Surgery | 1: No, 2: Yes | |
| B8 | Family History of Stone Disease | 1: No, 2: Yes | |
| B9 | Daily Water Consumption | L/day | |
| B10 | Daily Physical Activity | 0: Low, 1: Routine Daily Activities, 2: Regular Exercise | |
| B11 | Tea Consumption | 0: None, 1: 1–2 cups/day, 2: 4–5 cups/day, 3: >5 cups/day | |
| B12 | Coffee Consumption | 0: None, 1: 1–2 cups/day, 2: 4–5 cups/day, 3: >5 cups/day | |
| B13 | Salt Intake | 0: None, 1: Low, 2: Normal, 3: High | |
| B14 | Animal Protein Intake | 0: Low, 1: Normal, 2: High | |
| B15 | Climate Type | 0: Mild, 1: Cold, 2: Hot | |
| B16 | Occupation Type | 1: Active, 2: Sedentary | |
| B17 | Medication for Stone Formation | 0: No, 1: Yes | |
| B18 | Sweating Level | 0: None, 1: Little, 2: High | |
| B19 | Smoking | 0: No, 1: Yes | |
| B20 | Alcohol Consumption | 0: No, 1: Yes | |
| Part C | C1 | Body Mass Index | BMI |
| C2 | A Body Shape Index | ABSI | |
| C3 | Body Roundness Index | BRI | |
| C4 | Waist-to-Height Ratio | WHtR | |
| C5 | Conicity Index | CI | |
| C6 | Waist-to-Hip Ratio | WHR |
| Classifiers | Classes | Accuracy | Precision | Recall | F1-Score |
|---|---|---|---|---|---|
| XGBoost | Recurrence (−) | 71.18 | 79.00 | 71.00 | 75.00 |
| Recurrence (+) | 79.62 | 72.00 | 80.00 | 75.00 | |
| Cubic SVM | Recurrence (−) | 72.88 | 67.00 | 73.00 | 70.00 |
| Recurrence (+) | 61.11 | 67.00 | 61.00 | 64.00 | |
| Fine KNN | Recurrence (−) | 69.49 | 72.00 | 69.00 | 71.00 |
| Recurrence (+) | 70.37 | 68.00 | 70.00 | 69.00 | |
| DT | Recurrence (−) | 71.18 | 75.00 | 71.00 | 73.00 |
| Recurrence (+) | 74.07 | 70.00 | 74.00 | 72.00 |
| Model | Autoencoder | Accuracy | Precision | Recall | F1-Score |
|---|---|---|---|---|---|
| Cubic SVM | No | 58.41 | 58.64 | 58.41 | 57.14 |
| Fine KNN | No | 68.14 | 68.00 | 62.96 | 65.38 |
| DT | No | 64.60 | 62.96 | 62.96 | 62.96 |
| XGBoost | No | 71.68 | 71.15 | 68.52 | 69.81 |
| Cubic SVM | Yes | 67.26 | 67.35 | 61.11 | 64.10 |
| Fine KNN | Yes | 69.91 | 67.86 | 70.37 | 69.11 |
| DT | Yes | 72.57 | 70.18 | 74.07 | 72.04 |
| XGBoost | Yes | 75.22 | 71.67 | 79.63 | 75.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasar, H.; Yildirim, K.; Karaduman, M.; Kolcu, B.; Ezer, M.; Suceken, F.Y.; Bicaklioğlu, F.; Aydin, M.E.; Kaya, C.; Yildirim, M.; et al. Evaluation of the Risk of Urinary System Stone Recurrence Using Anthropometric Measurements and Lifestyle Behaviors in a Developed Artificial Intelligence Model. Diagnostics 2025, 15, 2643. https://doi.org/10.3390/diagnostics15202643
Yasar H, Yildirim K, Karaduman M, Kolcu B, Ezer M, Suceken FY, Bicaklioğlu F, Aydin ME, Kaya C, Yildirim M, et al. Evaluation of the Risk of Urinary System Stone Recurrence Using Anthropometric Measurements and Lifestyle Behaviors in a Developed Artificial Intelligence Model. Diagnostics. 2025; 15(20):2643. https://doi.org/10.3390/diagnostics15202643
Chicago/Turabian StyleYasar, Hikmet, Kadir Yildirim, Mucahit Karaduman, Bayram Kolcu, Mehmet Ezer, Ferhat Yakup Suceken, Fatih Bicaklioğlu, Mehmet Erhan Aydin, Coskun Kaya, Muhammed Yildirim, and et al. 2025. "Evaluation of the Risk of Urinary System Stone Recurrence Using Anthropometric Measurements and Lifestyle Behaviors in a Developed Artificial Intelligence Model" Diagnostics 15, no. 20: 2643. https://doi.org/10.3390/diagnostics15202643
APA StyleYasar, H., Yildirim, K., Karaduman, M., Kolcu, B., Ezer, M., Suceken, F. Y., Bicaklioğlu, F., Aydin, M. E., Kaya, C., Yildirim, M., & Sarica, K. (2025). Evaluation of the Risk of Urinary System Stone Recurrence Using Anthropometric Measurements and Lifestyle Behaviors in a Developed Artificial Intelligence Model. Diagnostics, 15(20), 2643. https://doi.org/10.3390/diagnostics15202643







