Diagnostic Utility of PRAME Expression in Melanocytic Lesions: Cut-Off Threshold Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Immunohistochemistry
2.3. Evaluation
3. Results
3.1. Clinical and Histopathological Results
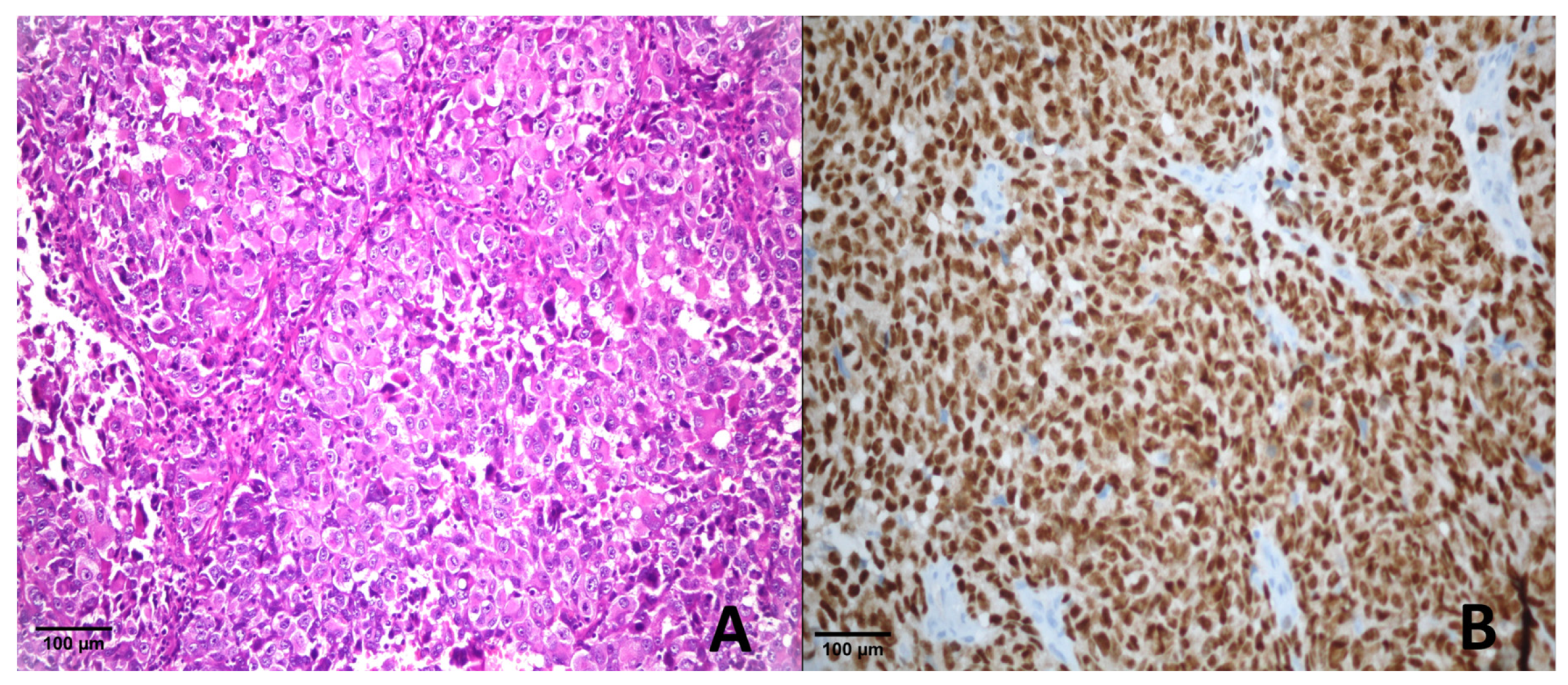
3.2. Immunohistochemical Results
3.2.1. Comparison of PRAME Cut-Off Groups in Melanoma and Benign Lesions
3.2.2. PRAME Immunoreactivity in Melanoma Subtypes
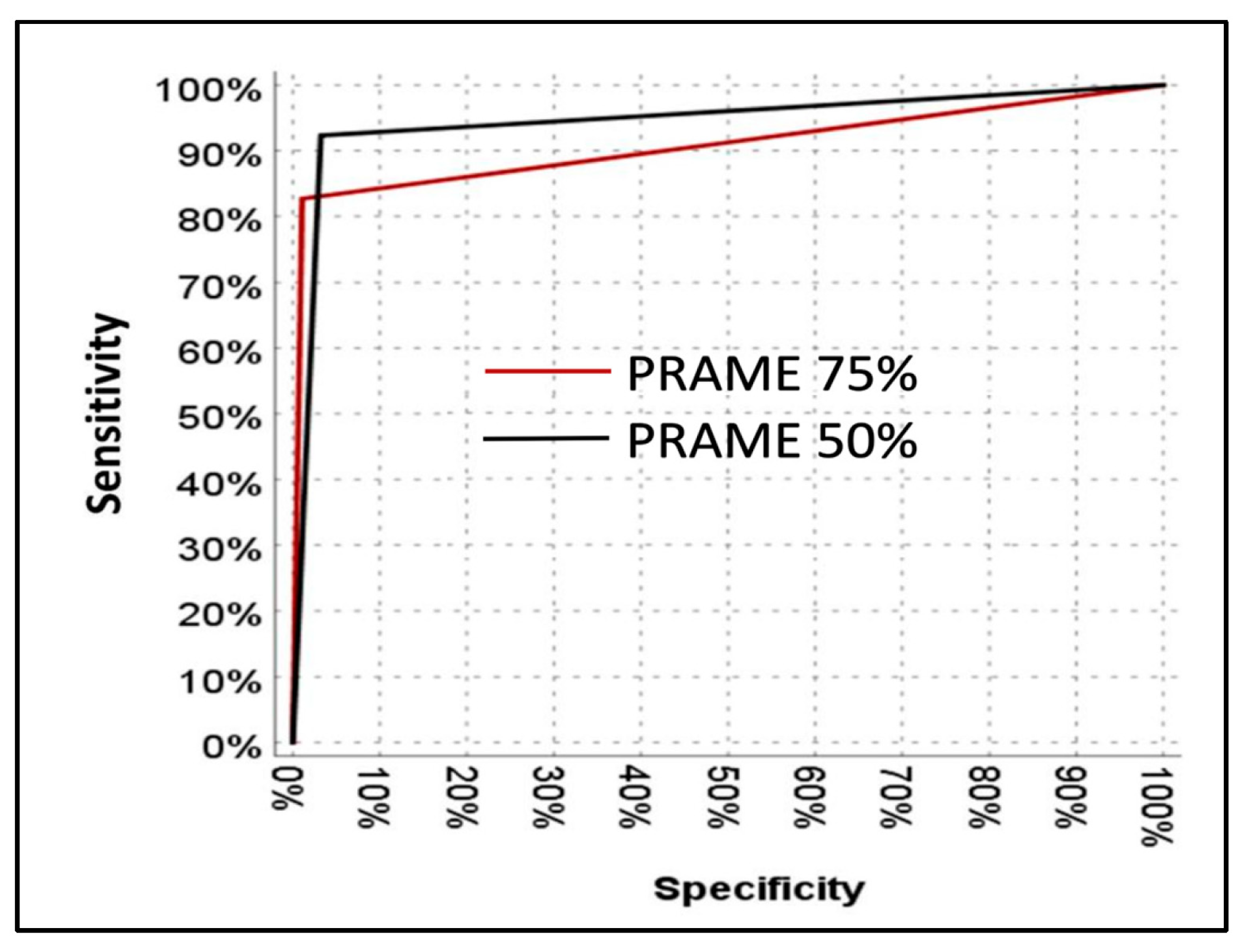
3.2.3. Sensitivity and Specificity of PRAME in Melanoma vs. Benign Melanocytic Lesions
4. Discussion
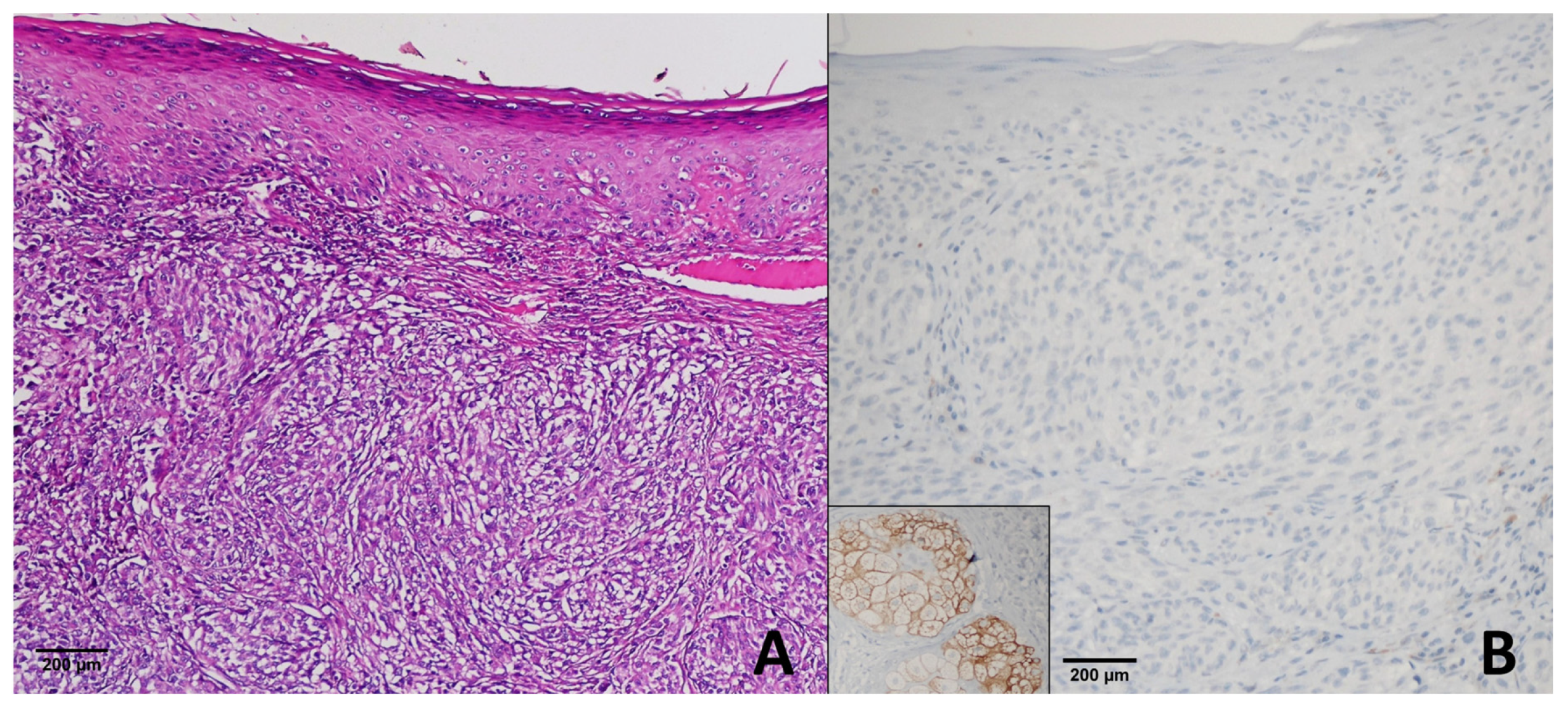
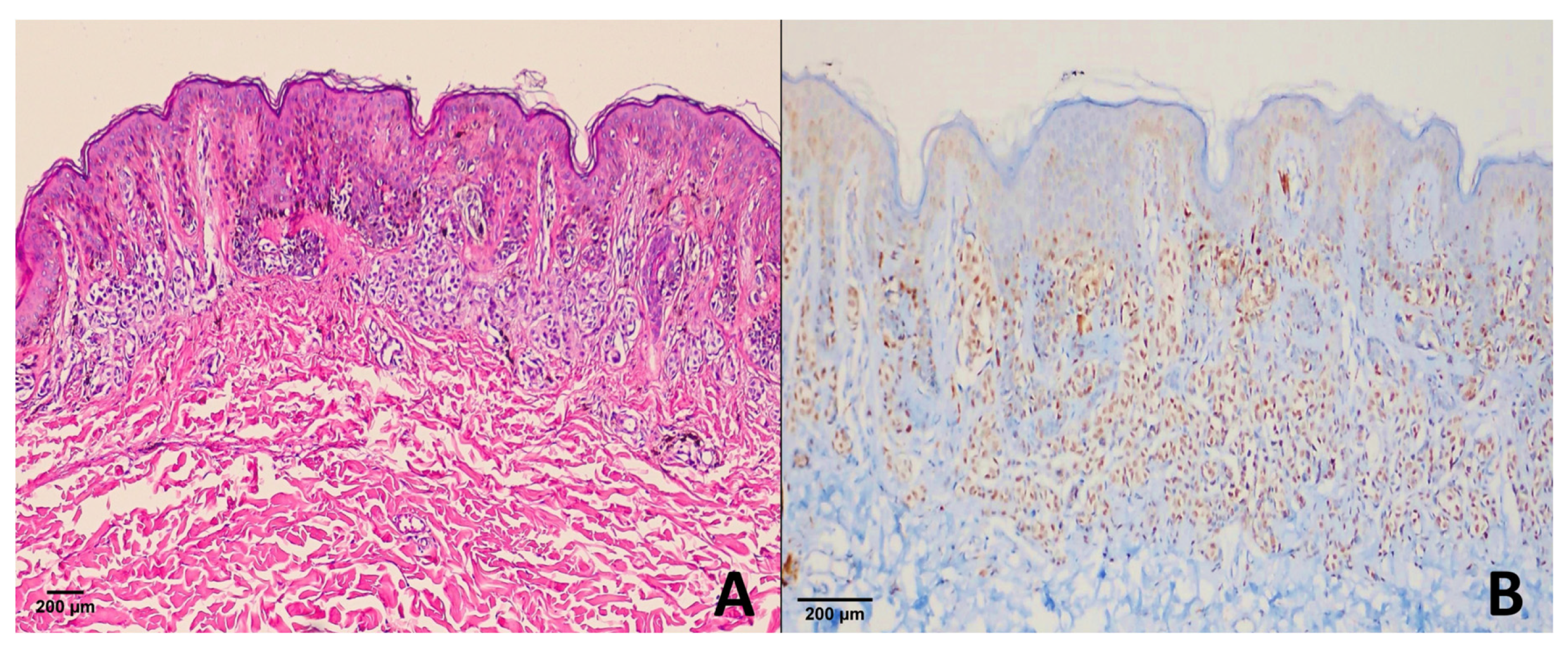
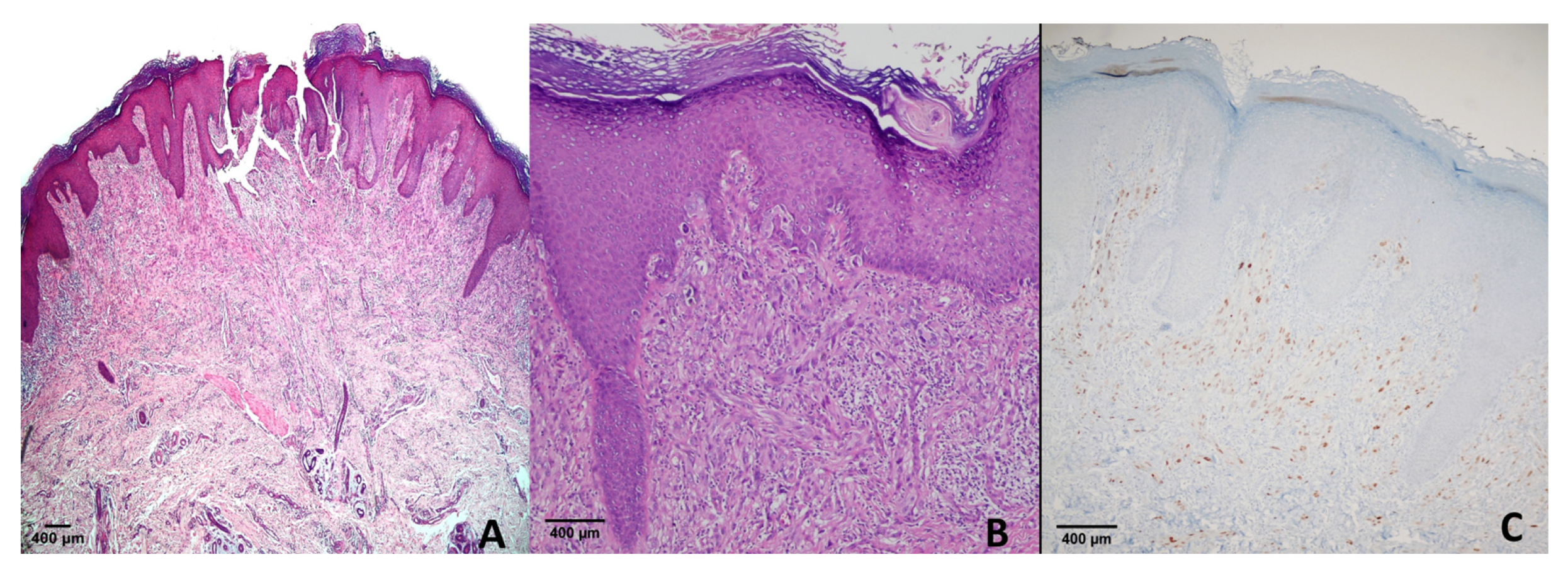
4.1. Dysplastic Nevi
4.2. Congenital, Blue and Benign Nevi
4.3. Spitz Nevus
4.4. Melanoma
4.4.1. Metastatic Melanoma
4.4.2. Primary Melanoma
Mucosal Melanoma
Cutaneous Melanoma Subtypes
4.5. Threshold Selection
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALM | Acral Lentiginous Melanoma |
| CI | Confidence Interval |
| H & E | Hematoxylin and Eosin |
| LM | Lentigo Maligna |
| LMM | Lentigo Maligna Melanoma |
| NM | Nodular Melanoma |
| NPV | Negative Predictive Value |
| PRAME | Preferentially Expressed Antigen in Melanoma |
| PV | Positive Predictive Value |
| RA | Retinoic Acid |
| SSM | Superficial Spreading Melanoma |
References
- Carr, S.; Smith, C.; Wernberg, J. Epidemiology and Risk Factors of Melanoma. Surg. Clin. 2020, 100, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Markovic, S.N.; Erickson, L.A.; Rao, R.D.; McWilliams, R.R.; Kottschade, L.A.; Creagan, E.T.; Weenig, R.H.; Hand, J.L.; Pittelkow, M.R.; Pockaj, B.A. Malignant Melanoma in the 21st Century, Part 1: Epidemiology, Risk Factors, Screening, Prevention, and Diagnosis; Elsevier: Amsterdam, The Netherlands, 2007; Volume 82, pp. 364–380. [Google Scholar]
- Gheoca Mutu, D.-E.; Avino, A.; Balcangiu-Stroescu, A.-E.; Mehedințu, M.; Bălan, D.G.; Brîndușe, L.A.; Popescu, A.-M.; Ionescu, D.; Cristea, B.-M.; Tomescu, L.F.; et al. Histopathological Evaluation of Cutaneous Malignant Melanoma: A Retrospective Study. Exp. Ther. Med. 2022, 23, 402. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.; Spatz, A.; Robert, C. Cutaneous Melanoma. Lancet 2014, 383, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- Elder, D.E. Histology of the Skin-Melanocytes. In Lever’s Histopathology of the Skin; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2015; pp. 38–43. ISBN 978-1-4511-9037-3. [Google Scholar]
- Elder, D.E.; Xu, X. The Approach to the Patient with a Difficult Melanocytic Lesion. Pathology 2004, 36, 428–434. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Oh, K.S.; Alexis, J. Potential Diagnostic Utility of PRAME and P16 Immunohistochemistry in Melanocytic Nevi and Malignant Melanoma. J. Cutan. Pathol. 2023, 50, 763–772. [Google Scholar] [CrossRef]
- Lam, G.T.; Prabhakaran, S.; Sorvina, A.; Martini, C.; Ung, B.S.-Y.; Karageorgos, L.; Hickey, S.M.; Lazniewska, J.; Johnson, I.R.D.; Williams, D.B.; et al. Pitfalls in Cutaneous Melanoma Diagnosis and the Need for New Reliable Markers. Mol. Diagn. Ther. 2023, 27, 49–60. [Google Scholar] [CrossRef]
- Ikeda, H.; Lethé, B.; Lehmann, F.; Baren, N.V.; Baurain, J.-F.; Smet, C.D.; Chambost, H.; Vitale, M.; Moretta, A.; Boon, T.; et al. Characterization of an Antigen That Is Recognized on a Melanoma Showing Partial HLA Loss by CTL Expressing an NK Inhibitory Receptor. Immunity 1997, 6, 199–208. [Google Scholar] [CrossRef]
- Wadelin, F.; Fulton, J.; McEwan, P.A.; Spriggs, K.A.; Emsley, J.; Heery, D.M. Leucine-Rich Repeat Protein PRAME: Expression, Potential Functions and Clinical Implications for Leukaemia. Mol. Cancer 2010, 9, 226. [Google Scholar] [CrossRef]
- Epping, M.T.; Wang, L.; Edel, M.J.; Carlée, L.; Hernandez, M.; Bernards, R. The Human Tumor Antigen PRAME Is a Dominant Repressor of Retinoic Acid Receptor Signaling. Cell 2005, 122, 835–847. [Google Scholar] [CrossRef]
- Gezgin, G.; Luk, S.J.; Cao, J.; Dogrusöz, M.; van der Steen, D.M.; Hagedoorn, R.S.; Krijgsman, D.; van der Velden, P.A.; Field, M.G.; Luyten, G.P.M.; et al. PRAME as a Potential Target for Immunotherapy in Metastatic Uveal Melanoma. JAMA Ophthalmol. 2017, 135, 541–549. [Google Scholar] [CrossRef]
- Lohman, M.E.; Steen, A.J.; Grekin, R.C.; North, J.P. The Utility of PRAME Staining in Identifying Malignant Transformation of Melanocytic Nevi. J. Cutan. Pathol. 2021, 48, 856–862. [Google Scholar] [CrossRef]
- O’Connor, M.K.; Dai, H.; Fraga, G.R. PRAME Immunohistochemistry for Melanoma Diagnosis: A STARD-Compliant Diagnostic Accuracy Study. J. Cutan. Pathol. 2022, 49, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Zboraș, I.; Ungureanu, L.; Șenilă, S.; Petrushev, B.; Zamfir, P.; Crișan, D.; Zaharie, F.A.; Vesa, Ș.C.; Cosgarea, R. PRAME Immunohistochemistry in Thin Melanomas Compared to Melanocytic Nevi. Diagnostics 2024, 14, 2015. [Google Scholar] [CrossRef] [PubMed]
- Salih, R.; Ismail, F.; Orchard, G.E. Double Immunohistochemical Labelling of PRAME and Melan A in Slow Mohs Biopsy Margin Assessment of Lentigo Maligna and Lentigo Maligna Melanoma. Br. J. Biomed. Sci. 2024, 81, 12319. [Google Scholar] [CrossRef]
- Cassalia, F.; Danese, A.; Tudurachi, I.; Federico, S.; Zambello, A.; Guidotti, A.; Franceschin, L.; Bolzon, A.; Naldi, L.; Belloni Fortina, A. PRAME Updated: Diagnostic, Prognostic, and Therapeutic Role in Skin Cancer. Int. J. Mol. Sci. 2024, 25, 1582. [Google Scholar] [CrossRef]
- Enevoldsen, J.; Brogård, M.B.; Lade-Keller, J.; Christensen, K.B.; Georgsen, J.B.; Nielsen, P.S.; Steiniche, T. Digital Quantification of PRAME for Distinguishing Melanoma from Nevi Compared to Manual Assessment. Pathol.—Res. Pract. 2024, 262, 155543. [Google Scholar] [CrossRef] [PubMed]
- Lezcano, C.; Jungbluth, A.A.; Nehal, K.S.; Hollmann, T.J.; Busam, K.J. PRAME Expression in Melanocytic Tumors. Am. J. Surg. Pathol. 2018, 42, 1456–1465. [Google Scholar] [CrossRef]
- Raghavan, S.S.; Wang, J.Y.; Kwok, S.; Rieger, K.E.; Novoa, R.A.; Brown, R.A. PRAME Expression in Melanocytic Proliferations with Intermediate Histopathologic or Spitzoid Features. J. Cutan. Pathol. 2020, 47, 1123–1131. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Zhang, W.-W.; Qiu, Y.-T.; Ke, L.-F.; Chen, H.; Chen, G. PRAME Is a Useful Marker for the Differential Diagnosis of Melanocytic Tumours and Histological Mimics. Histopathology 2023, 82, 285–295. [Google Scholar] [CrossRef]
- Gassenmaier, M.; Hahn, M.; Metzler, G.; Bauer, J.; Yazdi, A.S.; Keim, U.; Garbe, C.; Wagner, N.B.; Forchhammer, S. Diffuse PRAME Expression Is Highly Specific for Thin Melanomas in the Distinction from Severely Dysplastic Nevi but Does Not Distinguish Metastasizing from Non-Metastasizing Thin Melanomas. Cancers 2021, 13, 3864. [Google Scholar] [CrossRef]
- Cazzato, G.; Mangialardi, K.; Falcicchio, G.; Colagrande, A.; Ingravallo, G.; Arezzo, F.; Giliberti, G.; Trilli, I.; Loizzi, V.; Lettini, T.; et al. Preferentially Expressed Antigen in Melanoma (PRAME) and Human Malignant Melanoma: A Retrospective Study. Genes 2022, 13, 545. [Google Scholar] [CrossRef]
- Kline, N.; Menge, T.D.; Hrycaj, S.M.; Andea, A.A.; Patel, R.M.; Harms, P.W.; Chan, M.P.; Bresler, S.C. PRAME Expression in Challenging Dermal Melanocytic Neoplasms and Soft Tissue Tumors With Melanocytic Differentiation. Am. J. Dermatopathol. 2022, 44, 404. [Google Scholar] [CrossRef] [PubMed]
- Rasic, D.; Korsgaard, N.; Marcussen, N.; Precht Jensen, E.M. Diagnostic Utility of Combining PRAME and HMB-45 Stains in Primary Melanocytic Tumors. Ann. Diagn. Pathol. 2023, 67, 152211. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.S.; Lau, S.K.; Scapa, J.V.; Cassarino, D.S. PRAME Immunohistochemistry of Spitzoid Neoplasms. J. Cutan. Pathol. 2022, 49, 709–716. [Google Scholar] [CrossRef]
- Googe, P.B.; Flanigan, K.L.; Miedema, J.R. Preferentially Expressed Antigen in Melanoma Immunostaining in a Series of Melanocytic Neoplasms. Am. J. Dermatopathol. 2021, 43, 794. [Google Scholar] [CrossRef]
- Gradecki, S.E.; Slingluff, C.L., Jr.; Gru, A.A. PRAME Expression in 155 Cases of Metastatic Melanoma. J. Cutan. Pathol. 2021, 48, 479–485. [Google Scholar] [CrossRef]
- Kaczorowski, M.; Chłopek, M.; Kruczak, A.; Ryś, J.; Lasota, J.; Miettinen, M. PRAME Expression in Cancer. A Systematic Immunohistochemical Study of >5800 Epithelial and Nonepithelial Tumors. Am. J. Surg. Pathol. 2022, 46, 1467. [Google Scholar] [CrossRef] [PubMed]
- Scheurleer, W.F.J.; Braunius, W.W.; Tijink, B.M.; Suijkerbuijk, K.P.M.; Dierselhuis, M.P.; Meijers, R.W.J.; Blokx, W.A.M.; de Bree, R.; Breimer, G.E.; Rijken, J.A. PRAME Staining in Sinonasal Mucosal Melanoma: A Single-Center Experience. Head Neck Pathol. 2023, 17, 401–408. [Google Scholar] [CrossRef]
- Toyama, A.; Siegel, L.; Nelson, A.C.; Najmuddin, M.; Bu, L.; LaRue, R.; Henzler, C.; Caicedo-Granados, E.; Giubellino, A.; Li, F. Analyses of Molecular and Histopathologic Features and Expression of PRAME by Immunohistochemistry in Mucosal Melanomas. Mod. Pathol. 2019, 32, 1727–1733. [Google Scholar] [CrossRef]
- Kunc, M.; Żemierowska, N.; Skowronek, F.; Biernat, W. Diagnostic Test Accuracy Meta-Analysis of PRAME in Distinguishing Primary Cutaneous Melanomas from Benign Melanocytic Lesions. Histopathology 2023, 83, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Alomari, A.K.; Tharp, A.W.; Umphress, B.; Kowal, R.P. The Utility of PRAME Immunohistochemistry in the Evaluation of Challenging Melanocytic Tumors. J. Cutan. Pathol. 2021, 48, 1115–1123. [Google Scholar] [CrossRef]
- Rawson, R.V.; Shteinman, E.R.; Ansar, S.; Vergara, I.A.; Thompson, J.F.; Long, G.V.; Scolyer, R.A.; Wilmott, J.S. Diagnostic Utility of PRAME, P53 and 5-hmC Immunostaining for Distinguishing Melanomas from Naevi, Neurofibromas, Scars and Other Histological Mimics. Pathology 2022, 54, 863–873. [Google Scholar] [CrossRef] [PubMed]
- See, S.H.C.; Finkelman, B.S.; Yeldandi, A.V. The Diagnostic Utility of PRAME and P16 in Distinguishing Nodal Nevi from Nodal Metastatic Melanoma. Pathol. Res. Pract. 2020, 216, 153105. [Google Scholar] [CrossRef] [PubMed]
| Age Range | 6.0–93.0 | |
|---|---|---|
| Average Age | 41.2 | |
| Gender | Female | 69/145 |
| Male | 76/145 | |
| Diagnosis | Primary malignant melanoma | 40/145 |
| Metastatic Melanoma | 12/145 | |
| Dysplastic Nevus | 27/145 | |
| Blue Nevus | 23/145 | |
| Compound Nevus | 15/145 | |
| Spitz Nevus | 23/145 | |
| Congenital Nevus | 5/145 | |
| Location | Trunk | 56/145 |
| Extremity | 47/145 | |
| Head and Neck | 34/145 | |
| Lymph node | 5/145 | |
| Internal organs | 3/145 | |
| Score | Metastatic Melanoma | Melanoma (Primary) | Dysplastic Nevus | Congenital Nevus | Compound Nevus | Blue Nevus | Spitz Nevus | Atypical Spitz Nevus |
|---|---|---|---|---|---|---|---|---|
| +4 | 8/12 | 35/40 | 1/27 | 0/5 | 0/15 | 0/23 | 0/11 | 0/12 |
| +3 | 2/12 | 3/40 | 0/27 | 0/5 | 0/15 | 0/23 | 0/11 | 2/12 |
| +2 | 2/12 | 1/40 | 0/27 | 0/5 | 0/15 | 0/23 | 0/11 | 0/12 |
| +1 | 0/12 | 0/40 | 2/27 | 0/5 | 1/15 | 0/23 | 1/11 | 0/12 |
| 0 | 0/12 | 1/40 | 24/27 | 5/5 | 14/15 | 23/23 | 10/11 | 10/12 |
| Benign | Malignant | Sensitivity | PPV | Specificity | NPV | p | ||
|---|---|---|---|---|---|---|---|---|
| PRAME | <75% | 92 | 9 | 82.7% | 97.7% | 98.9% | 91.1% | <0.001 |
| ≥75% | 1 | 43 | ||||||
| PRAME | <50% | 90 | 4 | 92.3% | 94.1% | 96.8% | 95.7% | <0.001 |
| ≥50% | 3 | 48 |
| PRAME ≥ 75% | PRAME ≥ 50% | |
|---|---|---|
| Metastatic melanoma | 8/12 (66.7%) | 10/12 (83.3%) |
| Superficial spreading melanoma | 7/10 (70%) | 8/10 (80%) |
| Nodular melanoma | 4/5 (80%) | 5/5 (100%) |
| Acral lentiginous melanoma | 5/5 (100%) | 5/5 (100%) |
| Lentigo maligna melanoma | 3/3 (100%) | 3/3 (100%) |
| Lentigo maligna (in situ) | 9/10 (90%) | 9/10 (90%) |
| Mucosal melanoma | 2/2 (100%) | 2/2 (100%) |
| Study | Threshold (%) | Sensitivity (%) | Specificity (%) | Clone |
|---|---|---|---|---|
| Lezcano et al. [20] | 75 | 83 | 100 | EPR20330 |
| Raghavan et al. [21] | 60 | 92 | 98 | EPR20330 |
| Alomari et al. [34] | 75 | 53 | 86 | EPR20330 |
| Googe et al. [28] | 75 | 79 | 99 | EPR20330 |
| Gassenmaeier et al. [23] | 75 | 59 | 98 | QR005 |
| Lohman et al. [14] | 75 | 67 | 100 | EPR20330 |
| Lohman et al. [14] | 50 | 75 | 97 | EPR20330 |
| Gradecki et al. [29] | 75 | 41.3 | - | EPR20330 |
| Gradecki et al. [29] | 50 | 81 | - | EPR20330 |
| See et al. [36] | 50 | 94 | 100 | EPR20330 |
| Kaczorowski et al. [30] | 80 | 75 | - | EP461 |
| Our study | 75 | 82.7 | 98.9 | EP461 |
| Our study | 50 | 92.3 | 96.8 | EP461 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noyan Mod, B.; Leblebici, C. Diagnostic Utility of PRAME Expression in Melanocytic Lesions: Cut-Off Threshold Analysis. Diagnostics 2025, 15, 2595. https://doi.org/10.3390/diagnostics15202595
Noyan Mod B, Leblebici C. Diagnostic Utility of PRAME Expression in Melanocytic Lesions: Cut-Off Threshold Analysis. Diagnostics. 2025; 15(20):2595. https://doi.org/10.3390/diagnostics15202595
Chicago/Turabian StyleNoyan Mod, Beste, and Cem Leblebici. 2025. "Diagnostic Utility of PRAME Expression in Melanocytic Lesions: Cut-Off Threshold Analysis" Diagnostics 15, no. 20: 2595. https://doi.org/10.3390/diagnostics15202595
APA StyleNoyan Mod, B., & Leblebici, C. (2025). Diagnostic Utility of PRAME Expression in Melanocytic Lesions: Cut-Off Threshold Analysis. Diagnostics, 15(20), 2595. https://doi.org/10.3390/diagnostics15202595





