1. Introduction
Despite rapid advances in imaging, chest radiography (CXR) remains a pivotal aspect of medical diagnostics across the globe [
1,
2,
3,
4,
5]. The aortic knob, a landmark on posteroanterior CXRs, is viewed as an early indicator of aortic pathology [
3]. Historically, a widened aortic knob has been a subjective marker for pathological conditions like thoracic aortic aneurysms. Although some studies have explored normal dimensions [
2,
3,
5], there is no consensus on specific CXR thresholds for further evaluation. The decision making process for more advanced follow-up imaging like CTA remains based on individual expertise of the reader [
6]. At the same time, the presence of multiple imaging modalities makes development of standardized operating procedures a difficult task [
6,
7,
8].
The subjective interpretation of the aortic knob contributes to the known limitations of CXR in diagnosing aortic disease, with studies reporting variable sensitivity and specificity [
9,
10,
11]. Given that thoracic aortic aneurysms are often asymptomatic until they become life-threatening, early detection is crucial [
12]. While CTA is the gold standard for diagnosis, it is not suitable for general population screening.
This study evaluates the clinical utility of CXR-based measurements, focusing on aortic knob width in different orientations. We compared CXR parameters with CTA-based aortic arch dimensions to evaluate potential diagnostic performance and establish clinical relevance as a screening tool for aortic dilation.
2. Materials and Methods
This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. The research protocol received approval from the OIL Chamber of Physicians Ethical Council in Krakow (Approval No. 53/KBL/OIL/2025 from 15 July 2025).
2.1. Population
A retrospective search was conducted using the institutional PACS database to identify emergency department (ED) patients who had undergone both PA CXR and chest CTA within a 7-day interval. Exclusion criteria included: patient age < 18 years, inadequate chest CTA image quality (due to low contrast enhancement or motion artifacts), chest wall deformities, significant scoliosis, history of prior aortic surgery or endovascular repair, and acute aortic syndromes.
2.2. Measurement and Definitions
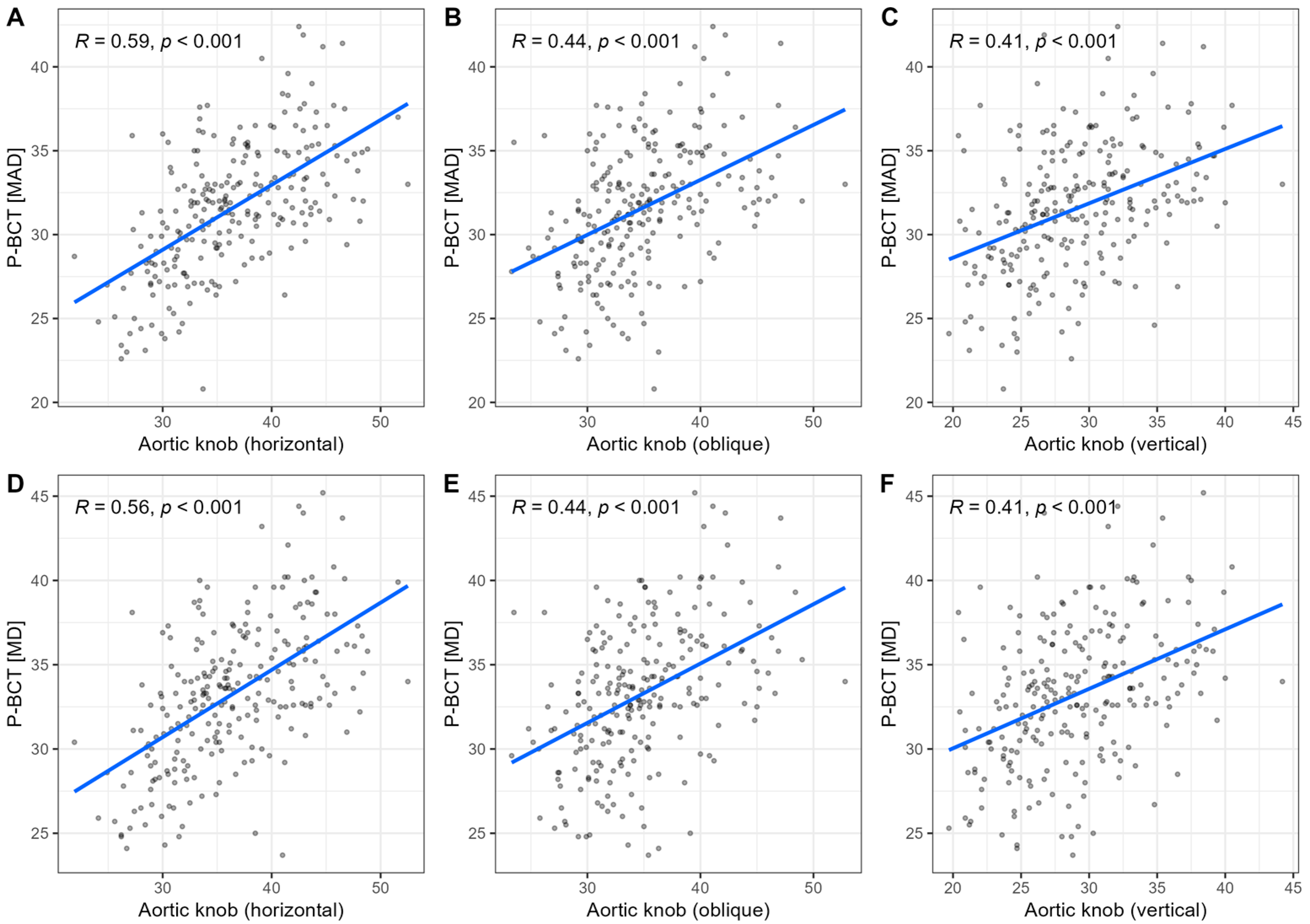
Two thoracic aortic levels were selected for CTA analysis: (1) the ascending aorta just proximal to the origin of the brachiocephalic trunk (P-BCT level) and (2) the aortic arch just distal to the origin of the left subclavian artery (D-LSA level). These segments represent the regions of the aortic arch that form the aortic knob silhouette on chest radiographs.
On CXR, the aortic knob was measured in three dimensions (see
Figure 1):
The horizontal diameter was defined as the maximum distance between the left border of the trachea and the most lateral projection of the aortic knob.
The oblique diameter was measured as the longest distance between any two visible borders of the knob.
The vertical diameter extended from the uppermost margin of the knob to the lowest visible shadow indicating the downward bend of the aortic arch.
Additional radiographic parameters included patient rotation, assessed by comparing the distance between each sternal end of clavicle and the closest spinous process; chest width, measured at the level of the diaphragm; and the presence of aortic arch calcifications, evaluated visually. On CTAs, we further assessed the span of the aortic arch by measuring the longest horizontal and vertical distances between visible aortic segments at the axial slice corresponding to the bifurcation of the pulmonary trunk. Calcifications of the arch were also recorded.
All image measurements were independently performed by three radiologists with expertise in interpreting chest radiographs and CT angiography. CXR measurements were conducted using RadiAnt DICOM Viewer (Medixant, Poznań, Poland), while CTA measurements were carried out using syngo.via software version VB80 (Siemens Healthineers, Erlangen, Germany). Representative examples of the measurement methodology are shown in
Figure 1.
2.3. Statistical Analysis
Statistical analyses were conducted using R version 4.4.1 (R Core Team, 2024; R Foundation for Statistical Computing, Vienna, Austria). Nominal variables were presented as counts and percentages (N, %). The distribution of continuous variables was assessed through density plots, the Shapiro-Wilk & Anderson-Darling tests, with supplemental measures of skewness. Continuous data were summarized as either mean (standard deviation) or median (interquartile range), as deemed appropriate. Robust regression models were developed using generalized additive models (GAMs using the mgcv package). Variable selection was guided by a combination of domain expertise and machine learning techniques. Random forest (RF) models were constructed using default settings to minimize overfitting, given sample size constraints and the avoidance of split-sample tuning. A parsimonious set of candidate predictors from 2D chest X-ray measurements was derived based on joint team discussions and the analysis of global variable importance plots.
No a priori sample size calculation was performed for this exploratory study. We provide confidence intervals and acknowledge that our findings require external validation. For tree-based predictive models or variable selection, no standardized methods for sample size calculation are available. These algorithms benefit from larger sample size and strong predictor variables, but multiple dataset characteristics can affect performance [
13].
2.4. Modeling Approach
We employed GAMs to analyze the relationship between chest radiography and CT measurements. These models are flexible enough to capture both linear and non-linear relationships in the data. Prior to GAM modeling, we utilized random forest (an aggregate decision tree model) to identify the most important predictive model features in a robust manner. Random forest algorithms enable complex, nonlinear modeling of relationships and are established as best-in-class tools for tabular data of moderate size [
14,
15]. Model optimization was performed using internal cross-validation techniques. To maximize interpretability, we report R
2 as the primary measure of model fit.
4. Discussion
This study demonstrates that simple, quantitative measurements of the aortic knob on routine posteroanterior chest radiographs can provide clinically useful insights. The salient findings of this report are: (1) CT-based aortic arch dimensions are moderately correlated with various measurements of aortic knob width on CXR; (2) the horizontal dimension is most closely related to P-BCT and D-LSA dimensions; (3) combining CXR measures with demographic information into a simple linear regression equation with square root transformation provides a readily accessible tool with high negative predictive value, pending further validation. If similar results are reported in various clinical populations, CXR assessments will be able to provide additional clinical reassurance and guide more advanced imaging decision making with high confidence.
When examining the three CXR dimensions of the aortic knob (horizontal, vertical, and oblique assessments), we observed that all measurements showed moderate strength inter-relationships with CT-based aortic parameters. However, the horizontal knob width was consistently observed to be most closely related to 3D dimensions. Our observations extend the findings of prior studies, in which the horizontal (transverse) or left mediastinal width was described as a useful and reliable measurement, contrasting with other CXR-based parameters or orientations [
3,
18]. Moderate-to-strong correlations between aortic knob measurements across different radiographic projections are likely to reflect three-dimensional aortic arch geometry. The square root transformation was also selected because aortic dimensions scale allometrically with body surface area. In contrast, weak correlations between transverse chest width and knob measurements suggest that aortic knob size is independent of overall thoracic dimensions and reflects intrinsic vascular geometry.
A major finding of our report is that the final model with age and sex-adjustment and square root transformation of horizontal knob width enables calculation of a prediction threshold that successfully identifies all cases of CT-detected aortic dilatation. We favored a simple model over the more mathematically complex GAM due to several theoretical considerations [
16,
17]. It should be emphasized that there was a low prevalence of dilation in our cohort, which necessitates caution (low pre-test probability) and reflects our approach to favor a very conservative rule, wherein sensitivity is prioritized over specificity. We also observed that inter- and intra-rater reliability for radiographic knob measurements, as well as CT-based aortic assessments was very high, which strengthens the confidence in reproducibility and clinical implications of this pilot study. Our findings support future efforts to incorporate standardized aortic knob assessment as an inherent part of routine CXR description protocols, particularly in older adults or patients with CV risk factors.
Our study focused on the segment of the thoracic aorta corresponding anatomically with the aortic knob silhouette; the distal ascending aorta and the transverse arch (i.e., between the origin of the P-BCT and D-LSA). We excluded aneurysms of the descending thoracic aorta from analysis, as they are much more likely to affect aortic shadow than just the knob itself. By limiting CT evaluations to these arch-specific region, we are likely to more confidently attribute knob enlargement on CXR to dilation of the arch, rather than adjacent structures. However, it should be kept in mind that aneurysms confined to the ascending aorta proximal to the brachiocephalic trunk may remain radiographically occult [
11,
19], as this portion usually does not significantly influence knob contour. At the same time, the prevalence of ascending aortic aneurysms is high [
20,
21,
22,
23].
Beyond age and sex considerations [
23], data from literature indicate that aortic knob width on CXR is associated with the presence of CV risk factors and overt CV disease. Therefore, when interpreting aortic knob measures, it is important to consider the clinical context. For example, in hypertensive populations, a prominent aortic knob is a common finding. Rayner et al. observed that patients with long-standing hypertension have significantly greater aortic knob widths on average (~3.7 cm), when compared to normotensive individuals (~3.3 cm) [
2]. Prior studies demonstrate associations between aortic knob width and hypertension-related markers of organ damage [
2], aortic pulse pressure [
24] as well as left ventricle diastolic function [
25]. Moreover, relationships with nontraditional CV risk factors, such as obstructive sleep apnea, should be considered [
26]. Due to these factors, the potential for selection bias represents a significant limitation of this report and our results require validation in heterogeneous clinical populations.
CXR based measurements of aortic dimensions have been reportd as useful in predicting aortic dissection [
18]. More recent studies incorporating machine-learning tools, such as neural networks, report on high detection accuracy of aortic dissection based on CXR data [
27]. However, the diagnostic limitations of CXR in detecting thoracic aortic aneurysms need to be emphasized [
9,
28]. Prior studies on CXR report sensitivity of 67% for overt dissection and 61% for non-dissecting aneurysm [
11]. Substantial overlap in knob dimensions between normal and aneurysmal cases remains a relevant risk. CXR cannot be used as a confirmatory tool but should be viewed as an initial screening test with moderate-to-high NPV value. Real-life cases emphasize the importance of maintaining a high index of suspicion when examining CXRs; even in younger individuals without overt risk factors, as aortic dissection may occur in persons of all ages, with identifiable early signs on CXR [
29].
With the ongoing search for CV biomarkers [
30], investigators have linked increased knob width to markers of atherosclerosis [
31,
32]. Some authors have described its adjunct value for predicting mortality in special populations, such as hemodialysis patients [
33]. Lee et al. demonstrated that aortic knob enlargement is independently associated with metabolic syndrome, implicating it as a surrogate for systemic atherosclerotic burden [
34]. Similarly, in patients with essential hypertension, a larger aortic knob correlates with greater carotid intima-media thickness, an ultrasound measure of arterial stiffness and atherosclerosis [
35]. These findings support the concept that an enlarged aortic knob on chest radiography represents not only an anatomic variant but also a potential indicator of broader CV risk.