Comparison of ADMIRE, SAFIRE, and Filtered Back Projection in Standard and Low-Dose Non-Enhanced Head CT
Abstract
1. Introduction
2. Materials and Methods
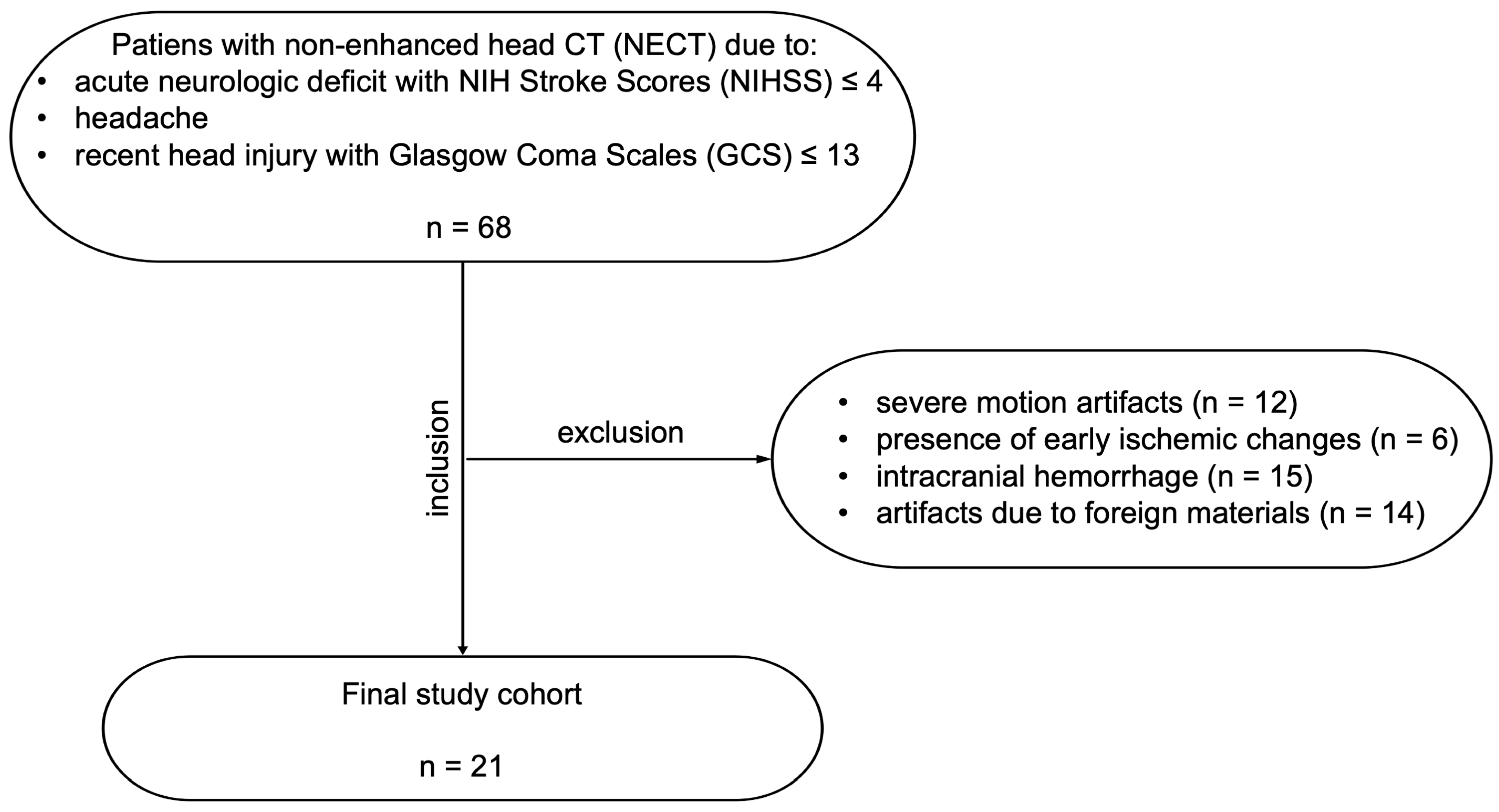
2.1. Study Design
2.2. Scanning Protocol and Reconstruction Parameters
2.3. Objective Assessment of Image Noise
2.4. Subjective Image Quality
2.5. Objective Image Quality
2.6. Statistical Analysis
3. Results
3.1. Study Population and Radiation Dose
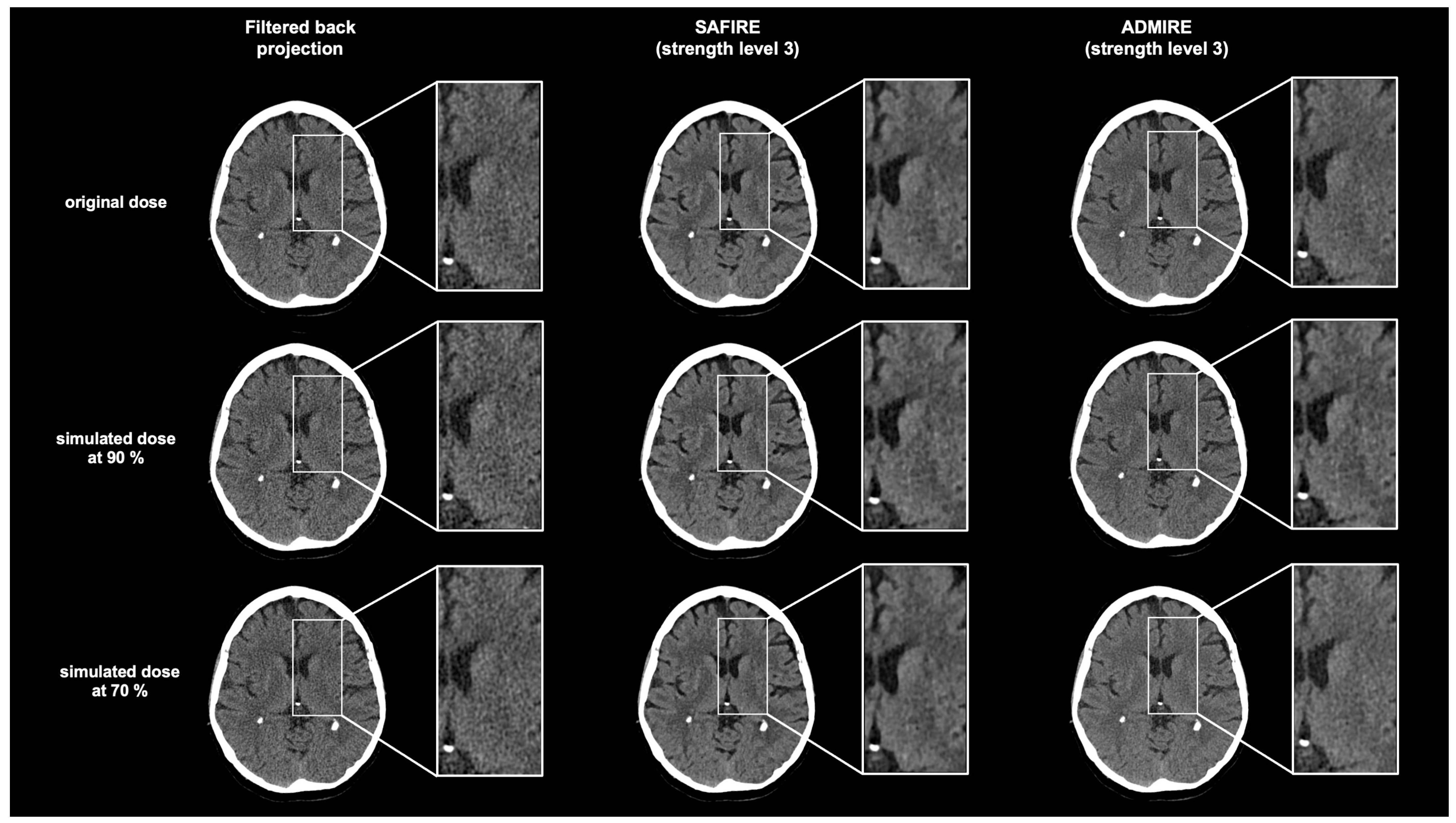
3.2. Image Noise
3.3. Subjective Image Quality Analysis
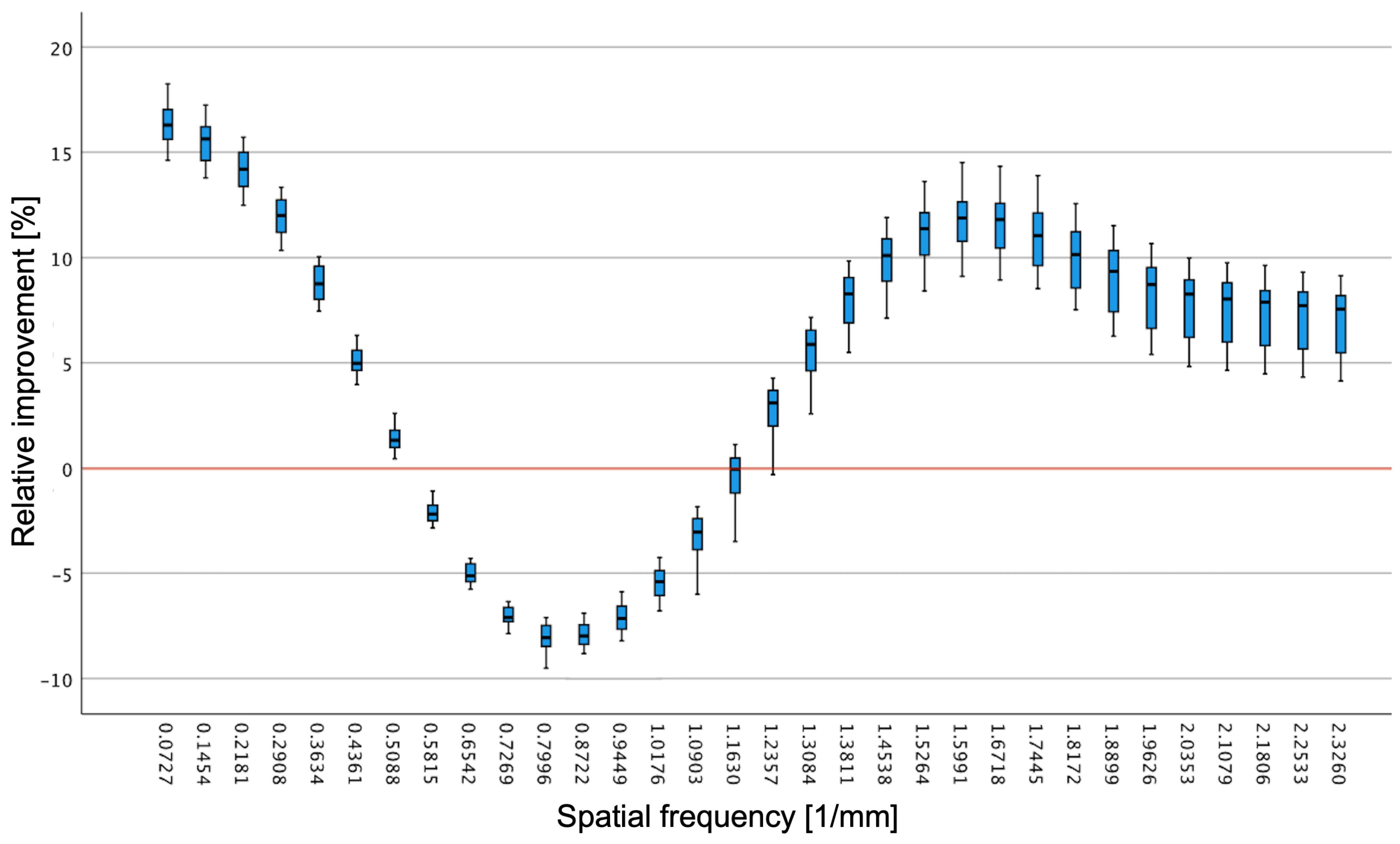
3.4. Objective Image Quality Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMIRE | Advanced modeled iterative reconstruction |
| ALARA | As low as reasonably achievable |
| CNR | Contrast-to-noise ratio |
| CT | Computed tomography |
| CTDI | Computed tomography dose index |
| DLP | Dose length product |
| FBP | Filtered back projection |
| IR | Iterative reconstruction |
| NECT | Non-enhanced head CT |
| NPS | Noise power spectrum |
| SAFIRE | Sinogram-affirmed iterative reconstruction |
References
- Rehani, M.M.; Nacouzi, D. Higher Patient Doses through X-Ray Imaging Procedures. Phys. Med. 2020, 79, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Rehani, M.M. Challenges in Radiation Protection of Patients for the 21st Century. AJR Am. J. Roentgenol. 2013, 200, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Rehani, M.M.; Srimahachota, S. Skin Injuries in Interventional Procedures. Radiat. Prot. Dosim. 2011, 147, 8–12. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Bushberg, J.T.; Fletcher, J.G.; Eckel, L.J. Answers to Common Questions About the Use and Safety of CT Scans. Mayo Clin. Proc. 2015, 90, 1380–1392. [Google Scholar] [CrossRef]
- McCollough, C.H.; Guimarães, L.; Fletcher, J.G. In Defense of Body CT. AJR Am. J. Roentgenol. 2009, 193, 28–39. [Google Scholar] [CrossRef]
- Kaul, D.; Kahn, J.; Huizing, L.; Wiener, E.; Grupp, U.; Böning, G.; Ghadjar, P.; Renz, D.M.; Streitparth, F. Reducing Radiation Dose in Adult Head CT Using Iterative Reconstruction—A Clinical Study in 177 Patients. Rofo 2016, 188, 155–162. [Google Scholar] [CrossRef]
- Nakai, Y.; Miyazaki, O.; Kitamura, M.; Imai, R.; Okamoto, R.; Tsutsumi, Y.; Miyasaka, M.; Ogiwara, H.; Miura, H.; Yamada, K.; et al. Evaluation of Radiation Dose Reduction in Head CT Using the Half-Dose Method. Jpn. J. Radiol. 2023, 41, 872–881. [Google Scholar] [CrossRef]
- Wu, D.; Wang, G.; Bian, B.; Liu, Z.; Li, D. Benefits of Low-Dose CT Scan of Head for Patients With Intracranial Hemorrhage. Dose Response 2020, 18, 1559325820909778. [Google Scholar] [CrossRef]
- Udayasankar, U.K.; Braithwaite, K.; Arvaniti, M.; Tudorascu, D.; Small, W.C.; Little, S.; Palasis, S. Low-Dose Nonenhanced Head CT Protocol for Follow-Up Evaluation of Children with Ventriculoperitoneal Shunt: Reduction of Radiation and Effect on Image Quality. Am. J. Neuroradiol. 2008, 29, 802–806. [Google Scholar] [CrossRef]
- Cho, H.-H.; Lee, S.M.; You, S.K. Pediatric Head Computed Tomography with Advanced Modeled Iterative Reconstruction: Focus on Image Quality and Reduction of Radiation Dose. Pediatr. Radiol. 2020, 50, 242–251. [Google Scholar] [CrossRef]
- Ren, Q.; Dewan, S.K.; Li, M.; Li, J.; Mao, D.; Wang, Z.; Hua, Y. Comparison of Adaptive Statistical Iterative and Filtered Back Projection Reconstruction Techniques in Brain CT. Eur. J. Radiol. 2012, 81, 2597–2601. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.I.; Uder, M.; Lell, M.M. Reaching for Better Image Quality and Lower Radiation Dose in Head and Neck CT: Advanced Modeled and Sinogram-Affirmed Iterative Reconstruction in Combination with Tube Voltage Adaptation. Dentomaxillofac. Radiol. 2017, 46, 20160131. [Google Scholar] [CrossRef] [PubMed]
- Ryska, P.; Kvasnicka, T.; Jandura, J.; Klzo, L.; Grepl, J.; Zizka, J. Reduction of Effective Dose and Organ Dose to the Eye Lens in Head MDCT Using Iterative Image Reconstruction and Automatic Tube Current Modulation. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2014, 158, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Komlosi, P.; Zhang, Y.; Leiva-Salinas, C.; Ornan, D.; Patrie, J.T.; Xin, W.; Grady, D.; Wintermark, M. Adaptive Statistical Iterative Reconstruction Reduces Patient Radiation Dose in Neuroradiology CT Studies. Neuroradiology 2014, 56, 187–193. [Google Scholar] [CrossRef]
- Solomon, J.B.; Li, X.; Samei, E. Relating Noise to Image Quality Indicators in CT Examinations with Tube Current Modulation. AJR Am. J. Roentgenol. 2013, 200, 592–600. [Google Scholar] [CrossRef]
- Smith, A.B.; Dillon, W.P.; Gould, R.; Wintermark, M. Radiation Dose-Reduction Strategies for Neuroradiology CT Protocols. AJNR Am. J. Neuroradiol. 2007, 28, 1628–1632. [Google Scholar] [CrossRef]
- Fletcher, J.G.; DeLone, D.R.; Kotsenas, A.L.; Campeau, N.G.; Lehman, V.T.; Yu, L.; Leng, S.; Holmes, D.R.; Edwards, P.K.; Johnson, M.P.; et al. Evaluation of Lower-Dose Spiral Head CT for Detection of Intracranial Findings Causing Neurologic Deficits. AJNR Am. J. Neuroradiol. 2019, 40, 1855–1863. [Google Scholar] [CrossRef]
- Willemink, M.J.; Noël, P.B. The Evolution of Image Reconstruction for CT-from Filtered Back Projection to Artificial Intelligence. Eur. Radiol. 2019, 29, 2185–2195. [Google Scholar] [CrossRef]
- Agostini, A.; Borgheresi, A.; Carotti, M.; Ottaviani, L.; Badaloni, M.; Floridi, C.; Giovagnoni, A. Third-Generation Iterative Reconstruction on a Dual-Source, High-Pitch, Low-Dose Chest CT Protocol with Tin Filter for Spectral Shaping at 100 kV: A Study on a Small Series of COVID-19 Patients. Radiol. Med. 2021, 126, 388–398. [Google Scholar] [CrossRef]
- Geyer, L.L.; Schoepf, U.J.; Meinel, F.G.; Nance, J.W.; Bastarrika, G.; Leipsic, J.A.; Paul, N.S.; Rengo, M.; Laghi, A.; De Cecco, C.N. State of the Art: Iterative CT Reconstruction Techniques. Radiology 2015, 276, 339–357. [Google Scholar] [CrossRef]
- Kilic, K.; Erbas, G.; Guryildirim, M.; Arac, M.; Ilgit, E.; Coskun, B. Lowering the Dose in Head CT Using Adaptive Statistical Iterative Reconstruction. AJNR Am. J. Neuroradiol. 2011, 32, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Rapalino, O.; Kamalian, S.; Kamalian, S.; Payabvash, S.; Souza, L.C.S.; Zhang, D.; Mukta, J.; Sahani, D.V.; Lev, M.H.; Pomerantz, S.R. Cranial CT with Adaptive Statistical Iterative Reconstruction: Improved Image Quality with Concomitant Radiation Dose Reduction. AJNR Am. J. Neuroradiol. 2012, 33, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Ellmann, S.; Kammerer, F.; Brand, M.; Allmendinger, T.; May, M.S.; Uder, M.; Lell, M.M.; Kramer, M. A Novel Pairwise Comparison-Based Method to Determine Radiation Dose Reduction Potentials of Iterative Reconstruction Algorithms, Exemplified Through Circle of Willis Computed Tomography Angiography. Investig. Radiol. 2016, 51, 331–339. [Google Scholar] [CrossRef]
- Örgel, A.; Bier, G.; Hennersdorf, F.; Richter, H.; Ernemann, U.; Hauser, T.-K. Image Quality of CT Angiography of Supra-Aortic Arteries: Comparison Between Advanced Modelled Iterative Reconstruction (ADMIRE), Sinogram Affirmed Iterative Reconstruction (SAFIRE) and Filtered Back Projection (FBP) in One Patients’ Group. Clin. Neuroradiol. 2020, 30, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Raslau, F.D.; Escott, E.J.; Smiley, J.; Adams, C.; Feigal, D.; Ganesh, H.; Wang, C.; Zhang, J. Dose Reduction While Preserving Diagnostic Quality in Head CT: Advancing the Application of Iterative Reconstruction Using a Live Animal Model. AJNR Am. J. Neuroradiol. 2019, 40, 1864–1870. [Google Scholar] [CrossRef]
- Kayun, Z.; Karim, M.K.A.; Muhammad, N.A.; Aljewaw, O.B.; Chew, M.T.; Harun, H.H. Implication of Applying Iterative Reconstruction on Low Contrast Detectability in CT Brain Examination. Radiat. Phys. Chem. 2021, 188, 109676. [Google Scholar] [CrossRef]
- McCollough, C.H.; Primak, A.N.; Braun, N.; Kofler, J.; Yu, L.; Christner, J. Strategies for Reducing Radiation Dose in CT. Radiol. Clin. N. Am. 2009, 47, 27–40. [Google Scholar] [CrossRef]
- Korn, A.; Fenchel, M.; Bender, B.; Danz, S.; Hauser, T.K.; Ketelsen, D.; Flohr, T.; Claussen, C.D.; Heuschmid, M.; Ernemann, U.; et al. Iterative Reconstruction in Head CT: Image Quality of Routine and Low-Dose Protocols in Comparison with Standard Filtered Back-Projection. AJNR Am. J. Neuroradiol. 2012, 33, 218–224. [Google Scholar] [CrossRef]
- Kramer, M.; Ellmann, S.; Allmendinger, T.; Eller, A.; Kammerer, F.; May, M.S.; Baigger, J.F.; Uder, M.; Lell, M.M. Computed Tomography Angiography of Carotid Arteries and Vertebrobasilar System: A Simulation Study for Radiation Dose Reduction. Medicine 2015, 94, e1058. [Google Scholar] [CrossRef]
- Winkelmann, M.T.; Afat, S.; Walter, S.S.; Stock, E.; Schwarze, V.; Brendlin, A.; Kolb, M.; Artzner, C.P.; Othman, A.E. Diagnostic Performance of Different Simulated Low-Dose Levels in Patients with Suspected Cervical Abscess Using a Third-Generation Dual-Source CT Scanner. Diagnostics 2020, 10, 1072. [Google Scholar] [CrossRef]
- Aissa, J.; Bölke, E.; Sawicki, L.M.; Appel, E.; Thomas, C.; Heusch, P.; Sedlmair, M.; Krzymyk, K.; Kröpil, P.; Antoch, G.; et al. Noise Insertion in CT for Cocaine Body Packing: Where Is the Limit of Extensive Dose Reduction? Eur. J. Med. Res. 2018, 23, 59. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.; Marin, D.; Roy Choudhury, K.; Patel, B.; Samei, E. Effect of Radiation Dose Reduction and Reconstruction Algorithm on Image Noise, Contrast, Resolution, and Detectability of Subtle Hypoattenuating Liver Lesions at Multidetector CT: Filtered Back Projection versus a Commercial Model-Based Iterative Reconstruction Algorithm. Radiology 2017, 284, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, M.T.; Walter, S.S.; Stock, E.; Brendlin, A.; Kolb, M.; Othman, A.E.; Afat, S. Effects of Radiation Dose Reduction on Diagnostic Performance of 3rd Generation Dual Source CT Pulmonary Angiography. Eur. J. Radiol. 2021, 134, 109426. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Dalehaug, I.; Bolstad, K.N.; Aadnevik, D.; Flataboe, S.; Pettersen, H.E.S. Admire vs. Safire: Objective Comparison of CT Reconstruction Algorithms and Their Noise Properties 2017. arxiv 2017. https://arxiv.org/abs/1708.09616.
- Yu, L.; Vrieze, T.J.; Leng, S.; Fletcher, J.G.; McCollough, C.H. Technical Note: Measuring Contrast- and Noise-Dependent Spatial Resolution of an Iterative Reconstruction Method in CT Using Ensemble Averaging. Med. Phys. 2015, 42, 2261–2267. [Google Scholar] [CrossRef]
- Raslau, F.D.; Escott, E.J.; Elbelasi, H.; Adams, C.; Smiley, J.; Zhang, J. Iterative Reconstruction in Dose Reduction of A Head CT Examination and Corresponding Acquisition Parameter Selection. Radiol. Technol. 2022, 93, 462–472. [Google Scholar]
- Rivers-Bowerman, M.D.; Shankar, J.J.S. Iterative Reconstruction for Head CT: Effects on Radiation Dose and Image Quality. Can. J. Neurol. Sci. 2014, 41, 620–625. [Google Scholar] [CrossRef]
- Rabinowich, A.; Shendler, G.; Ben-Sira, L.; Shiran, S.I. Pediatric Low-Dose Head CT: Image Quality Improvement Using Iterative Model Reconstruction. Neuroradiol. J. 2023, 36, 555–562. [Google Scholar] [CrossRef]
- Shin, J.-B.; Yoon, D.-K.; Pak, S.; Kwon, Y.-H.; Suh, T.S. Comparative Performance Analysis for Abdominal Phantom ROI Detectability According to CT Reconstruction Algorithm: ADMIRE. J. Appl. Clin. Med. Phys. 2020, 21, 136–143. [Google Scholar] [CrossRef]
- McCollough, C.H.; Leng, S. Use of Artificial Intelligence in Computed Tomography Dose Optimisation. Ann. ICRP 2020, 49, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Diwakar, M.; Kumar, M. A Review on CT Image Noise and Its Denoising. Biomed. Signal Process. Control 2018, 42, 73–88. [Google Scholar] [CrossRef]
- Kang, E.; Min, J.; Ye, J.C. A Deep Convolutional Neural Network Using Directional Wavelets for Low-Dose X-Ray CT Reconstruction. Med. Phys. 2017, 44, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Moloney, F.; Kavanagh, R.G.; Ronan, N.J.; Grey, T.M.; Joyce, S.; Ryan, D.J.; Moore, N.; O’Connor, O.J.; Plant, B.J.; Maher, M.M. Ultra-Low-Dose Thoracic CT with Model-Based Iterative Reconstruction (MBIR) in Cystic Fibrosis Patients Undergoing Treatment with Cystic Fibrosis Transmembrane Conductance Regulators (CFTR). Clin. Radiol. 2021, 76, 393.e9–393.e17. [Google Scholar] [CrossRef]
- Karakaş, H.M.; Yıldırım, G.; Çiçek, E.D. The Reliability of Low-Dose Chest CT for the Initial Imaging of COVID-19: Comparison of Structured Findings, Categorical Diagnoses and Dose Levels. Diagn. Interv. Radiol. 2021, 27, 607–614. [Google Scholar] [CrossRef]
- Klug, M.; Sobeh, T.; Green, M.; Mayer, A.; Kirshenboim, Z.; Konen, E.; Marom, E.M. Denoised Ultra-Low-Dose Chest CT to Assess Pneumonia in Individuals Who Are Immunocompromised. Radiol. Cardiothorac. Imaging 2025, 7, e240189. [Google Scholar] [CrossRef]
- Jensen, C.T.; Gupta, S.; Saleh, M.M.; Liu, X.; Wong, V.K.; Salem, U.; Qiao, W.; Samei, E.; Wagner-Bartak, N.A. Reduced-Dose Deep Learning Reconstruction for Abdominal CT of Liver Metastases. Radiology 2022, 303, 90–98. [Google Scholar] [CrossRef]

| Scanning Parameters | |
|---|---|
| scan mode | spiral |
| stellar detector configuration | 128 × 0.6 (64 × 0.6 = 38.4 mm) |
| slice collimation (mm) | 40 × 0.6 |
| effective reference tube voltage (kV) | 100 |
| effective tube current-time product (mAs) | 401.6 ± 28.7 (350–445) |
| pitch | 0.55 |
| rotation time (s) | 1 |
| Category | Reconstruction Method | Radiation Dose Simulation | p-Value Original vs. 90% | p-Value Original vs. 70% | p-Value 90% vs. 70% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Original | 90% | 70% | ||||||||
| Mdn (IQR) | Mean ± SD | Mdn (IQR) | Mean ± SD | Mdn (IQR) | Mean ± SD | |||||
| Overall image quality | FBP | 4 (4–5) | 4.48 ± 0.51 | 4 (4–4) | 3.95 ± 0.25 | 3 (2–3) | 2.71 ± 0.46 | 0.001 | <0.001 | <0.001 |
| SAFIRE | 8 (7–8) | 7.57 ± 0.51 | 8 (8–8) | 7.95 ± 0.21 | 7 (7–7) | 6.95 ± 0.20 | 0.005 | 0.001 | <0.001 | |
| ADMIRE | 9 (9–9) | 8.95 ± 0.22 | 9 (9–9) | 8.95 ± 0.22 | 8 (8–8) | 7.95 ± 0.19 | 1 | < 0.001 | <0.001 | |
| Image detail | FBP | 4 (3–4) | 3.57 ± 0.51 | 3 (3–3) | 2.86 ± 0.36 | 2 (2–2.5) | 2.24 ± 0.44 | 0.001 | <0.001 | 0.001 |
| SAFIRE | 7 (7–7) | 6.95 ± 0.21 | 7 (7–7) | 7.05 ± 0.22 | 6 (6–6) | 5.95 ± 0.23 | 0.157 | <0.001 | <0.001 | |
| ADMIRE | 9 (9–9) | 8.95 ± 0.18 | 8 (8–9) | 8.48 ± 0.51 | 7 (7–7) | 6.95 ± 0.22 | 0.002 | <0.001 | <0.001 | |
| Image noise | FBP | 4 (4–4) | 3.95 ± 0.22 | 4 (4–4) | 3.95 ± 0.23 | 3 (3–3) | 2.95 ± 0.22 | 1 | <0.001 | <0.001 |
| SAFIRE | 9 (8–9) | 8.67 ± 0.48 | 8 (7.5–8) | 7.76 ± 0.44 | 7 (7–7) | 6.95 ± 0.22 | <0.001 | <0.001 | <0.001 | |
| ADMIRE | 9 (8.5–9) | 8.76 ± 0.44 | 8 (8–8) | 8.05 ± 0.22 | 6 (6–6) | 6.00 ± 0.45 | <0.001 | <0.001 | <0.001 | |
| Contrast | FBP | 5 (5–5) | 4.95 ± 0.24 | 4 (4–5) | 4.48 ± 0.51 | 3 (3–3) | 2.95 ± 0.24 | 0.004 | <0.001 | <0.001 |
| SAFIRE | 9 (9–9) | 9.05 ± 0.22 | 9 (9–9) | 8.95 ± 0.22 | 8 (8–8) | 7.95 ± 0.24 | 0.157 | <0.001 | <0.001 | |
| ADMIRE | 7 (7–7) | 6.95 ± 0.24 | 7 (7–7) | 6.95 ± 0.22 | 6 (6–6) | 5.95 ± 0.22 | 1 | <0.001 | <0.001 | |
| Artifacts | FBP | 7 (7–7) | 6.95 ± 0.22 | 6 (5–6) | 5.48 ± 0.93 | 5 (5–5) | 4.95 ± 0.21 | <0.001 | <0.001 | 0.036 |
| SAFIRE | 5 (5–6) | 5.48 ± 0.51 | 4 (4–4) | 4.19 ± 0.40 | 4 (4–4) | 3.95 ± 0.22 | <0.001 | <0.001 | 0.059 | |
| ADMIRE | 9 (9–9) | 8.95 ± 0.22 | 9 (9–9) | 8.95 ± 0.21 | 8 (8–8) | 7.95 ± 0.24 | 1 | <0.001 | <0.001 | |
| ROI Localization | Reconstruction Algorithm | Dose Simulation | Hounsfield Units (Mean ± SD) | p-Value |
|---|---|---|---|---|
| GM | FBP | original | 39.916 ± 1.275 | 0.001 |
| SAFIRE | original | 41.254 ± 1.251 | ||
| ADMIRE | original | 39.967 ± 1.180 | ||
| WM | FBP | original | 33.452 ± 1.424 | 0.347 |
| SAFIRE | original | 32.913 ± 1.703 | ||
| ADMIRE | original | 33.542 ± 1.472 | ||
| GM | FBP | 90% | 39.795 ± 1.280 | 0.001 |
| SAFIRE | 90% | 41.182 ± 1.335 | ||
| ADMIRE | 90% | 39.870 ± 1.197 | ||
| WM | FBP | 90% | 33.339 ± 1.421 | 0.392 |
| SAFIRE | 90% | 32.847 ± 1.673 | ||
| ADMIRE | 90% | 33.444 ± 1.494 | ||
| GM | FBP | 70% | 39.815 ± 1.246 | < 0.001 |
| SAFIRE | 70% | 41.176 ± 1.317 | ||
| ADMIRE | 70% | 39.843 ± 1.153 | ||
| WM | FBP | 70% | 33.325 ± 1.384 | 0.378 |
| SAFIRE | 70% | 32.843 ± 1.626 | ||
| ADMIRE | 70% | 33.417 ± 1.490 |
| ROI Localization | Algorithm Group 1 vs. Group 2 | Dose Simulation | Hounsfield Units (Mean Difference) | 95% Confidence Interval | p-Value | SEM | |
|---|---|---|---|---|---|---|---|
| GM | FBP | SAFIRE | original | −1.338 | −2.212 to −0.460 | 0.001 | 0.356 |
| ADMIRE | original | −0.051 | −0.926 to 0.823 | 1 | |||
| SAFIRE | FBP | original | 1.338 | 0.463 to 2.212 | 0.001 | ||
| ADMIRE | original | 1.286 | 0.412 to 2.161 | 0.002 | |||
| ADMIRE | FBP | original | 0.051 | −0.823 to 0.926 | 1 | ||
| SAFIRE | original | −1.286 | −2.161 to −0.412 | 0.002 | |||
| WM | FBP | SAFIRE | original | 0.539 | −0.550 to 1.627 | 0.685 | 0.443 |
| ADMIRE | original | −0.090 | −1.179 to 0.998 | 1 | |||
| SAFIRE | FBP | original | −0.539 | −1.627 to 0.550 | 0.685 | ||
| ADMIRE | original | −0.629 | −1.717 to 0.460 | 0.481 | |||
| ADMIRE | FBP | original | 0.090 | −0.998 to 1.179 | 1 | ||
| SAFIRE | original | 0.629 | −0.460 to 1.717 | 0.481 | |||
| GM | FBP | SAFIRE | 90% | −1.386 | −2.286 to −0.486 | 0.001 | 0.366 |
| ADMIRE | 90% | −0.074 | −0.975 to 0.826 | 1 | |||
| SAFIRE | FBP | 90% | 1.386 | 0.486 to 2.286 | 0.001 | ||
| ADMIRE | 90% | 1.312 | 0.412 to 2.212 | 0.002 | |||
| ADMIRE | FBP | 90% | 0.074 | −0.826 to 0.975 | 1 | ||
| SAFIRE | 90% | −1.312 | −2.212 to −0.412 | 0.002 | |||
| WM | FBP | SAFIRE | 90% | 0.491 | −0.594 to 1.576 | 0.810 | 0.442 |
| ADMIRE | 90% | −0.106 | −1.191 to 0.979 | 1 | |||
| SAFIRE | FBP | 90% | −0.491 | −1.576 to 0.594 | 0.810 | ||
| ADMIRE | 90% | −0.597 | −1.682 to 0.488 | 0.543 | |||
| ADMIRE | FBP | 90% | 0.106 | −0.979 to 1.191 | 1 | ||
| SAFIRE | 90 % | 0.597 | −0.488 to 1.682 | 0.543 | |||
| GM | FBP | SAFIRE | 70% | −1.361 | −2.259 to −0.463 | 0.001 | 0.366 |
| ADMIRE | 70% | −0.027 | −0.926 to 0.871 | 1 | |||
| SAFIRE | FBP | 70% | 1.361 | 0.463 to 2.259 | 0.001 | ||
| ADMIRE | 70% | 1.334 | 0.435 to 2.232 | 0.002 | |||
| ADMIRE | FBP | 70% | 0.027 | −0.871 to 0.926 | 1 | ||
| SAFIRE | 70% | −1.334 | −2.232 to −0.435 | 0.002 | |||
| WM | FBP | SAFIRE | 70% | 0.482 | −0.597 to 1.562 | 0.829 | 0.439 |
| ADMIRE | 70% | −0.092 | −1.172 to 0.987 | 1 | |||
| SAFIRE | FBP | 70% | −0.482 | −1.562 to 0.597 | 0.829 | ||
| ADMIRE | 70% | −0.575 | −1.654 to 0.505 | 0.587 | |||
| ADMIRE | FBP | 70% | 0.092 | −0.987 to 1.172 | 1 | ||
| SAFIRE | 70% | 0.575 | −0.505 to 1.654 | 0.587 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gohla, G.; Örgel, A.; Klose, U.; Brendlin, A.; Bongers, M.N.; Bender, B.; Staber, D.; Ernemann, U.; Hauser, T.-K.; Ruff, C. Comparison of ADMIRE, SAFIRE, and Filtered Back Projection in Standard and Low-Dose Non-Enhanced Head CT. Diagnostics 2025, 15, 1541. https://doi.org/10.3390/diagnostics15121541
Gohla G, Örgel A, Klose U, Brendlin A, Bongers MN, Bender B, Staber D, Ernemann U, Hauser T-K, Ruff C. Comparison of ADMIRE, SAFIRE, and Filtered Back Projection in Standard and Low-Dose Non-Enhanced Head CT. Diagnostics. 2025; 15(12):1541. https://doi.org/10.3390/diagnostics15121541
Chicago/Turabian StyleGohla, Georg, Anja Örgel, Uwe Klose, Andreas Brendlin, Malte Niklas Bongers, Benjamin Bender, Deborah Staber, Ulrike Ernemann, Till-Karsten Hauser, and Christer Ruff. 2025. "Comparison of ADMIRE, SAFIRE, and Filtered Back Projection in Standard and Low-Dose Non-Enhanced Head CT" Diagnostics 15, no. 12: 1541. https://doi.org/10.3390/diagnostics15121541
APA StyleGohla, G., Örgel, A., Klose, U., Brendlin, A., Bongers, M. N., Bender, B., Staber, D., Ernemann, U., Hauser, T.-K., & Ruff, C. (2025). Comparison of ADMIRE, SAFIRE, and Filtered Back Projection in Standard and Low-Dose Non-Enhanced Head CT. Diagnostics, 15(12), 1541. https://doi.org/10.3390/diagnostics15121541








