Assessment of the Relationship Between Haller Cells, Accessory Maxillary Ostium, and Maxillary Sinus Pathologies: A Cross-Sectional CBCT Study
Abstract
1. Introduction
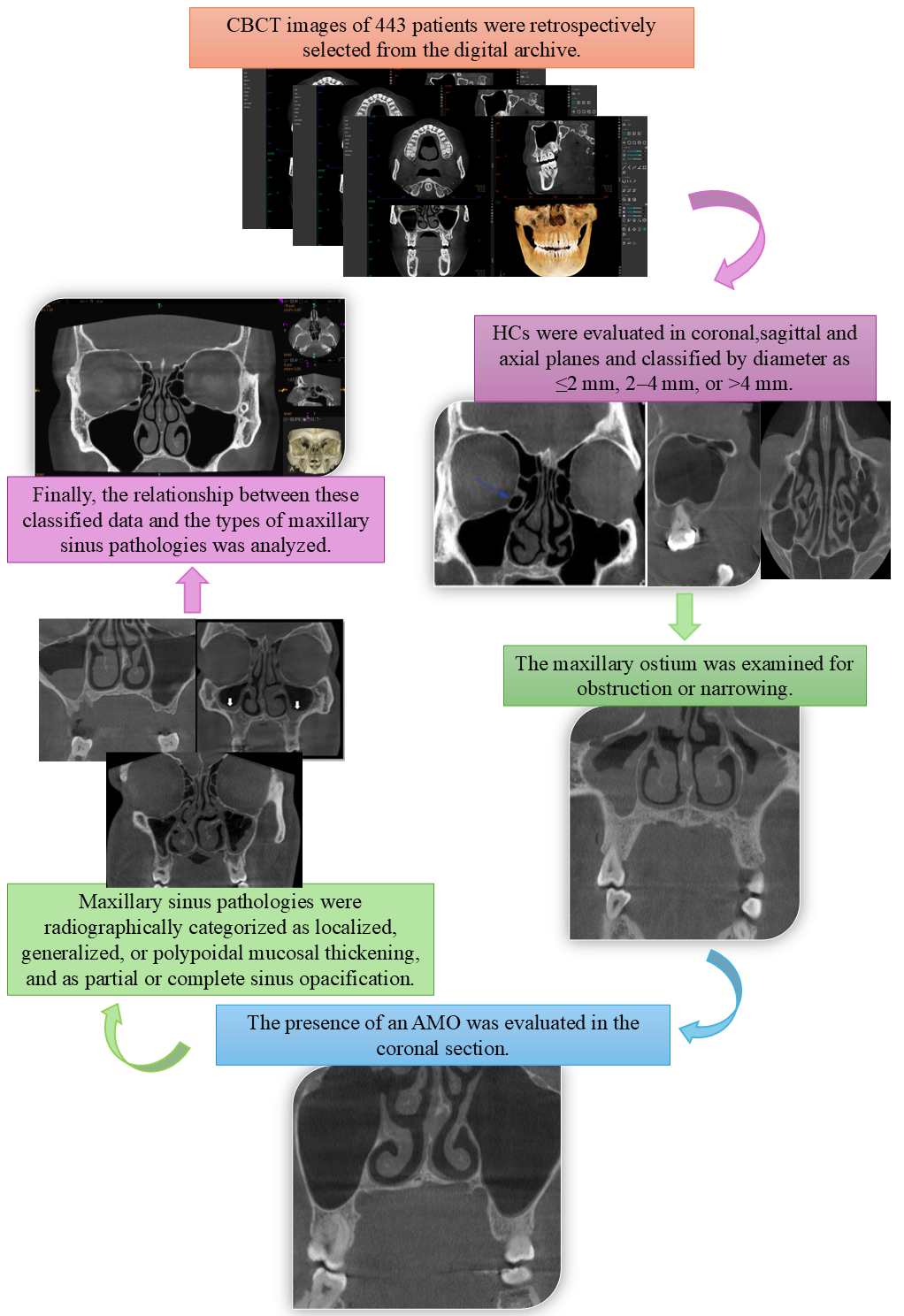
2. Materials and Methods
Statistical Analysis
3. Results
| Absent | Localized Mucosal Thickening | Generalized Mucosal Thickening | Polypoidal Mucosal Thickening | Partial Opacification | Total Opacification | p F | ||
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
| For Right | ||||||||
| Haller cell | − | 99 (66) | 116 (65.2) | 20 (80) | 15 (50) | 37 (67.3) | 3 (60) | 0.330 |
| + | 52 (34) | 61 (34.8) | 5 (20) | 15 (50) | 18 (32.7) | 2 (40) | ||
| Ostium narrowing | − | 97 (64.7) | 115 (64.6) | 15 (60) | 16 (53.3) | 37 (67.3) | 5 (100) | 0.480 |
| + | 54 (35.3) | 62 (35.4) | 10 (40) | 14 (46.7) | 18 (32.7) | 0 (0) | ||
| Accessory maxillary ostium | − | 107 (71.3) a | 94 (52.8) b | 13 (52) a,b | 17 (56.7) a,b | 32 (58.2) a,b | 5 (100) a,b | 0.005 * |
| + | 44 (28.7) a | 83 (47.2) b | 12 (48) a,b | 13 (43.3) a,b | 23 (41.8) a,b | 0 (0) a,b | ||
| Ostium obstruction | + | 149 (99.3) a | 159 (89.3) b | 20 (80) b,c | 29 (96.7) a,b | 32 (58.2) c,d | 0 (0) d | 0.001 * |
| − | 2 (0.7) a | 18 (10.7) b | 5 (20) b,c | 1 (3.3) a,b | 23 (41.8) c,d | 5 (1.2) d | ||
| For Left | ||||||||
| Haller cell | − | 90 (58.8) | 102 (58.6) | 20 (71.4) | 20 (62.5) | 30 (60) | 6 (100) | 0.336 |
| + | 63 (41.2) | 72 (41.4) | 8 (28.6) | 12 (37.5) | 20 (40) | 0 (0) | ||
| Ostium narrowing | − | 105 (68.6) | 103 (59.2) | 21 (75) | 22 (68.8) | 33 (66) | 6 (100) | 0.162 |
| + | 48 (31.4) | 71 (40.8) | 7 (25) | 10 (31.3) | 17 (34) | 0 (0) | ||
| Accessory maxillary ostium | − | 107 (69.9) a | 104 (59.8) a,b | 19 (67.9) a,b | 13 (40.6) b | 23 (46) b | 5 (83.3) a,b | 0.004 * |
| + | 46 (30.1) a | 70 (40.2) a,b | 9 (32.1) a,b | 19 (59.4) b | 27 (54) b | 1 (16.7) a,b | ||
| Ostium obstruction | + | 151 (98.7) a | 165 (94.8) a | 18 (64.3) b,c | 30 (93.8) a,c | 27 (54) b | 0 (0) b | 0.001 * |
| − | 2 (1.3) a | 9 (5.2) a | 10 (35.7) b,c | 2 (6.3) a,c | 23 (46) b | 6 (100) b |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HC | Haller Cell |
| AMO | Accessory Maxillary Ostium |
| CBCT | Cone-Beam Computed Tomography |
| FOV | Field of View |
| ENT | Otolaryngological |
References
- Stammberger, H.; Wolf, G. Headaches and sinus disease: The endoscopic approach. Ann. Otol. Rhinol. Laryngol. 1988, 97 (Suppl. S5), 3–23. [Google Scholar] [CrossRef]
- Kantarci, M.; Karasen, R.M.; Alper, F.; Onbas, O.; Okur, A.; Karaman, A. Remarkable anatomic variations in paranasal sinus region and their clinical importance. Eur. J. Radiol. 2004, 50, 296–302. [Google Scholar] [CrossRef]
- Mathew, R.; Omami, G.; Hand, A.; Fellows, D.; Lurie, A. Cone beam CT analysis of Haller cells: Prevalence and clinical significance. Dentomaxillofac. Radiol. 2013, 42, 20130055. [Google Scholar] [CrossRef]
- Ahmad, M.; Khurana, N.; Jaberi, J.; Sampair, C.; Kuba, R.K. Prevalence of infraorbital ethmoid (Haller’s) cells on panoramic radiographs. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Asantoğrol, F.; Coşgunarslan, A.; Soydan Çabuk, D.; Amuk, M. The prevalence of Haller cell and its effect on maxillary sinusitis and obstruction of osteomeatal complex. Turk. Klin. J. Dent. Sci. 2021, 27, 191–198. [Google Scholar] [CrossRef]
- Wanamaker, H.H. Role of Haller’s cell in headache and sinus disease: A case report. Otolaryngol. Head Neck Surg. 1996, 114, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Denny, C.; Shenoy, N.; Shruthi, P. Clinical significance of Haller cells: A cone beam computed tomography study. World J. Dent. 2018, 9, 225–230. [Google Scholar] [CrossRef]
- Patil, K.; Sanjay, C.; Surya, L.K.; Mahima, V.; Doggalli, N.; Doddawad, V. Ubiquity and attribution of Haller cells: A CBCT study. Eur. J. Anat. 2023, 27, 25–32. [Google Scholar] [CrossRef]
- Özcan, İ.; Göksel, S.; Çakır-Karabaş, H.; Ünsal, G. CBCT analysis of Haller cells: Relationship with accessory maxillary ostium and maxillary sinus pathologies. Oral Radiol. 2021, 37, 502–506. [Google Scholar] [CrossRef]
- Sarna, A.; Hayman, L.A.; Laine, F.J.; Taber, K.H. Coronal imaging of the osteomeatal unit: Anatomy of 24 variants. J. Comput. Assist. Tomogr. 2002, 26, 153–157. [Google Scholar] [CrossRef]
- Jones, N.S. CT of the paranasal sinuses: Correlation with clinical, surgical and histopathological findings. Clin. Otolaryngol. 2002, 27, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, R. Revisiting human nose anatomy: Phylogenic and ontogenic perspectives. Laryngoscope 2011, 121, 2461–2467. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, E.; Ozturk, E.M.A. Association between accessory maxillary ostium, Haller cell, and sinus pathologies in cone-beam computed tomography. J. Stomatol. 2022, 75, 187–194. [Google Scholar] [CrossRef]
- Caversaccio, M.; Boschung, U.; Mudry, A. Historical review of Haller’s cells. Ann. Anat. 2011, 193, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Orhan, K.; Kusakci Seker, B.; Aksoy, S.; Bayindir, H.; Berberoğlu, A.; Seker, E. Cone beam CT evaluation of maxillary sinus septa prevalence, height, location and morphology in children and an adult population. Med. Princ. Pract. 2013, 22, 47–53. [Google Scholar] [CrossRef]
- Devi, A.N.; Basavaraju, S.M.; Singh, D.N.; Chungkham, S.; Ashem, A.; Yumnam, R. Retrospective study on the prevalence of Haller cells using cone beam computed tomography. Cureus 2024, 16, e67200. [Google Scholar] [CrossRef]
- Tepordei, R.T.; Haliciu, A.; Popa, C.G.; Vicoleanu, S.A.P.; Statescu, G.; Popa, R.A.; Moraru, M.C.; Ursaru, M. Haller cells and their association with chronic rhinosinusitis: A comprehensive review. Rom. J. Oral Rehabil. 2025, 17, 276–284. [Google Scholar]
- Kaya, S. Evaluation of the relationship between Haller cell and concha bullosa with cone beam computed tomography: Cross-sectional study. Turk. Klin. J. Dent. Sci. 2024, 30, 406–413. [Google Scholar] [CrossRef]
- Ali, I.K.; Sansare, K.; Karjodkar, F.R.; Vanga, K.; Salve, P.; Pawar, A.M. Cone-beam computed tomography analysis of accessory maxillary ostium and Haller cells: Prevalence and clinical significance. Imaging Sci. Dent. 2017, 47, 33–37. [Google Scholar] [CrossRef]
- Namdar Pekiner, F. Diş Hekimliğinde Radyolojinin Esasları; Özcan, I., Ed.; İstanbul Tıp Kitabevleri: İstanbul, Turkey, 2017; pp. 513–540. [Google Scholar]
- Dogan, M.E.; Uluısık, N.; Yuvarlakbaş, S.D. Retrospective analysis of pathological changes in the maxillary sinus with CBCT. Sci. Rep. 2024, 14, 15529. [Google Scholar] [CrossRef]
- Valizadeh, S.; Ahsaie, M.G.; Firoozabad, F.F.; Aryanezhad, S.S.; Arianezhad, S.M. Exploring maxillary sinus ostium characteristics and insights for pathology prediction and anatomical variations: A CBCT analysis. J. Dent. Sch. 2025, 45, 29. [Google Scholar]
- Mahapatra, S.; Hebbale, M.; Tepan, M.; Halli, R.; Singh, S.; Modak, R. Prevalence of Haller cell and accessory maxillary ostium: A CBCT study. J. Indian. Acad. Oral Med. Radiol. 2022, 34, 466–469. [Google Scholar] [CrossRef]
- Moshfeghi, M.; Dehini, H.; Ghazizadeh Ahsaie, M. Cone beam CT analysis of Haller cells: Prevalence and relationship with orbital floor dehiscence. Int. J. Dent. 2023, 2023, 5200152. [Google Scholar] [CrossRef] [PubMed]
- Yılmazsoy, Y.; Arslan, S. Assessment of the prevalence of Haller cell variation and its relation with maxillary sinusitis. J. Health Sci. Med. 2018, 1, 54–58. [Google Scholar]
- Ayyıldız, H.; Akgünlü, F. Are maxillary sinus variations related to maxillary sinus diameters? Oral Radiol. 2023, 39, 425–436. [Google Scholar] [CrossRef]
- Türker, N.; Göller Bulut, D. Frequency, localization, and diameter of the accessory maxillary ostium and its relationship with sinus pathologies and nasal cavity variations: An anatomical study based on cone beam computed tomography. Folia Morphol. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Kamdi, P.; Nimma, V.; Ramchandani, A.; Ramaswami, E.; Gogri, A.; Umarji, H. Evaluation of Haller cell on CBCT and its association with maxillary sinus pathologies. J. Indian. Acad. Oral Med. Radiol. 2018, 30, 41–45. [Google Scholar] [CrossRef]
- Razavi, M.; Mohebiniya, M.; Moridi, M. The prevalence of Haller’s cells in cone-beam computer tomography. Med. Sci. 2020, 24, 1500–1506. [Google Scholar]
- Park, I.H.; Song, J.S.; Choi, H.; Lee, S.H.; Hwang, E. The analysis of maxillary sinus aeration according to nasal septal deviation. Auris Nasus Larynx. 2011, 38, 770–774. [Google Scholar]
- Alkholaiwi, F.M.; Almutairi, R.R.; Alrajhi, D.M.; Alturki, B.A.; Almutairi, A.G.; Binyousef, F.H. Occupational and environmental exposures, the association with chronic sinusitis. Saudi Med. J. 2022, 43, 125. [Google Scholar] [CrossRef]
- Haylaz, E.; Geduk, G.; Şeker, Ç. Evaluation of the frequency localization and relationship of maxillary sinus pathologies with dental pathologies by Cone Beam Computed Tomography (CBCT). J. Contemp. Med. 2024, 14, 94–100. [Google Scholar] [CrossRef]
- Bani-Ata, M.; Aleshawi, A.; Khatatbeh, A.; Al-Domaidat, D.; Alnussair, B.; Al-Shawaqfeh, R.; Allouh, M. Accessory maxillary ostia: Prevalence of an anatomical variant and association with chronic sinusitis. Int. J. Gen. Med. 2020, 13, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Serindere, G.; Gunduz, K.; Avsever, H. The relationship between an accessory maxillary ostium and variations in structures adjacent to the maxillary sinus without polyps. Int. Arch. Otorhinolaryngol. 2022, 26, e548–e555. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Al Bayatti, S.W.; Al-Rawi, N.H.; Samsudin, R.; Marei, H.; Shetty, R.; Abdelmagyd, H.A.; Reddy, S. A study on the association between accessory maxillary ostium and maxillary sinus mucosal thickening using cone beam computed tomography. Head Face Med. 2021, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Kıpçak, Ö.A. Evaluation of maxillary sinus pathologies and schneider membrane changes in relation to odontogenic factors using cone beam computed tomography. KOU Sag. Bil. Derg. 2021, 7, 296–303. [Google Scholar]
- Raitz, R.; Traiano, A.P.; e Silva, M.B.F. Relação entre lesões periapicais e espessamento mucoso dos seios maxilares por meio de tomografia computadorizada de feixe cônico. Clin. Lab. Res. Den. 2019, 1–6. [Google Scholar] [CrossRef]
- Aust, R.; Drettner, B. The Functional Size of the Human Maxillary Ostium in Vivo. Acta Oto-Laryngol. 1974, 78, 432–435. [Google Scholar] [CrossRef]
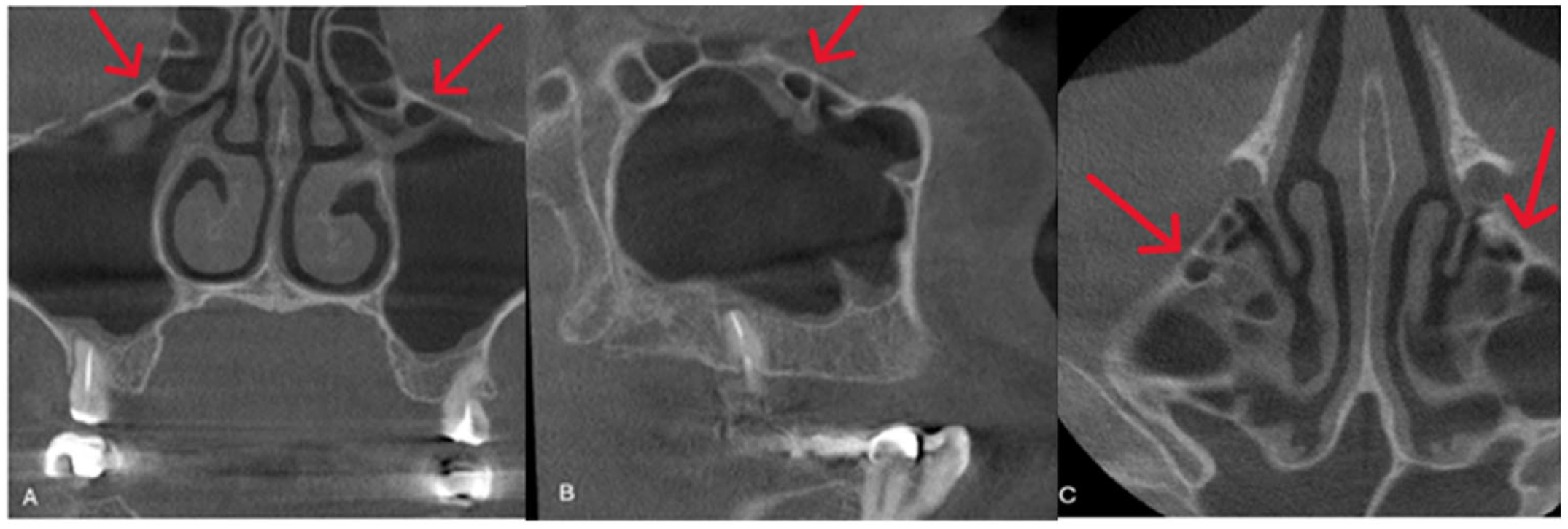
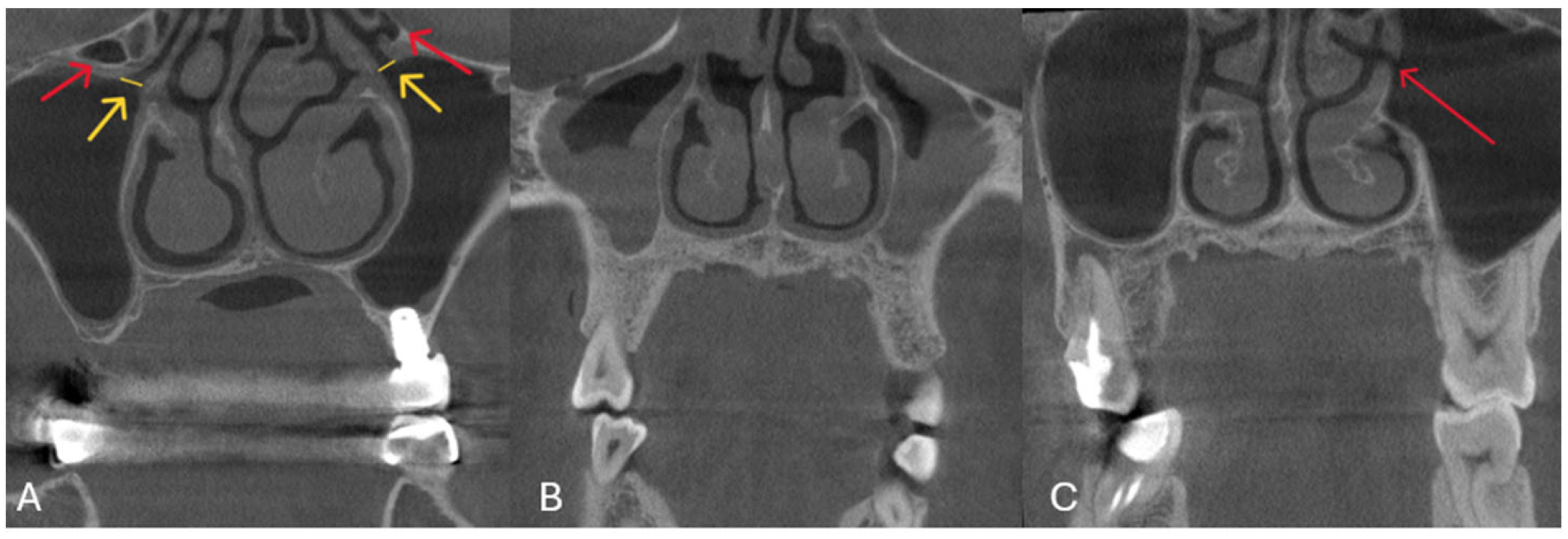

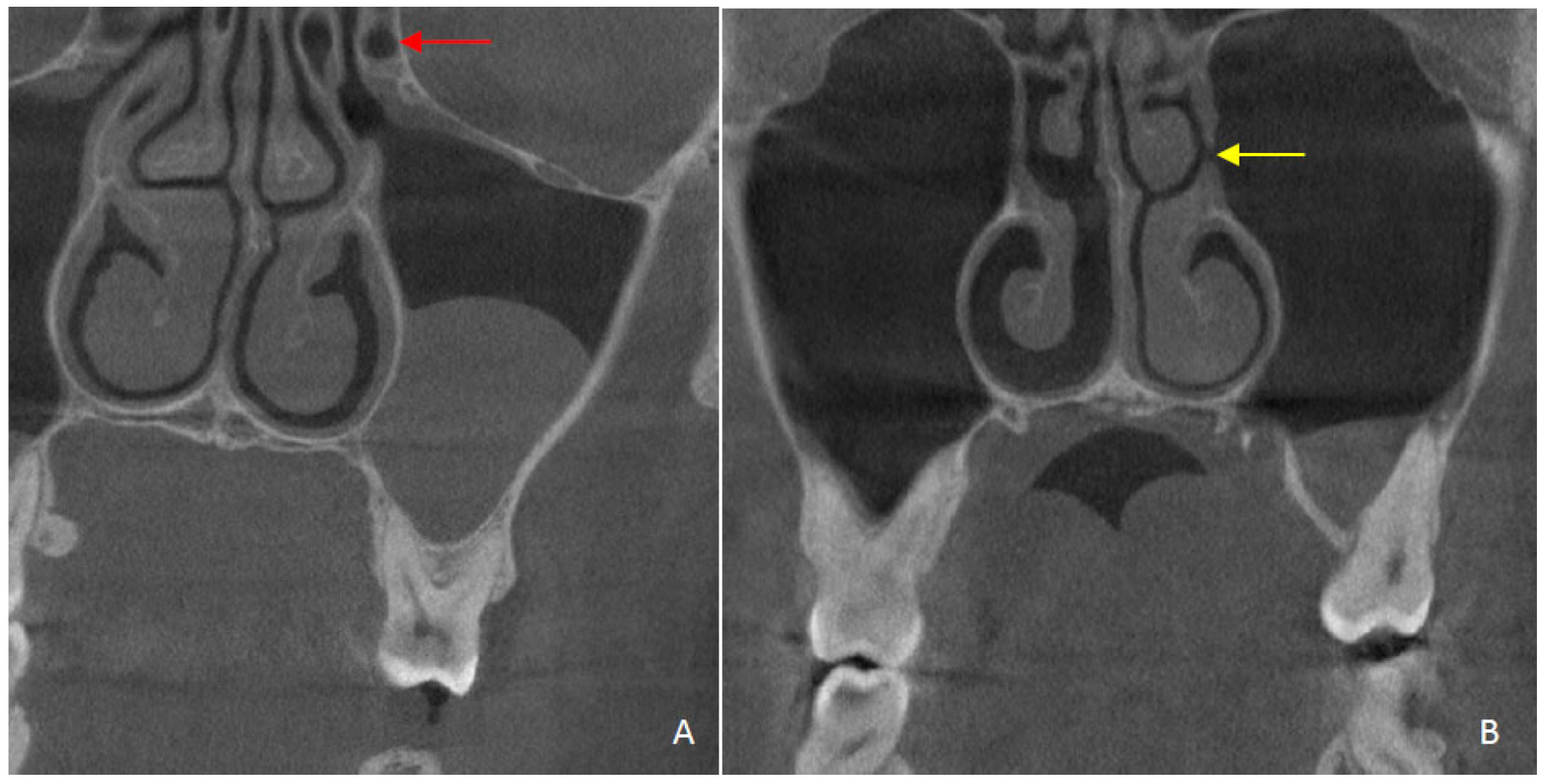
| Variables | Right | Left | ||
|---|---|---|---|---|
| Absent, n (%) | Present, n (%) | Absent, n (%) | Present, n (%) | |
| Haller cell | 290 (65.5) | 153 (34.5) | 268 (60.5) | 175 (39.5) |
| Ostium narrowing | 285 (64.3) | 158 (35.7) | 290 (65.5) | 153 (34.5) |
| Accessory maxillary ostium | 268 (60.5) | 175 (39.5) | 271 (61.2) | 172 (38.8) |
| Maxillary sinus pathology | 151 (34.1) | 292 (65.9) | 153 (34.5) | 290 (65.5) |
| Subgroups | n (%) | Subgroups | n (%) | |
| Haller dimension types | Absent | 290 (65.5) | Absent | 268 (60.5) |
| 0–2 mm | 9 (2) | 0–2 mm | 6 (1.4) | |
| 2–4 mm | 74 (16.7) | 2–4 mm | 78 (17.6) | |
| >4 mm | 70 (15.8) | >4 mm | 91 (20.5) | |
| Subgroups | n (%) | Subgroups | n (%) | |
| Maxillary sinus pathology types | Localized mucosal thickening | 177 (40) | Localized mucosal thickening | 174 (39.3) |
| Generalized mucosal thickening | 25 (5.6) | Generalized mucosal thickening | 28 (6.3) | |
| Polypoidal mucosal thickening | 30 (6.8) | Polypoidal mucosal thickening | 32 (7.2) | |
| Partial opacification | 55 (12.4) | Partial opacification | 50 (11.3) | |
| Total opacification | 5 (1.1) | Total opacification | 6 (1.4) | |
| Subgroups | n (Mean ± SD) | Subgroups | n (Mean ± SD) | |
| Ostium dimensions | Male | 226 (2.05 ± 1.01) | Male | 226 (2.10 ± 0.94) |
| Female | 217 (2.16 ± 0.84) | Female | 217 (2.18 ± 0.86) | |
| Open, n (%) | Closed, n (%) | Open, n (%) | Closed, n (%) | |
| Ostium obstruction | 389 (87.8) | 54 (12.2) | 391 (88.3) | 52 (11.7) |
| Variables | Subgroups | n | Mean ± SD (Median) | p M | |
|---|---|---|---|---|---|
| For Right | |||||
| Sex | Male | 226 | 2.05 ± 1.01 (2.38) | 0.594 | |
| Female | 217 | 2.16 ± 0.84 (2.42) | |||
| Haller cell | Absent | 290 | 2.16 ± 0.95 (2.49) | 0.001 * | |
| Present | 153 | 1.99 ± 0.86 (2.05) | |||
| Ostium narrowing | Absent | 285 | 2.22 ± 1.11 (2.63) | 0.001 * | |
| Present | 158 | 1.89 ± 0.31 (1.94) | |||
| Accessory maxillary ostium | Absent | 268 | 2.15 ± 0.88 (2.45) | 0.516 | |
| Present | 175 | 2.03 ± 0.99 (2.32) | |||
| Maxillary sinus pathology | Absent | 151 | 2.45 ± 0.5 (2.52) | 0.001 * | |
| Present | 292 | 1.92 ± 1.04 (2.21) | |||
| For Left | |||||
| Sex | Male | 226 | 2.10 ± 0.94 (2.43) | 0.649 | |
| Female | 217 | 2.18 ± 0.86 (2.42) | |||
| Haller cell | Absent | 268 | 2.17 ± 0.97 (2.5) | 0.002 * | |
| Present | 175 | 2.09 ± 0.78 (2.21) | |||
| Ostium narrowing | Absent | 290 | 2.25 ± 1.08 (2.65) | 0.001 * | |
| Present | 153 | 1.93 ± 0.27 (1.94) | |||
| Accessory maxillary ostium | Absent | 271 | 2.21 ± 0.87 (2.46) | 0.082 | |
| Present | 172 | 2.04 ± 0.94 (2.34) | |||
| Maxillary sinus pathology | Absent | 153 | 2.43 ± 0.62 (2.49) | 0.001 * | |
| Present | 290 | 1.99 ± 0.98 (2.26) |
| Variables | Right | Left | |||||
|---|---|---|---|---|---|---|---|
| Male (n) | Female (n) | p χ2 | Male (n) | Female (n) | p χ2 | ||
| Haller cell | Absent | 151 | 139 | 0.542 | 144 | 124 | 0.157 |
| Present | 75 | 78 | 82 | 93 | |||
| Ostium narrowing | Absent | 150 | 135 | 0.361 | 151 | 139 | 0.542 |
| Present | 76 | 82 | 75 | 78 | |||
| Accessory maxillary ostium | Absent | 118 a | 150 b | 0.001 * | 130 | 141 | 0.108 |
| Present | 108 a | 67 b | 96 | 76 | |||
| Maxillary sinus pathology | Absent | 49 a | 102 b | 0.001 * | 54 a | 99 b | 0.001 * |
| Present | 177 a | 115 b | 172 a | 118 b | |||
| Haller dimension types | Absent | 151 | 139 | 0.339 F | 114 | 124 | 0.138 F |
| 0–2 mm | 2 | 7 | 1 | 5 | |||
| 2–4 mm | 39 | 35 | 41 | 37 | |||
| >4 mm | 34 | 36 | 40 | 51 | |||
| Maxillary sinus pathology types | Absent | 49 a | 102 b | 0.001 *,F | 54 a | 99 b | 0.001 *,F |
| Localized mucosal thickening | 103 a | 74 b | 94 a | 80 a | |||
| Generalized mucosal thickening | 19 a | 6 b | 22 a | 6 b | |||
| Polypoidal mucosal thickening | 18 a | 12 a | 22 a | 10 b | |||
| Partial opacification | 35 a | 20 b | 30 a | 20 a | |||
| Total opacification | 2 a | 3 a | 4 a | 2 a | |||
| Ostium obstruction | Open | 192 | 197 | 0.061 | 194 | 197 | 0.106 |
| Closed | 34 | 20 | 32 | 20 | |||
| For Right | Ostium Narrowing | Accessory Maxillary Ostium | Maxillary Sinus Pathology | Ostium Obstruction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | p | n (%) | n (%) | p | n (%) | n (%) | p | n (%) | n (%) | p | ||
| − | + | − | + | − | + | + | − | ||||||
| Haller cell | − | 218 (76.5) a | 72 (45.6) b | * | 185 (69) | 105 (60) | x | 98 (64.9) | 192 (65.8) | x | 253 (65) | 37 (68.5) | x |
| + | 67 (23.5) a | 86 (54.4) b | 83 (31) | 70 (40) | 53 (35.1) | 100 (34.2) | 136 (35) | 17 (31.5) | |||||
| Ostium narrowing | − | 175 (65.3) | 110 (62.9) | x | 97 (64.2) | 188 (64.4) | x | ||||||
| + | 93 (34.7) | 65 (62.4) | 54 (35.8) | 104 (35.6) | |||||||||
| Accessory maxillary ostium | − | 107 (70.9) a | 161 (55.1) b | * | 240 (61.7) | 28 (51.9) | x | ||||||
| + | 44 (29.1) a | 131 (44.9) b | 149 (38.3) | 26 (48.1) | |||||||||
| Maxillary sinus pathology | − | 151 (38.8) a | 0 (0) b | * | |||||||||
| + | 238 (61.2) a | 54 (100) b | |||||||||||
| For Left | Ostium narrowing | Accessory maxillary ostium | Maxillary sinus pathology | Ostium obstruction | |||||||||
| n (%) | n (%) | p | n (%) | n (%) | p | n (%) | n (%) | p | n (%) | n (%) | p | ||
| − | + | − | + | − | + | + | − | ||||||
| Haller cell | − | 201 (69.3) a | 67 (43.8) b | * | 173 (63.8) | 95 (55.2) | x | 90 (58.8) | 178 (61.4) | x | 230 (58.8) a | 38 (73.1) b | * |
| + | 89 (30.7) a | 86 (56.2) b | 98 (36.2) | 77 (44.8) | 63 (41.2) | 112 (38.6) | 161 (41.2) a | 14 (26.9) b | |||||
| Ostium narrowing | − | 180 (66.4) | 110 (64) | x | 105 (68.6) | 185 (63.8) | x | ||||||
| + | 91 (33.6) | 62 (36) | 48 (31.4) | 105 (36.2) | |||||||||
| Accessory maxillary ostium | − | 107 (69.9) a | 164 (56.6) b | * | 242 (61.9) | 29 (55.8) | x | ||||||
| + | 46 (30.1) a | 126 (43.4) b | 149 (38.1) | 23 (44.2) | |||||||||
| Maxillary sinus pathology | − | 151 (38.6) a | 2 (3.8) b | * | |||||||||
| + | 240 (61.4) a | 50 96.2) b | |||||||||||
| Parameter | For Right | For Left | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Sex | 0.335 | 0.22–0.51 | <0.001 * | 0.384 | 0.25–0.57 | <0.001 * |
| Haller cell | 0.933 | 0.59–1.47 | 0.767 | 0.852 | 0.55–1.31 | 0.467 |
| Ostium narrowing | 1.059 | 0.67–1.66 | 0.805 | 1.33 | 0.85–2.07 | 0.212 |
| Accessory maxillary ostium | 1.698 | 1.09–2.63 | 0.018 * | 1.713 | 1.11–2.63 | 0.014 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çapar, İ.; Şeker, Ç.; Cicek, O. Assessment of the Relationship Between Haller Cells, Accessory Maxillary Ostium, and Maxillary Sinus Pathologies: A Cross-Sectional CBCT Study. Diagnostics 2025, 15, 2557. https://doi.org/10.3390/diagnostics15202557
Çapar İ, Şeker Ç, Cicek O. Assessment of the Relationship Between Haller Cells, Accessory Maxillary Ostium, and Maxillary Sinus Pathologies: A Cross-Sectional CBCT Study. Diagnostics. 2025; 15(20):2557. https://doi.org/10.3390/diagnostics15202557
Chicago/Turabian StyleÇapar, İsmail, Çiğdem Şeker, and Orhan Cicek. 2025. "Assessment of the Relationship Between Haller Cells, Accessory Maxillary Ostium, and Maxillary Sinus Pathologies: A Cross-Sectional CBCT Study" Diagnostics 15, no. 20: 2557. https://doi.org/10.3390/diagnostics15202557
APA StyleÇapar, İ., Şeker, Ç., & Cicek, O. (2025). Assessment of the Relationship Between Haller Cells, Accessory Maxillary Ostium, and Maxillary Sinus Pathologies: A Cross-Sectional CBCT Study. Diagnostics, 15(20), 2557. https://doi.org/10.3390/diagnostics15202557







