Differences in Vaginal Microbiota Composition Between Infertile and Fertile Patients: A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Study Design and Subjects
2.3. Sample Collection, Bacterial DNA Extraction, and RNA Sequencing
2.4. Statistical Data Analysis
3. Results
3.1. Flow Chart Pertaining to Study Design and Demographics
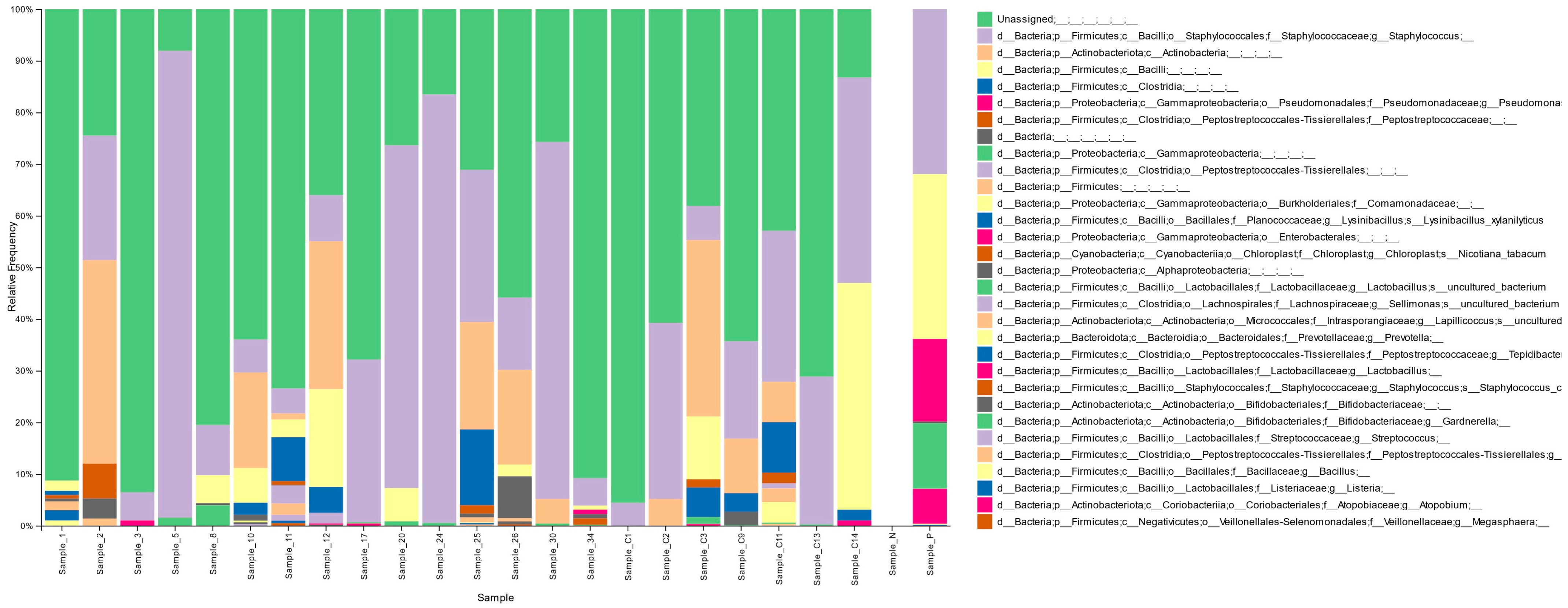
3.2. Vaginal Microbiota Distribution in the Cohort
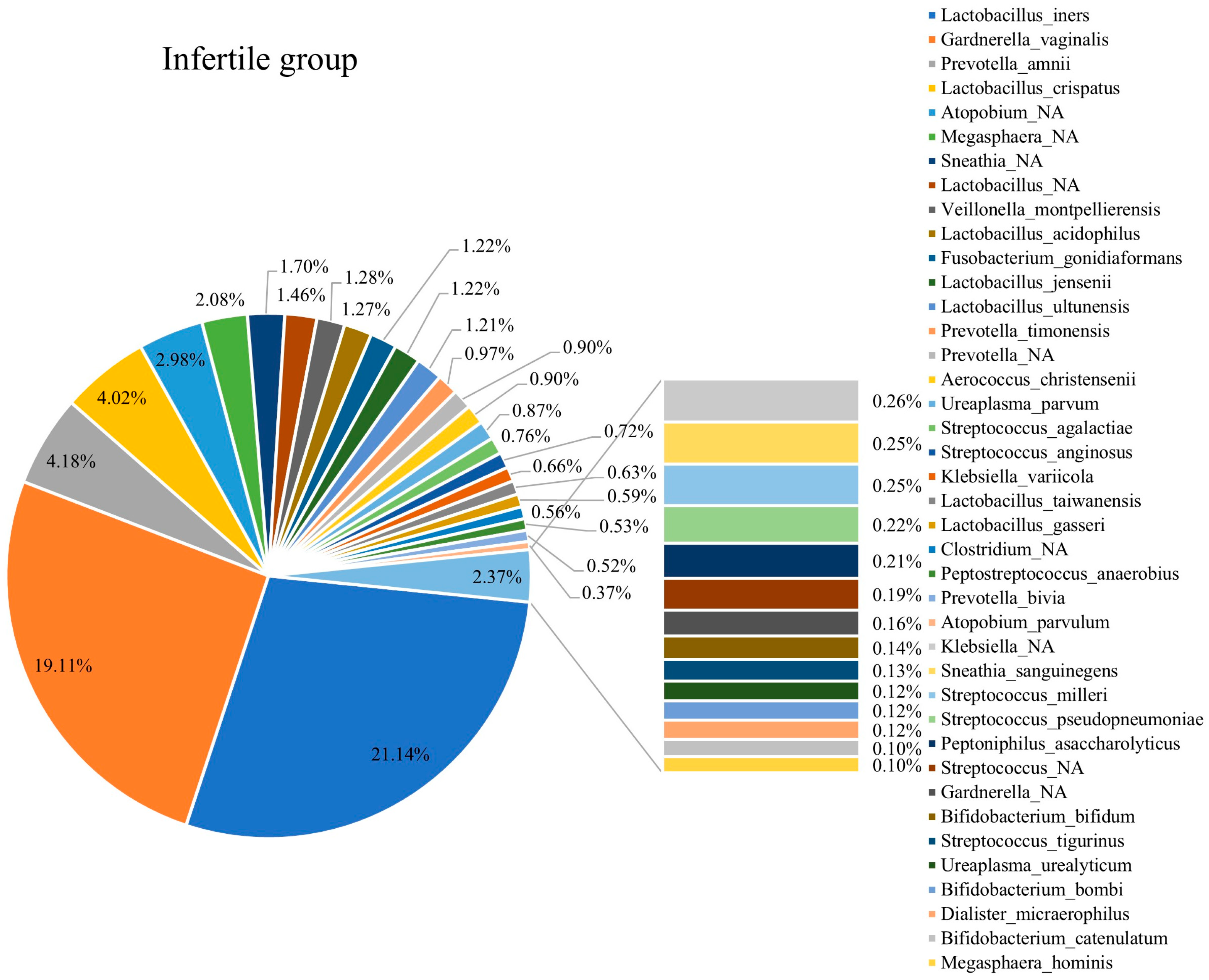
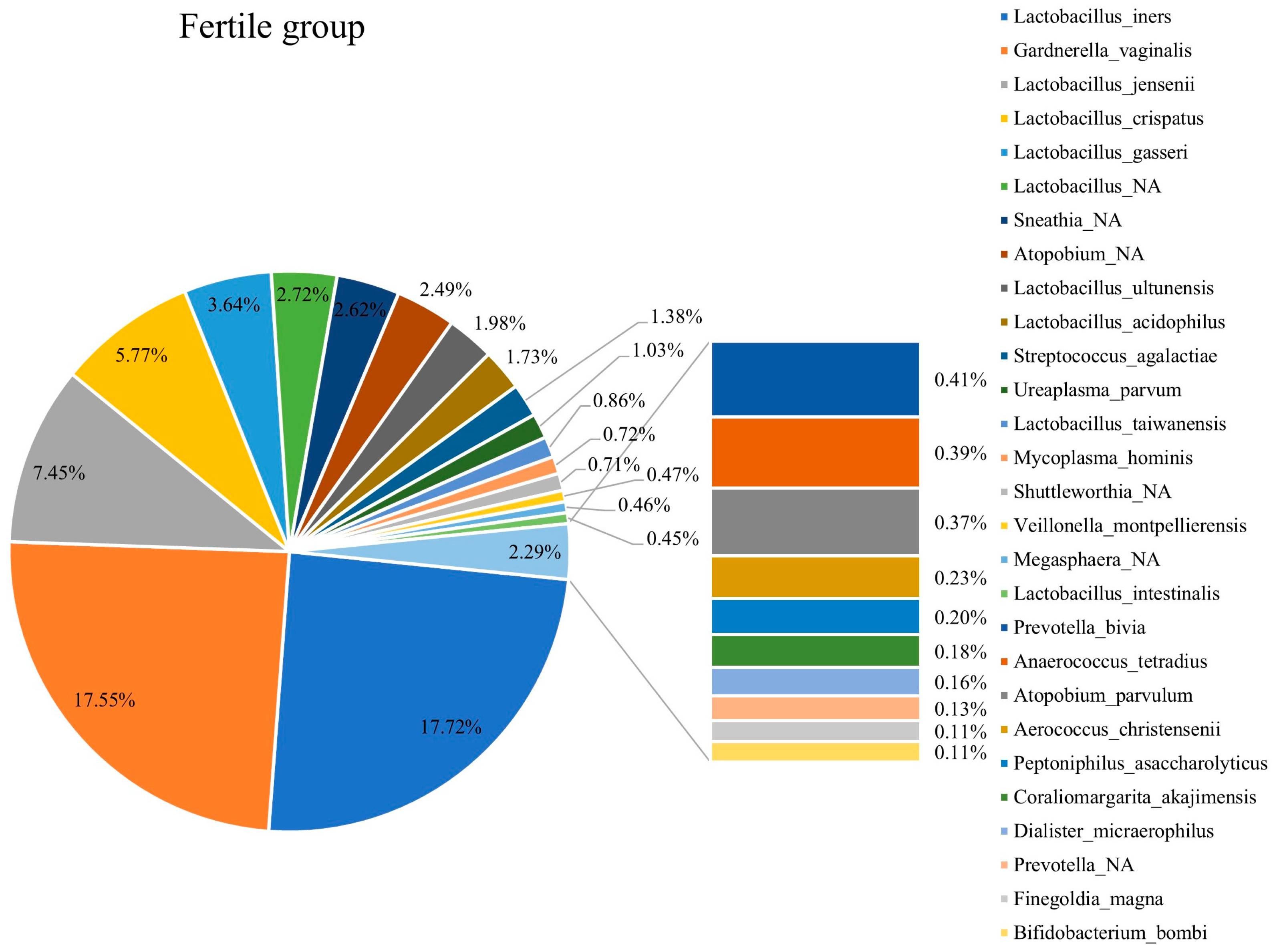
3.3. Composition of Vaginal Microbiota in Fertile and Infertile Women
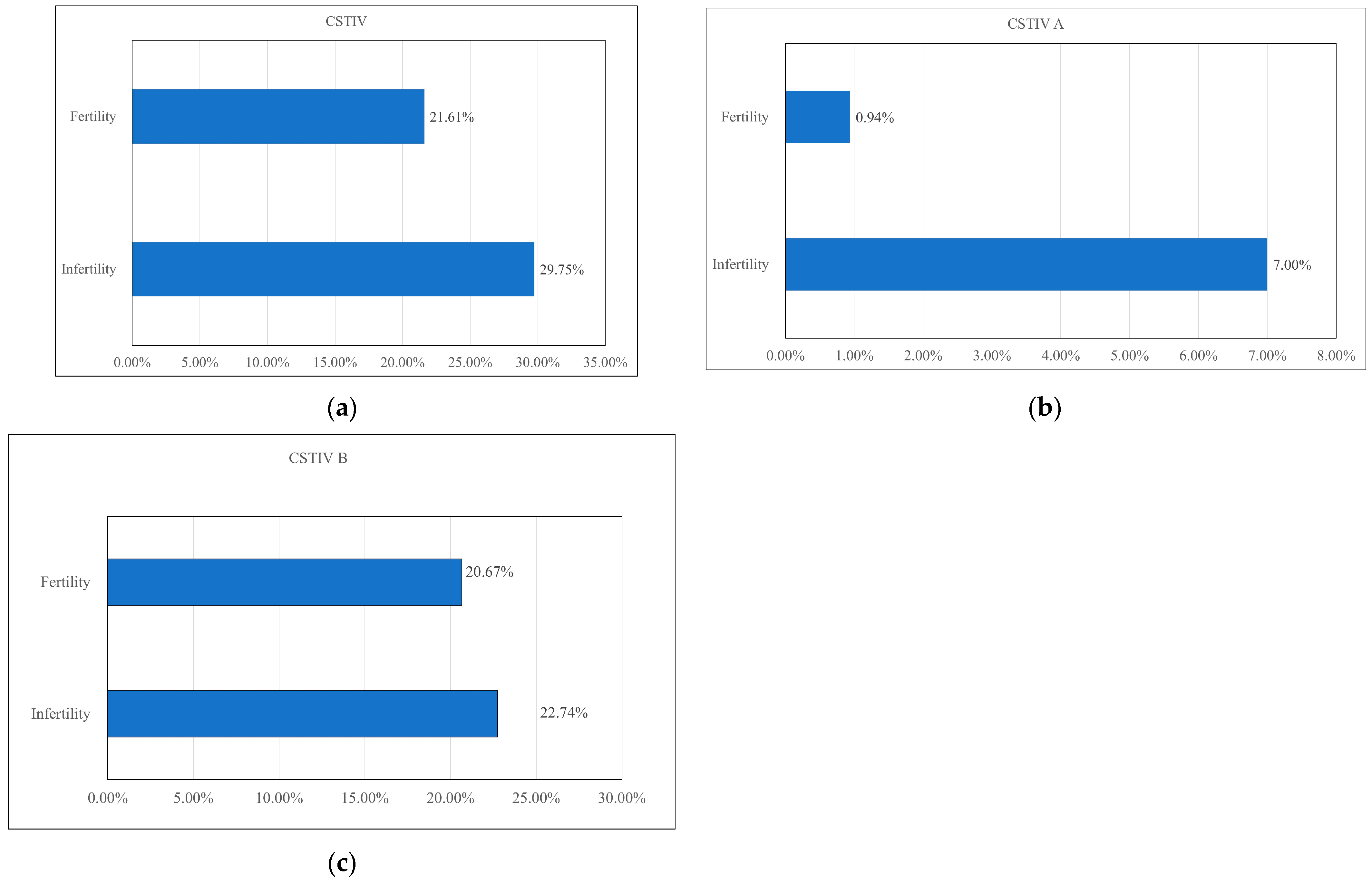
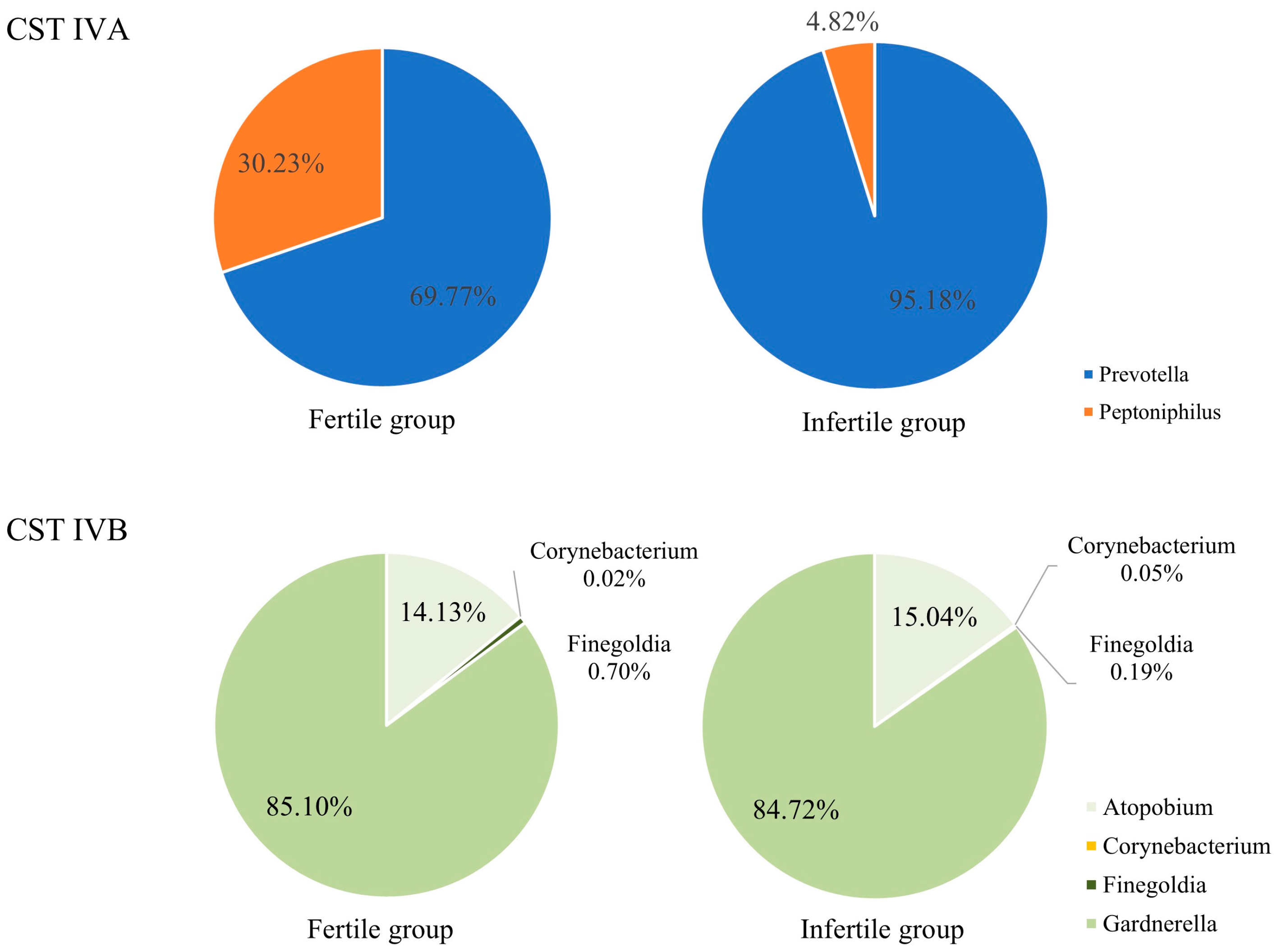
3.4. CST IV Composition Between the Infertile and Fertile Groups
3.5. Comparison of Vaginal Microbiota Compositions in Infertile and Fertile Females
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CST | Community state type |
| BMI | Body mass index |
| PCR | Polymerase chain reaction |
| NGS | Next-generation sequencing |
| SD | Standard deviation |
| BV | Bacterial vaginosis |
References
- Inhorn, M.C.; Patrizio, P. Infertility around the Globe: New Thinking on Gender, Reproductive Technologies and Global Movements in the 21st Century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef]
- Bourrion, B.; Panjo, H.; Bithorel, P.-L.; de La Rochebrochard, E.; François, M.; Pelletier-Fleury, N. The Economic Burden of Infertility Treatment and Distribution of Expenditures Overtime in France: A Self-Controlled Pre-Post Study. BMC Health Serv. Res. 2022, 22, 512. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and Infertility: Definition and Epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Infertility Workup for the Women’s Health Specialist: ACOG Committee Opinion Summary, Number 781. Obstet. Gynecol. 2019, 133, 1294–1295. [CrossRef] [PubMed]
- Walker, M.H.; Tobler, K.J. Female Infertility; StatPearls Publishing: Petersburg, FL, USA, 2021. [Google Scholar]
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility. JAMA 2021, 326, 65. [Google Scholar] [CrossRef]
- Hong, X.; Ma, J.; Yin, J.; Fang, S.; Geng, J.; Zhao, H.; Zhu, M.; Ye, M.; Zhu, X.; Xuan, Y.; et al. The Association between Vaginal Microbiota and Female Infertility: A Systematic Review and Meta-Analysis. Arch. Gynecol. Obstet. 2020, 302, 569–578. [Google Scholar] [CrossRef]
- Eijkemans, M.J.C.; van Poppel, F.; Habbema, D.F.; Smith, K.R.; Leridon, H.; te Velde, E.R. Too Old to Have Children? Lessons from Natural Fertility Populations. Hum. Reprod. 2014, 29, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Moreno, I.; Simón, C. Bacterial Vaginosis and Its Association with Infertility, Endometritis, and Pelvic Inflammatory Disease. Am. J. Obstet. Gynecol. 2021, 224, 251–257. [Google Scholar] [CrossRef]
- Chopra, C.; Kumar, V.; Kumar, M.; Bhushan, I. Role of Vaginal Microbiota in Idiopathic Infertility: A Prospective Study. Microbes Infect. 2024, 26, 105308. [Google Scholar] [CrossRef]
- Patel, N.; Patel, N.; Pal, S.; Nathani, N.; Pandit, R.; Patel, M.; Patel, N.; Joshi, C.; Parekh, B. Distinct Gut and Vaginal Microbiota Profile in Women with Recurrent Implantation Failure and Unexplained Infertility. BMC Women’s Health 2022, 22, 113. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef]
- Xu, J.; Bian, G.; Zheng, M.; Lu, G.; Chan, W.-Y.; Li, W.; Yang, K.; Chen, Z.-J.; Du, Y. Fertility Factors Affect the Vaginal Microbiome in Women of Reproductive Age. Am. J. Reprod. Immunol. 2020, 83, e13220. [Google Scholar] [CrossRef]
- Tao, X.; Ge, S.-Q.; Chen, L.; Cai, L.-S.; Hwang, M.-F.; Wang, C.-L. Relationships between Female Infertility and Female Genital Infections and Pelvic Inflammatory Disease: A Population-Based Nested Controlled Study. Clinics 2018, 73, e364. [Google Scholar] [CrossRef]
- Takimoto, K.; Yamada, H.; Shimada, S.; Fukushi, Y.; Wada, S. Chronic Endometritis and Uterine Endometrium Microbiota in Recurrent Implantation Failure and Recurrent Pregnancy Loss. Biomedicines 2023, 11, 2391. [Google Scholar] [CrossRef]
- de Oliveira, M.S. Infertility and Vaginal Microbiome: Review Study. Braz. J. Sex. Transm. Dis. 2019, 31, 24–29. [Google Scholar]
- Gao, X.; Louwers, Y.V.; Laven, J.S.E.; Schoenmakers, S. Clinical Relevance of Vaginal and Endometrial Microbiome Investigation in Women with Repeated Implantation Failure and Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2024, 25, 622. [Google Scholar] [CrossRef]
- Green, K.A.; Zarek, S.M.; Catherino, W.H. Gynecologic Health and Disease in Relation to the Microbiome of the Female Reproductive Tract. Fertil. Steril. 2015, 104, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Venneri, M.A.; Franceschini, E.; Sciarra, F.; Rosato, E.; D’Ettorre, G.; Lenzi, A. Human Genital Tracts Microbiota: Dysbiosis Crucial for Infertility. J. Endocrinol. Investig. 2022, 45, 1151–1160. [Google Scholar] [CrossRef]
- Vitale, S.G.; Ferrari, F.; Ciebiera, M.; Zgliczyńska, M.; Rapisarda, A.M.C.; Vecchio, G.M.; Pino, A.; Angelico, G.; Knafel, A.; Riemma, G.; et al. The Role of Genital Tract Microbiome in Fertility: A Systematic Review. Int. J. Mol. Sci. 2021, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Kroon, S.J.; Ravel, J.; Huston, W.M. Cervicovaginal Microbiota, Women’s Health, and Reproductive Outcomes. Fertil. Steril. 2018, 110, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, S.; Chen, X.; Zhang, H.; Huang, X.; Xue, X.; Guo, Y.; Ruan, X.; Liu, X.; Deng, G.; et al. Association between Vaginal Gardnerella and Tubal Pregnancy in Women with Symptomatic Early Pregnancies in China: A Nested Case-Control Study. Front. Cell. Infect. Microbiol. 2021, 11, 761153. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J.; et al. Correction: The Composition and Stability of the Vaginal Microbiota of Normal Pregnant Women Is Different from That of Non-Pregnant Women. Microbiome 2014, 2, 10. [Google Scholar]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Fadrosh, D.; Chang, K.; Silver, M.I.; Viscidi, R.P.; Burke, A.E.; Ravel, J.; Gravitt, P.E. Association between the Vaginal Microbiota, Menopause Status, and Signs of Vulvovaginal Atrophy. Menopause 2014, 21, 450–458. [Google Scholar] [CrossRef]
- Chopra, C.; Bhushan, I.; Mehta, M.; Koushal, T.; Gupta, A.; Sharma, S.; Kumar, M.; Khodor, S.A.; Sharma, S. Vaginal Microbiome: Considerations for Reproductive Health. Future Microbiol. 2022, 17, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Wee, B.A.; Thomas, M.; Sweeney, E.L.; Frentiu, F.D.; Samios, M.; Ravel, J.; Gajer, P.; Myers, G.; Timms, P.; Allan, J.A.; et al. A Retrospective Pilot Study to Determine Whether the Reproductive Tract Microbiota Differs between Women with a History of Infertility and Fertile Women. Aust. N. Z. J. Obstet. Gynaecol. 2018, 58, 341–348. [Google Scholar] [CrossRef]
- ZymoBIOMICSTM DNA Miniprep Kit Instruction Manual Ver 1.4.1. 27 August 2020. 2020. Available online: https://zymoresearch.eu/products/zymobiomics-dna-miniprep-kit (accessed on 7 October 2025).
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S RRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Hsu, M.-H.; Tu, S.-J.; Yen, J.-C.; Lee, Y.-T.; Fang, H.-Y.; Chang, J.-G. Metatranscriptomic Analysis of Human Lung Metagenomes from Patients with Lung Cancer. Genes 2021, 12, 1458. [Google Scholar] [CrossRef]
- Burton, J.P.; Reid, G. Evaluation of the Bacterial Vaginal Flora of 20 Postmenopausal Women by Direct (Nugent Score) and Molecular (Polymerase Chain Reaction and Denaturing Gradient Gel Electrophoresis) Techniques. J. Infect. Dis. 2002, 186, 1770–1780. [Google Scholar] [CrossRef]
- Zhao, C.; Wei, Z.; Yang, J.; Zhang, J.; Yu, C.; Yang, A.; Zhang, M.; Zhang, L.; Wang, Y.; Mu, X.; et al. Characterization of the Vaginal Microbiome in Women with Infertility and Its Potential Correlation with Hormone Stimulation during in Vitro Fertilization Surgery. mSystems 2020, 5, e00450-20. [Google Scholar] [CrossRef] [PubMed]
- Haahr, T.; Zacho, J.; Bräuner, M.; Shathmigha, K.; Skov Jensen, J.; Humaidan, P. Reproductive Outcome of Patients Undergoing in Vitro Fertilisation Treatment and Diagnosed with Bacterial Vaginosis or Abnormal Vaginal Microbiota: A Systematic PRISMA Review and Meta-Analysis. BJOG 2019, 126, 200–207. [Google Scholar] [CrossRef]
- Campisciano, G.; Florian, F.; D’Eustacchio, A.; Stanković, D.; Ricci, G.; De Seta, F.; Comar, M. Subclinical Alteration of the Cervical-Vaginal Microbiome in Women with Idiopathic Infertility. J. Cell. Physiol. 2017, 232, 1681–1688. [Google Scholar] [CrossRef]
- Zhou, X.; Brown, C.J.; Abdo, Z.; Davis, C.C.; Hansmann, M.A.; Joyce, P.; Foster, J.A.; Forney, L.J. Differences in the Composition of Vaginal Microbial Communities Found in Healthy Caucasian and Black Women. ISME J. 2007, 1, 121–133. [Google Scholar] [CrossRef]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.E.; Zhong, X.; Koenig, S.S.K.; Fu, L.; Ma, Z.S.; Zhou, X.; et al. Temporal Dynamics of the Human Vaginal Microbiota. Sci. Transl. Med. 2012, 4, 132ra52. [Google Scholar] [CrossRef]
- El-Sayed, A.; Aleya, L.; Kamel, M. Microbiota’s Role in Health and Diseases. Environ. Sci. Pollut. Res. Int. 2021, 28, 36967–36983. [Google Scholar] [CrossRef] [PubMed]
- Koedooder, R.; Singer, M.; Schoenmakers, S.; Savelkoul, P.H.M.; Morré, S.A.; de Jonge, J.D.; Poort, L.; Cuypers, W.J.S.S.; Beckers, N.G.M.; Broekmans, F.J.M.; et al. The Vaginal Microbiome as a Predictor for Outcome of in Vitro Fertilization with or without Intracytoplasmic Sperm Injection: A Prospective Study. Hum. Reprod. 2019, 34, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Sezer, O.; Soyer Çalışkan, C.; Celik, S.; Kilic, S.S.; Kuruoglu, T.; Unluguzel Ustun, G.; Yurtcu, N. Assessment of Vaginal and Endometrial Microbiota by Real-Time PCR in Women with Unexplained Infertility. J. Obstet. Gynaecol. Res. 2022, 48, 129–139. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, W.; Yuan, Y.; Zhu, W.; Shang, A. Vaginal Microecological Characteristics of Women in Different Physiological and Pathological Period. Front. Cell. Infect. Microbiol. 2022, 12, 959793. [Google Scholar] [CrossRef]
- Cocomazzi, G.; De Stefani, S.; Del Pup, L.; Palini, S.; Buccheri, M.; Primiterra, M.; Sciannamè, N.; Faioli, R.; Maglione, A.; Baldini, G.M.; et al. The Impact of the Female Genital Microbiota on the Outcome of Assisted Reproduction Treatments. Microorganisms 2023, 11, 1443. [Google Scholar] [CrossRef]
- Lledo, B.; Fuentes, A.; Lozano, F.M.; Cascales, A.; Morales, R.; Hortal, M.; Sellers, F.; Palacios-Marques, A.; Bermejo, R.; Quereda, F.; et al. Identification of Vaginal Microbiome Associated with IVF Pregnancy. Sci. Rep. 2022, 12, 6807. [Google Scholar] [CrossRef]
- Zhang, F.; Dai, J.; Chen, T. Role of Lactobacillus in Female Infertility via Modulating Sperm Agglutination and Immobilization. Front. Cell. Infect. Microbiol. 2020, 10, 620529. [Google Scholar] [CrossRef] [PubMed]
- Baud, D.; Zuber, A.; Peric, A.; Pluchino, N.; Vulliemoz, N.; Stojanov, M. Impact of Semen Microbiota on the Composition of Seminal Plasma. Microbiol. Spectr. 2024, 12, e0291123. [Google Scholar] [CrossRef]
- Weng, S.-L.; Chiu, C.-M.; Lin, F.-M.; Huang, W.-C.; Liang, C.; Yang, T.; Yang, T.-L.; Liu, C.-Y.; Wu, W.-Y.; Chang, Y.-A.; et al. Bacterial Communities in Semen from Men of Infertile Couples: Metagenomic Sequencing Reveals Relationships of Seminal Microbiota to Semen Quality. PLoS ONE 2014, 9, e110152. [Google Scholar] [CrossRef]
- Zeyad, A.; Hamad, M.; Amor, H.; Hammadeh, M.E. Relationships between Bacteriospermia, DNA Integrity, Nuclear Protamine Alteration, Sperm Quality and ICSI Outcome. Reprod. Biol. 2018, 18, 115–121. [Google Scholar] [CrossRef]
- Pagliuca, C.; Cariati, F.; Bagnulo, F.; Scaglione, E.; Carotenuto, C.; Farina, F.; D’Argenio, V.; Carraturo, F.; D’Aprile, P.; Vitiello, M.; et al. Microbiological Evaluation and Sperm DNA Fragmentation in Semen Samples of Patients Undergoing Fertility Investigation. Genes 2021, 12, 654. [Google Scholar] [CrossRef] [PubMed]
- Campisciano, G.; Iebba, V.; Zito, G.; Luppi, S.; Martinelli, M.; Fischer, L.; De Seta, F.; Basile, G.; Ricci, G.; Comar, M. Lactobacillus Iners and Gasseri, Prevotella Bivia and HPV Belong to the Microbiological Signature Negatively Affecting Human Reproduction. Microorganisms 2020, 9, 39. [Google Scholar] [CrossRef]
- Mändar, R.; Punab, M.; Borovkova, N.; Lapp, E.; Kiiker, R.; Korrovits, P.; Metspalu, A.; Krjutškov, K.; Nõlvak, H.; Preem, J.-K.; et al. Complementary Seminovaginal Microbiome in Couples. Res. Microbiol. 2015, 166, 440–447. [Google Scholar] [CrossRef]
- Amato, V.; Papaleo, E.; Pasciuta, R.; Viganò, P.; Ferrarese, R.; Clementi, N.; Sanchez, A.M.; Quaranta, L.; Burioni, R.; Ambrosi, A.; et al. Differential Composition of Vaginal Microbiome, but Not of Seminal Microbiome, Is Associated with Successful Intrauterine Insemination in Couples with Idiopathic Infertility: A Prospective Observational Study. Open Forum Infect. Dis. 2020, 7, ofz525. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Kong, Y.; Chen, W.; Liang, D.; Xiao, Q.; Hu, L.; Tan, X.; Wei, J.; Liu, Y.; Deng, X.; et al. Improvement of Vaginal Probiotics Lactobacillus Crispatus on Intrauterine Adhesion in Mice Model and in Clinical Practice. BMC Microbiol. 2023, 23, 78. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Koedooder, R.; Bos, M.P.; Poort, L.; Schoenmakers, S.; Savelkoul, P.H.M.; Laven, J.S.E.; de Jonge, J.D.; Morré, S.A.; Budding, A.E. The Profiling of Microbiota in Vaginal Swab Samples Using 16S RRNA Gene Sequencing and IS-pro Analysis. BMC Microbiol. 2021, 21, 100. [Google Scholar] [CrossRef]

| Bacterial Taxa | Infertility (n = 15) | Fertility (n = 7) | Total | p-Value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Kitasatospora_NA | 0.000 | 0.000 | 0.078 | 0.081 | 0.025 | 0.057 | 0.001 |
| Oscillospira_eae | 0.004 | 0.017 | 0.041 | 0.055 | 0.016 | 0.037 | 0.025 |
| Alkaliphilus_crotonatoxidans | 0.000 | 0.000 | 0.018 | 0.031 | 0.005 | 0.019 | 0.030 |
| Lactobacillus_farciminis | 0.000 | 0.000 | 0.034 | 0.059 | 0.011 | 0.035 | 0.030 |
| Lactobacillus_brantae | 0.008 | 0.033 | 0.125 | 0.201 | 0.011 | 0.035 | 0.037 |
| Lactobacillus_Japonicus | 0.000 | 0.000 | 0.025 | 0.046 | 0.008 | 0.027 | 0.039 |
| Staphylococcus_aureus | 0.010 | 0.027 | 0.064 | 0.091 | 0.027 | 0.059 | 0.044 |
| Corynebacterium_atypicum | 0.000 | 0.000 | 0.085 | 0.158 | 0.027 | 0.094 | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-C.; Chen, S.-F.; Hung, W.-T.; Lin, Y.-Y.; Lin, L.-C.; Wang, J.-H.; Chen, P.-C. Differences in Vaginal Microbiota Composition Between Infertile and Fertile Patients: A Prospective Study. Diagnostics 2025, 15, 2544. https://doi.org/10.3390/diagnostics15192544
Chen P-C, Chen S-F, Hung W-T, Lin Y-Y, Lin L-C, Wang J-H, Chen P-C. Differences in Vaginal Microbiota Composition Between Infertile and Fertile Patients: A Prospective Study. Diagnostics. 2025; 15(19):2544. https://doi.org/10.3390/diagnostics15192544
Chicago/Turabian StyleChen, Pei-Chen, Shih-Fen Chen, Wei-Tung Hung, Yu-Ying Lin, Ling-Chun Lin, Jen-Hung Wang, and Pao-Chu Chen. 2025. "Differences in Vaginal Microbiota Composition Between Infertile and Fertile Patients: A Prospective Study" Diagnostics 15, no. 19: 2544. https://doi.org/10.3390/diagnostics15192544
APA StyleChen, P.-C., Chen, S.-F., Hung, W.-T., Lin, Y.-Y., Lin, L.-C., Wang, J.-H., & Chen, P.-C. (2025). Differences in Vaginal Microbiota Composition Between Infertile and Fertile Patients: A Prospective Study. Diagnostics, 15(19), 2544. https://doi.org/10.3390/diagnostics15192544







