Incidence, Clinical Profile, and Cardiac Manifestations of MIS-C in Children in Kuwait
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Data Sources
2.3. Classification of MIS-C
2.4. Data Collection
2.5. Quality and Reliability of Diagnosis
2.6. Case Ascertainment and Adjudication
2.7. Data Analysis
2.8. Ethics
3. Results
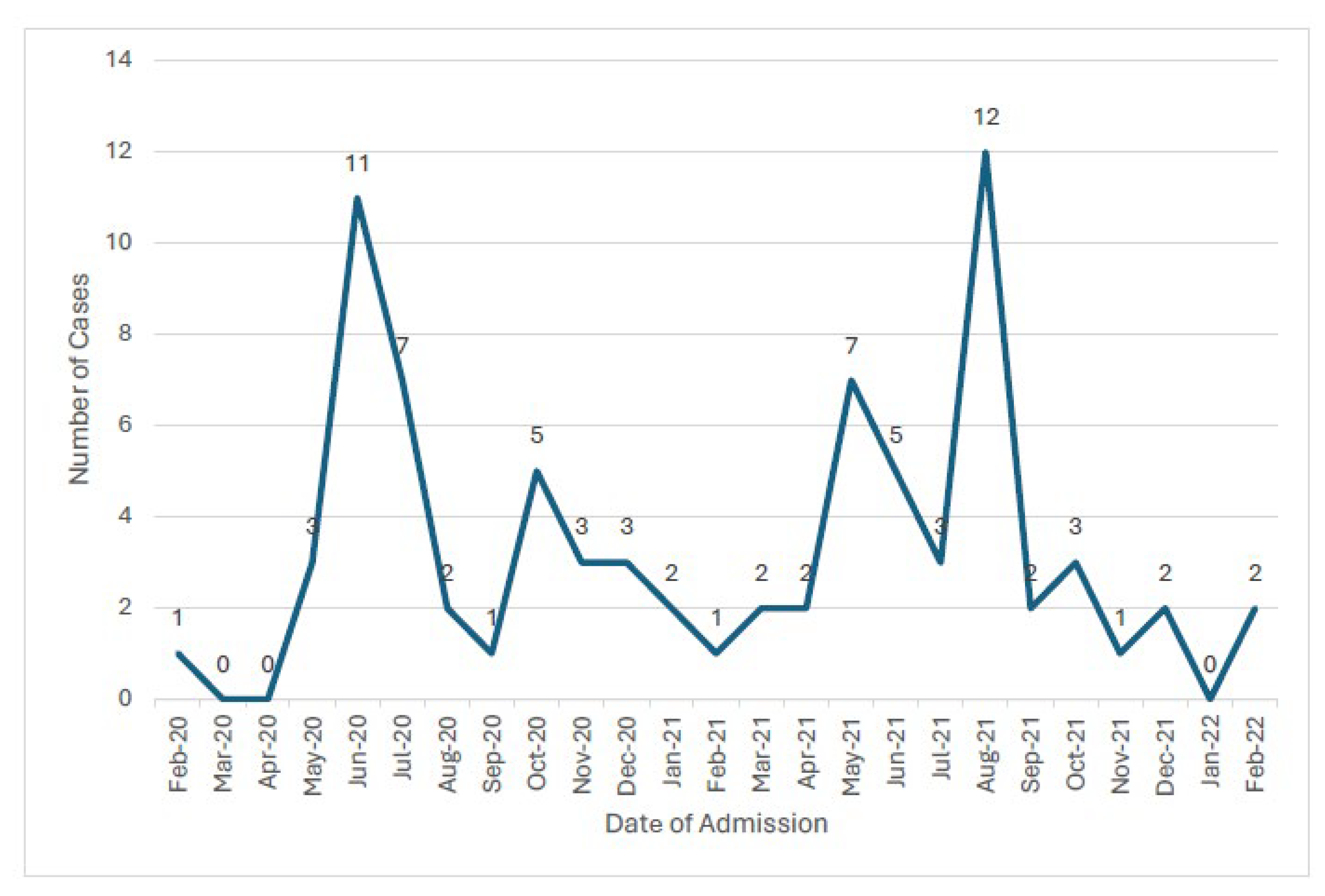
3.1. Incidence of MIS-C Amongst Children with SARS-CoV-2
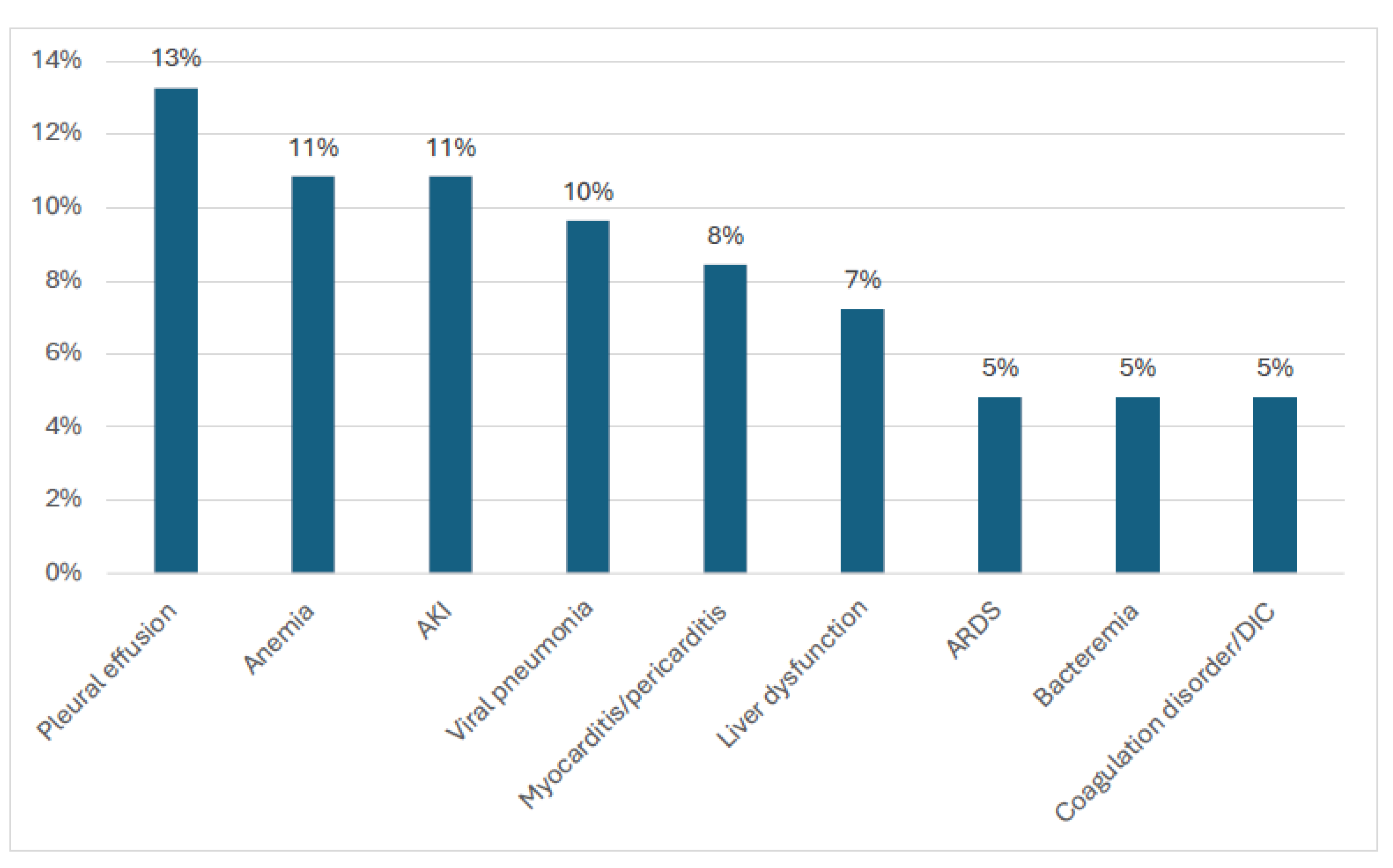
3.2. Demographic and Clinical Characteristics of the Children with MIS-C
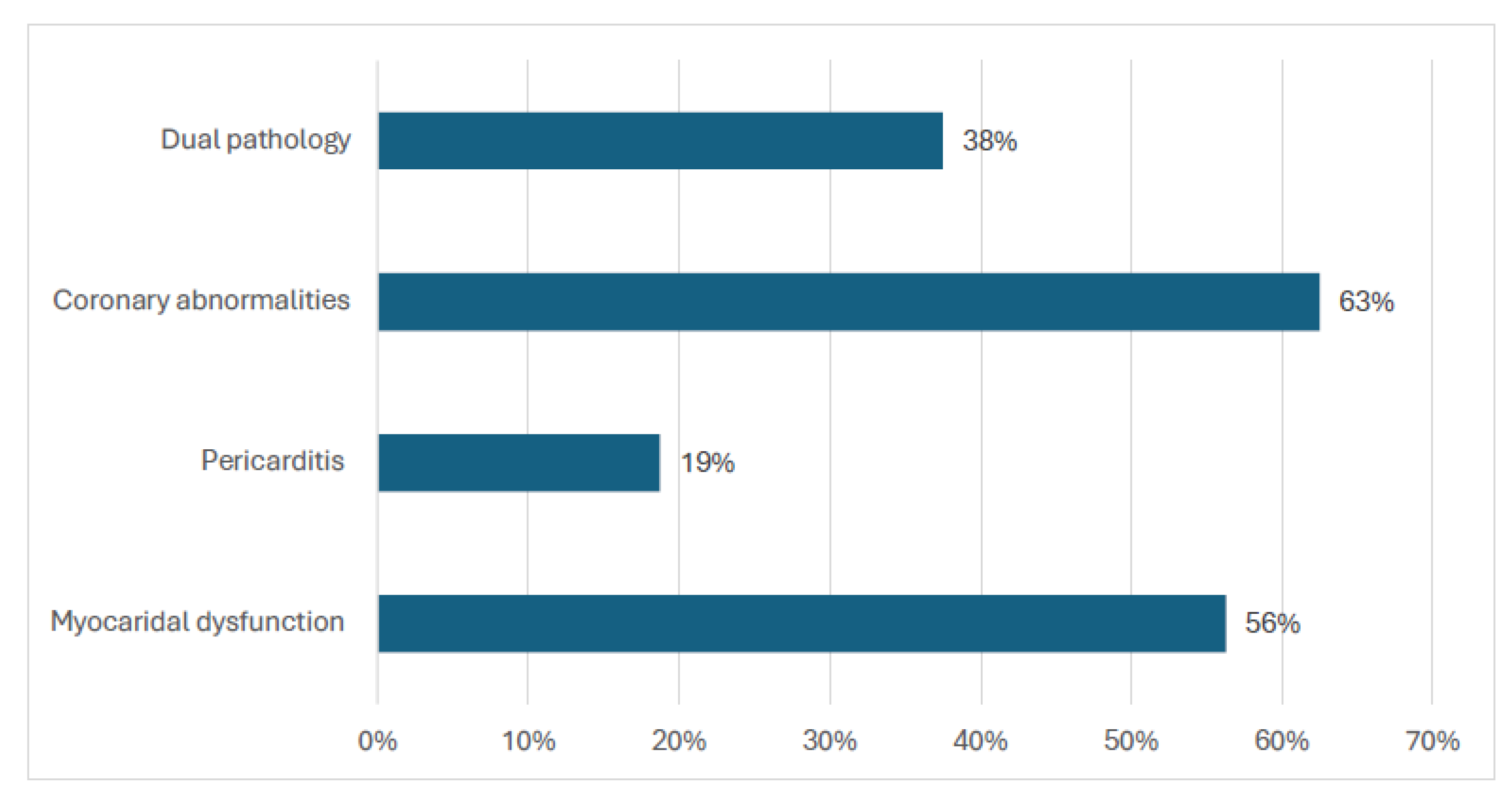
3.3. Echocardiogram Abnormalities
3.4. Demographic and Laboratory Profile of Patients with Cardiac Abnormalities
3.5. Treatment and Outcomes
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECG | Electrocardiogram |
| MIS-C | Multisystem inflammatory syndrome |
| PCR-Q8 | Pediatric COVID registry for Kuwait |
| PICU | Pediatric intensive care unit |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
References
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef]
- Gottlieb, M.; Bridwell, R.; Ravera, J.; Long, B. Multisystem inflammatory syndrome in children with COVID-19. Am. J. Emerg. Med. 2021, 49, 148–152. [Google Scholar] [CrossRef]
- Mizuno, S.; Ogawa, E.; Nozaki, M.; Cho, Y.; Kasai, M. Hospital burden and characteristics of pediatric COVID-19 based on a multicenter collaborative retrospective study in Japan. IJID Reg. 2023, 6, 108–112. [Google Scholar] [CrossRef]
- Lee, D.; Le Pen, J.; Yatim, A.; Dong, B.; Aquino, Y.; Ogishi, M.; Pescarmona, R.; Talouarn, E.; Rinchai, D.; Zhang, P.; et al. Inborn errors of OAS–RNase L in SARS-CoV-2–related multisystem inflammatory syndrome in children. Science 2022, 379, eabo3627. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.Y.; Godfred-Cato, S.E.; Oster, M.E.; Chow, E.J.; Koumans, E.H.; Bryant, B.; Leung, J.W.; Belay, E.D. Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2: A Systematic Review. J. Pediatr. 2020, 226, 45–54.e1. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, M.; Scheier, E.; Shavit, I. Multisystem inflammatory syndrome in children (MIS-C): A mini-review. Int. J. Emerg. Med. 2021, 14, 50. [Google Scholar] [CrossRef]
- Jiang, L.; Tang, K.; Irfan, O.; Li, X.; Zhang, E.; Bhutta, Z. Epidemiology, Clinical Features, and Outcomes of Multisystem Inflammatory Syndrome in Children (MIS-C) and Adolescents—A Live Systematic Review and Meta-analysis. Curr. Pediatr. Rep. 2022, 10, 19–30. [Google Scholar] [CrossRef]
- Sezer, M.; Çelikel, E.; Tekin, Z.E.; Aydın, F.; Kurt, T.; Tekgöz, N.; Karagöl, C.; Coşkun, S.; Kaplan, M.M.; Öner, N.; et al. Multisystem inflammatory syndrome in children: Clinical presentation, management, and short- and long-term outcomes. Clin. Rheumatol. 2022, 41, 3807–3816. [Google Scholar] [CrossRef]
- Rowley, A.H.; Shulman, S.T.; Arditi, M. Immune pathogenesis of COVID-19-related multisystem inflammatory syndrome in children. J. Clin. Investig. 2020, 130, 5619–5621. [Google Scholar] [CrossRef]
- Carter, M.J.; Fish, M.; Jennings, A.; Doores, K.J.; Wellman, P.; Seow, J.; Acors, S.; Graham, C.; Timms, E.; Kenny, J.; et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat. Med. 2020, 26, 1701–1707. [Google Scholar] [CrossRef]
- McMurray, J.C.; May, J.W.; Cunningham, M.W.; Jones, O.Y. Multisystem Inflammatory Syndrome in Children (MIS-C), a Post-viral Myocarditis and Systemic Vasculitis-A Critical Review of Its Pathogenesis and Treatment. Front Pediatr. 2020, 8, 626182. [Google Scholar] [CrossRef]
- Rhedin, S.; Lundholm, C.; Horne, A.; Smew, A.I.; Osvald, E.C.; Haddadi, A.; Alfvén, T.; Kahn, R.; Król, P.; Brew, B.H.; et al. Risk factors for multisystem inflammatory syndrome in children—A population-based cohort study of over 2 million children. Lancet Reg Health Eur. 2022, 19, 100443. [Google Scholar] [CrossRef]
- Fraser, L.K.; Miller, M.; Hain, R.; Norman, P.; Aldridge, J.; McKinney, P.A.; Parslow, R.C. Rising national prevalence of life-limiting conditions in children in England. Pediatrics 2012, 129, e923–e929. [Google Scholar] [CrossRef] [PubMed]
- Alkamali, A.; Al-Juailla, F.; Ahmed, K.E.; Qabazard, S.; Alkandari, A.; Alghounaim, M.; Al-Abdulrazzaq, D.; Al-Kandari, H.M. Epidemiology and Clinical Characteristics of sars-cov-2 Infection in Kuwait: Results of a National Registry. Pediatrics 2022, 149, 240. [Google Scholar]
- Qabazard, S.; Al-Abdulrazzaq, D.; Al-Kandari, H.; Ayed, M.; Alanezi, A.; Al-Shammari, N.; Alharbash, Z.; Al-Khabbaz, M.; Kalander, K.; Bin-Hassan, S.; et al. The Pediatric COVID-19 Registry in Kuwait: Methodology and Results of Pilot Phase. Med. Princ. Pract. 2022, 31, 471–479. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Multisystem Inflammatory Syndrome in Children and Adolescents Temporally Related to COVID-19; World Health Organisation: Geneva, Switzerland, 2020. [Google Scholar]
- Pouletty, M.; Borocco, C.; Ouldali, N.; Caseris, M.; Basmaci, R.; Lachaume, N.; Bensaid, P.; Pichard, S.; Kouider, H.; Morelle, G.; et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): A multicentre cohort. Ann. Rheum. Dis. 2020, 79, 999–1006. [Google Scholar] [CrossRef]
- McIntosh, A.M.; Tong, S.; Deakyne, S.J.; Davidson, J.A.; Scott, H.F. Validation of the vasoactive-inotropic score in pediatric sepsis. Pediatr. Crit. Care Med. 2017, 18, 750. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; National Institutes of Health: Bethesda, MD, USA, 2022. [Google Scholar]
- Ooms, C.; Mossong, J.; Vergison, A.; Biver, A.; Wagner, K.; Niel, O.; Parrish, A.; Abdelrahman, T.T.; de la Fuente Garcia, I. Multisystem inflammatory syndrome in children during the first two years of the COVID-19 pandemic in Luxembourg. Front. Pediatr. 2023, 11, 1141074. [Google Scholar] [CrossRef]
- Ptak, K.; Szymońska, I.; Olchawa-Czech, A.; Kukla, K.; Cisowska, M.; Kwinta, P. Comparison of the course of multisystem inflammatory syndrome in children during different pandemic waves. Eur. J. Pediatr. 2023, 182, 1647–1656. [Google Scholar] [CrossRef]
- Abraham, D.R.; Butters, C.; Abdulbari Yunis, N.; Lishman, J.; Scott, C.; van der Zalm, M.M.; Zühlke, L.; Rabie, H.; Webb, K. The Impact of SARS-CoV-2 Variants on the Clinical Phenotype and Severity of Multisystem Inflammatory Syndrome in Children in South Africa. Pediatr. Infect. Dis. J. 2022, 41, e510–e512. [Google Scholar] [CrossRef]
- Al-Harbi, S.; Kazzaz, Y.; Uddin, M.; Maghrabi, F.; Alnajjar, A.; Muzaffer, M.A.; Alnahdi, B.M.; Abusaif, S.M.; Alhejaili, A.; Aljohnei, R.; et al. Clinical characteristics and outcomes of Multisystem Inflammatory Syndrome in Children (MIS-C): A national multicenter cohort in Saudi Arabia. Curr. Pediatr. Res. 2021, 25, 904–913. [Google Scholar]
- Santos, M.O.; Gonçalves, L.C.; Silva, P.A.N.; Moreira, A.L.E.; Ito, C.R.M.; Peixoto, F.A.O.; Wastowski, I.J.; Carneiro, L.C.; Avelino, M.A. Multisystem inflammatory syndrome (MIS-C): A systematic review and meta-analysis of clinical characteristics, treatment, and outcomes. J. Pediatr. 2022, 98, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, M.S.; Burrows, H.; Joseph, J.P.; Leveille, J.; Nihtianova, S.; Amirian, E.S. Multisystem inflammatory syndrome in children (MIS-C) and the coronavirus pandemic: Current knowledge and implications for public health. J. Infect. Public Health 2021, 14, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Eaaswarkhanth, M.; Pathak, A.K.; Ongaro, L.; Montinaro, F.; Hebbar, P.; Alsmadi, O.; Metspalu, M.; Al-Mulla, F.; Thanaraj, T.A. Unraveling a fine-scale high genetic heterogeneity and recent continental connections of an Arabian Peninsula population. Eur. J. Hum. Genet. 2022, 30, 307–319. [Google Scholar] [CrossRef]
- Mamishi, S.; Olfat, M.; Pourakbari, B.; Eshaghi, H.; Abdolsalehi, M.R.; Shahbabaie, M.A.; Jalali, F.; Safari, F.; Mahmoudi, S. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in children: Update and new insights from the second report of an Iranian referral hospital. Epidemiol. Infect. 2022, 150, e179. [Google Scholar] [CrossRef]
- Ahmed, M.; Advani, S.; Moreira, A.; Zoretic, S.; Martinez, J.; Chorath, K.; Acosta, S.; Naqvi, R.; Burmeister-Morton, F.; Burmeister, F.; et al. Multisystem inflammatory syndrome in children: A systematic review. eClinicalMedicine 2020, 26, 100527. [Google Scholar] [CrossRef]
- Radia, T.; Williams, N.; Agrawal, P.; Harman, K.; Weale, J.; Cook, J.; Gupta, A. Multi-system inflammatory syndrome in children & adolescents (MIS-C): A systematic review of clinical features and presentation. Paediatr. Respir. Rev. 2021, 38, 51–57. [Google Scholar]
- Grimaud, M.; Starck, J.; Levy, M.; Marais, C.; Chareyre, J.; Khraiche, D.; Leruez-Ville, M.; Quartier, P.; Léger, P.L.; Geslain, G.; et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann. Intensive Care 2020, 10, 69. [Google Scholar] [CrossRef]
- Lee, P.Y.; Day-Lewis, M.; Henderson, L.A.; Friedman, K.G.; Lo, J.; Roberts, J.E.; Lo, M.S.; Platt, C.D.; Chou, J.; Hoyt, K.J.; et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J. Clin. Investig. 2020, 130, 5942–5950. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Tenforde, M.W.; Friedman, K.G.; Newhams, M.; Rose, E.B.; Dapul, H.; Soma, V.L.; Maddux, A.B.; Mourani, P.M.; Bowens, C.; et al. Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared with Severe Acute COVID-19. JAMA 2021, 325, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Hoste, L.; Van Paemel, R.; Haerynck, F. Multisystem inflammatory syndrome in children related to COVID-19: A systematic review. Eur. J. Pediatr. 2021, 180, 2019–2034. [Google Scholar] [CrossRef] [PubMed]
- Valverde, I.; Singh, Y.; Sanchez-de-Toledo, J.; Theocharis, P.; Chikermane, A.; Di Filippo, S.; Kucinska, B.; Mannarino, S.; Tamariz-Martel, A.; Gutierrez-Larraya, F.; et al. Acute Cardiovascular Manifestations in 286 Children With Multisystem Inflammatory Syndrome Associated With COVID-19 Infection in Europe. Circulation 2021, 143, 21–32. [Google Scholar] [CrossRef]
- Wu, E.Y.; Campbell, M.J. Cardiac Manifestations of Multisystem Inflammatory Syndrome in Children (MIS-C) Following COVID-19. Curr. Cardiol. Rep. 2021, 23, 168. [Google Scholar] [CrossRef]
- Binstadt, B.A.; Levine, J.C.; Nigrovic, P.A.; Gauvreau, K.; Dedeoglu, F.; Fuhlbrigge, R.C.; Weindling, S.N.; Newburger, J.W.; Sundel, R.P. Coronary Artery Dilation Among Patients Presenting with Systemic-Onset Juvenile Idiopathic Arthritis. Pediatrics 2005, 116, e89–e93. [Google Scholar] [CrossRef]
- Tissières, P.; Teboul, J.-L. SARS-CoV-2 post-infective myocarditis: The tip of COVID-19 immune complications? Ann. Intensive Care 2020, 10, 98. [Google Scholar] [CrossRef]
- Acevedo, L.; Piñeres-Olave, B.E.; Niño-Serna, L.F.; Vega, L.M.; Gomez, I.J.A.; Chacón, S.; Jaramillo-Bustamante, J.C.; Mulett-Hoyos, H.; González-Pardo, O.; Zemanate, E.; et al. Mortality and clinical characteristics of multisystem inflammatory syndrome in children (MIS-C) associated with covid-19 in critically ill patients: An observational multicenter study (MISCO study). BMC Pediatr. 2021, 21, 516. [Google Scholar] [CrossRef]
- Mannarino, S.; Raso, I.; Garbin, M.; Ghidoni, E.; Corti, C.; Goletto, S.; Nespoli, L.; Santacesaria, S.; Zoia, E.; Camporesi, A.; et al. Cardiac dysfunction in Multisystem Inflammatory Syndrome in Children: An Italian single-center study. Ital. J. Pediatr. 2022, 48, 25. [Google Scholar] [CrossRef]
- Algarni, A.S.; Alamri, N.M.; Khayat, N.Z.; Alabdali, R.A.; Alsubhi, R.S.; Alghamdi, S.H. Clinical practice guidelines in multisystem inflammatory syndrome (MIS-C) related to COVID-19: A critical review and recommendations. World J. Pediatr. 2022, 18, 83–90. [Google Scholar] [CrossRef]

| Children and adolescents 0–19 years of age with fever ≥3 days |
AND two of the following:
|
| AND Elevated markers of inflammation such as ESR, C-reactive protein, or procalcitonin. |
| AND No other obvious microbial cause of inflammation, including bacterial sepsis, staphylococcal or streptococcal shock syndromes. |
| AND Evidence of COVID-19 (RT-PCR, antigen test or serology positive) or likely contact with patients with COVID-19. |
| (a) | ||||||
|---|---|---|---|---|---|---|
| Variable | Characteristic | No Cardiac Abnormality | Cardiac Abnormality | Total (84) | Chi Squared | p Value |
| Gender | Male | 26 (43%) | 8 (10%) | 44 (52%) | 0.58 | 0.45 |
| Female | 30 (36%) | 10 (12%) | 40 (48%) | |||
| Nationality | Kuwait | 36 (43%) | 5 (6%) | 41 (49%) | 4.06 | 0.04 |
| Non-Kuwaiti | 30 (36%) | 13 (15%) | 43 (51%) | |||
| Age group | 0–3 years | 23 (27%) | 8 (10%) | 31 (37%) | 4.5 | 0.21 |
| 4 years–6 years | 20 (24%) | 3 (4%) | 23 (27%) | |||
| 7 years–9 years | 15 (18%) | 2 (2%) | 17 (20%) | |||
| >9 years | 8 (12%) | 5 (6%) | 13 (16%) | |||
| Disease severity | Mild | 46 (55%) | 7 (8%) | 53 (63%) | 7.61 | 0.02 * |
| Moderate | 15 (18%) | 6 (7%) | 21 (25%) | |||
| Severe | 5 (6%) | 5 (6%) | 10 (12%) | |||
| (b) | ||||||
| Laboratory Variables | MIS-C (Mean/SD) | Median (IQR) | Normal Values | |||
| Ferritin (μg/L) | 637 (790) | 414 (133–819) | 7–140 | |||
| C-reactive protein (mg/L) | 175 (114) | 157 (92–237) | 0–8 | |||
| Troponin T (ng/L) | 44 (104) | 10 (1.5–31) | <14 | |||
| D-dimer (μg/L) | 1953 (2103) | 1289 (803–1800) | <250 | |||
| Variable | Level | No Cardiac Abnormality n (%) | Cardiac Abnormality n (%) | Total n (%) | p-Value |
|---|---|---|---|---|---|
| Gender | 0 | 30 (36.6%) | 9 (11.0%) | 39 (47.6%) | 0.578 (Fisher) |
| 1 | 36 (43.9%) | 7 (8.5%) | 43 (52.4%) | ||
| Nationality | 0 | 36 (43.9%) | 7 (8.5%) | 43 (52.4%) | 0.578 (Fisher) |
| 1 | 30 (36.6%) | 9 (11.0%) | 39 (47.6%) | ||
| Age group | 0 | 14 (17.1%) | 3 (3.7%) | 17 (20.7%) | 0.630 (χ2) |
| 1 | 25 (30.5%) | 7 (8.5%) | 32 (39.0%) | ||
| 2 | 21 (25.6%) | 6 (7.3%) | 27 (32.9%) | ||
| 3 | 6 (7.3%) | 0 (0.0%) | 6 (7.3%) | ||
| Disease severity | Better | 2 (2.4%) | 0 (0.0%) | 2 (2.4%) | 0.630 (χ2) |
| Mild | 36 (43.9%) | 10 (12.2%) | 46 (56.1%) | ||
| Moderate | 17 (20.7%) | 5 (6.1%) | 22 (26.8%) | ||
| Severe | 11 (13.4%) | 1 (1.2%) | 12 (14.6%) | ||
| Measure | No Cardiac Abnormality Mean (SD) | Cardiac Abnormality Mean (SD) | Total Mean (SD) | p-Value | |
| Ferritin (µg/L) | 800.06 (1778.39) | 594.17 (952.58) | 756.71 (1634.13) | 0.277 | |
| C-reactive protein (mg/L) | 143.64 (107.27) | 192.64 (136.05) | 153.44 (114.30) | 0.236 | |
| Troponin (ng/L) | 37.87 (99.30) | 24.06 (33.71) | 35.30 (90.64) | 0.216 | |
| D-dimer (µg/L FEU) | 1608.06 (1586.02) | 2120.43 (2698.43) | 1715.12 (1860.15) | 0.982 | |
| Marker | Median (IQR) Normal | Median (IQR) Abnormal | Exact p |
|---|---|---|---|
| Troponin | 4.96 (0.45–22.84) | 12.00 (7.37–21.40) | 0.2161 |
| D-dimer | 1193.00 (753.00–1665.00) | 1087.00 (765.25–1817.25) | 0.9815 |
| Ferritin | 413.60 (167.00–697.00) | 192.75 (92.75–850.25) | 0.2773 |
| CRP | 138.00 (66.60–202.68) | 172.00 (87.20–286.00) | 0.2358 |
| Comparison LVEF vs. biomarkers | Spearman ρ | Exact p | |
| LVEF vs. Troponin | −0.199 | 0.3208 | |
| LVEF vs. D-dimer | 0.183 | 0.3245 | |
| LVEF vs. Ferritin | 0.111 | 0.5732 | |
| LVEF vs. CRP | −0.271 | 0.1045 | |
| Median (IQR) abnormal (LVEF vs. Echocardiogram) | Exact p | ||
| 56.00 (50.50–65.00) | 0.1003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahomed, O.; Alhadlaq, A.; Alsaeid, K.; Alsaqabi, A.; Othman, F.; Al-Shammari, S.; Al-Yaqoub, S.; Al-Daihani, A.; Alfraij, A.; Alafasy, K.; et al. Incidence, Clinical Profile, and Cardiac Manifestations of MIS-C in Children in Kuwait. Diagnostics 2025, 15, 2545. https://doi.org/10.3390/diagnostics15192545
Mahomed O, Alhadlaq A, Alsaeid K, Alsaqabi A, Othman F, Al-Shammari S, Al-Yaqoub S, Al-Daihani A, Alfraij A, Alafasy K, et al. Incidence, Clinical Profile, and Cardiac Manifestations of MIS-C in Children in Kuwait. Diagnostics. 2025; 15(19):2545. https://doi.org/10.3390/diagnostics15192545
Chicago/Turabian StyleMahomed, Ozayr, Adnan Alhadlaq, Khaled Alsaeid, Aisha Alsaqabi, Fouzeyah Othman, Saja Al-Shammari, Sarah Al-Yaqoub, Abdullah Al-Daihani, Abdulla Alfraij, Khalid Alafasy, and et al. 2025. "Incidence, Clinical Profile, and Cardiac Manifestations of MIS-C in Children in Kuwait" Diagnostics 15, no. 19: 2545. https://doi.org/10.3390/diagnostics15192545
APA StyleMahomed, O., Alhadlaq, A., Alsaeid, K., Alsaqabi, A., Othman, F., Al-Shammari, S., Al-Yaqoub, S., Al-Daihani, A., Alfraij, A., Alafasy, K., Al-Qallaf, M., Al-Hajeri, M., Al-Mutairi, N., Alenezi, A., Mohammed, S., Al-Sarraf, A., Al-Abdulrazzaq, D., & Al-Kandari, H. (2025). Incidence, Clinical Profile, and Cardiac Manifestations of MIS-C in Children in Kuwait. Diagnostics, 15(19), 2545. https://doi.org/10.3390/diagnostics15192545








