Prognostic Insights into Orbital Metastases: A Comprehensive Analysis of Clinical Features and Survival Outcomes
Abstract
1. Introduction
2. Materials and Methods
- Patient Cohort
- 2.
- Data Collection
- Demographic data: Age at diagnosis and gender.
- Clinical findings: Presenting symptoms, laterality of orbital involvement, and specific signs such as proptosis, pain, vision changes, and diplopia.
- Tumor characteristics: Primary tumor type, location of the orbital metastasis, and histopathological diagnosis.
- Treatment modalities: Information regarding systemic chemotherapy, radiotherapy, surgical intervention, or other treatments received for orbital metastasis.
- Survival outcomes: Overall survival time from the diagnosis of orbital metastasis until the last follow-up or death.
- 3.
- Subgroup Analyses
- Age groups: Pediatric (<18 years) and adult (≥19 years). Subgroups (0–19 years, 20–39 years, 40–59 years, 60–79 years, ≥80 years)
- Gender: Male and female.
- Primary tumor type: Major primary tumor categories, including breast cancer, lung cancer, neuroblastoma, and others, were analyzed to assess their impact on clinical presentation and survival.
- 4.
- Statistical Analysis
- 5.
- Ethical Considerations
3. Results
3.1. Patient Demographics and Tumor Characteristics
3.2. Primary Tumor Distribution
3.3. Age Distribution and Primary Tumor Correlation
3.4. Survival Outcomes
3.5. Time to Metastasis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shields, J.A.; Shields, C.L.; Brotman, H.K.; Carvalho, C.; Perez, N.; Eagle, R.C., Jr. Cancer metastatic to the orbit’ The 200 Robert M. Curts Lecture. Ophthalmic Plast. Reconstr. Surg. 2001, 17, 346–354. [Google Scholar] [CrossRef]
- Magliozzi, P.; Strianese, D.; Bonavolonta, P.; Ferrara, M.; Ruggiero, P.; Carandente, R.; Banavolonta, G.; Tranfa, F. Orbital metastases in Italy. Int. J. Ophthalmol. 2015, 8, 1018–1023. [Google Scholar] [PubMed]
- Zhao, Y.; Yu, S.; Mu, M.; Li, J.; Li, H.; Zhao, H. Clinical and Imaging Characterics of Metastatic Orbital Tumours in North China. J. Ophthalmol. 2024, 2024, 3394425. [Google Scholar] [CrossRef]
- Amemiya, T.; Hayashida, H.; Dake, Y. Metastatic orbital tumors in Japan: A review of the literature. Ophthalmic Epidemiol. 2002, 9, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Eldesouky, M.A.; Elbakary, M.A. Clinical and imaging characteristics of orbital metastatic lesions among Egyptian patients. Clin. Ophthalmol. 2015, 9, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, A.A.; Archibald, C.W.; Fleming, B.; Ong, L.; O’Donnell, B.; Crompton, J.J.; Selva, D.; McNab, A.A.; Sullivan, T.J. Orbital Metastasis: Clinical Features, Management and Outcome. Orbit 2009, 28, 153–159. [Google Scholar] [CrossRef]
- Kamieniarz, L.; Armeni, E.; O’Mahony, L.F.; Leigh, C.; Miah, L.; Narayan, A.; Bhatt, A.; Cox, N.; Mandair, D.; Navalkissoor, S.; et al. Orbital metastases from neuroendocrine neoplasms: Clinical implications and outcomes. Endocrine 2020, 67, 485–493. [Google Scholar] [CrossRef]
- Wladis, E.J.; Lee, K.W.; Nazeer, T. Metastases of systemic malignancies to the orbit: A major review. Orbit 2021, 40, 93–97. [Google Scholar] [CrossRef]
- Grajales-Alvarez, R.; Gutierrez-Mata, A. Orbital metastases from breast cancer: A retrospective analysis of 28 cases. Cancer Treat. Res. Commun. 2020, 24, 100184. [Google Scholar] [CrossRef]
- Tsagkaraki, I.M.; Kourouniotis, C.D.; Gomatou, G.L.; Syrigos, N.K.; Kotteas, E.A. Orbital metastases of invasive lobular breast carcinoma. Breast Dis. 2019, 38, 85–91. [Google Scholar] [CrossRef]
- Palmisciano, P.; Ferini, G.; Ogasawara, C.; Wahood, W.; Bin Alamer, O.; Gupta, A.D.; Scalia, G.; Larsen, A.M.G.; Yu, K.; Umana, G.E.; et al. Orbital Metastases: A Systematic Review of Clinical Characteristics, Management Strategies, and Treatment Outcomes. Cancers 2022, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.A.; Rootman, J. Clinical Characteristics of Metastatic Orbital Tumors. Ophthalmology 1990, 97, 620–624. [Google Scholar] [CrossRef]
- Günalp, I.; Gündüz, K. Metastatic orbital tumors. Jpn J. Opthalmol. 1995, 39, 65–70. [Google Scholar]
- Ahmad, S.M.; Esmaeli, B. Metastatic tumors of the orbit and ocular adnexa. Curr. Opin. Ophthalmol. 2007, 18, 405–413. [Google Scholar] [CrossRef]
- Allen, R.C. Orbital metastases: When to suspect? When to biopsy? Middle East Afr. J. Ophthalmol. 2018, 25, 60–64. [Google Scholar] [CrossRef]
- Gotwald, T.F.; Zinreich, S.J.; Schocke, M.; Frede, T.; Bellmann, R.; Zur Nedden, D. CT and MR Imaging of Orbital Metastasis from Islet Cell Carcinoma of the Pancreas. AJR Am. J. Roentgenol. 2000, 175, 475–476. [Google Scholar] [CrossRef] [PubMed]
- Peschiaroli, S.; Iuliano, A.; Cuffaro, G.; Leoni, F.M.Q.; Tartaglione, T.; Pagliara, M.M.; Sammarco, M.G.; Caputo, C.G.; Santoro, A.; Barchitta, M.; et al. Breast cancer orbital metastases: Clinical and histopathological characteristics, imaging features, and disease-related survival in a multicenter retrospective case series. Cancers 2025, 17, 1875. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Shields, J.A.; Gross, N.; Schwartz, G.P.; Lally, S.E. Survey of 520 uveal metastases. Ophthalmology 1997, 104, 1265–1276. [Google Scholar] [CrossRef]
- Garrity, J.A.; Henderson, J.W.; Cameron, J.D. Metastatic carcinomas. In Henderson’s Orbital Tumors, 4th ed.; Raven Press: New York, NY, USA, 2007; pp. 313–326. [Google Scholar]
- Thapa, B.; Arobelidze, S.; Clark, B.A.; Xuefei, J.; Daw, H.; Cheng, Y.C.; Patel, M.; Spiro, T.P.; Haddad, A. Metaplastic breast cancer: Characteristics and survival outcomes. Cureus 2022, 14, e28551. [Google Scholar] [CrossRef]
- Tsutsumi, W.D.; Rattanasuwan, A.; Aryasit, O. Clinical features and treatment outcomes of intraocular and ocular adnexal metastasis. Sci. Rep. 2024, 14, 15258. [Google Scholar] [CrossRef]
- Liu, X.; Yue, H.; Jiang, S.; Kong, L.; Xu, Y.; Chen, Y.; Wang, C.; Wang, Y.; Zhu, X.; Kong, Y.; et al. Clinical features and prognosis of patients with metastatic ocular and orbital melanoma: A bi-institutional study. Cancer Med. 2023, 12, 16163–16172. [Google Scholar] [CrossRef] [PubMed]
- Vlachostergios, P.J.; Voutsadakis, I.A.; Papandreou, C.N. Orbital metastasis of breast carcinoma. Breast Cancer 2009, 3, 91–97. [Google Scholar] [CrossRef] [PubMed]
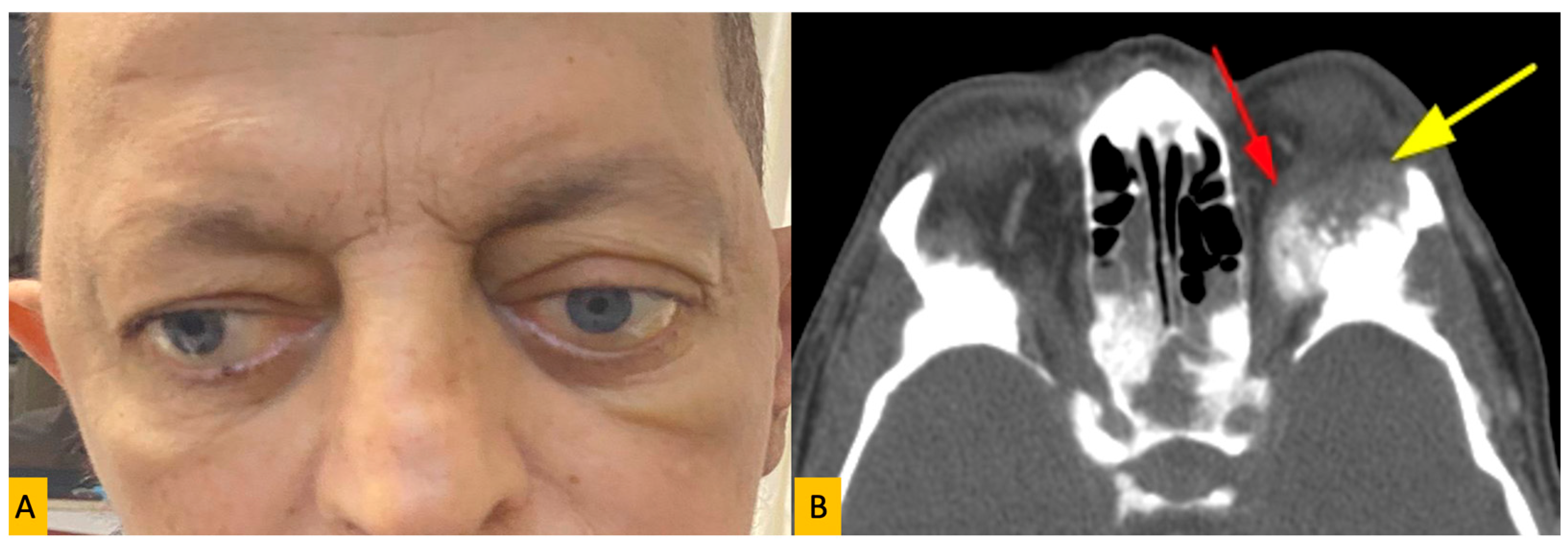
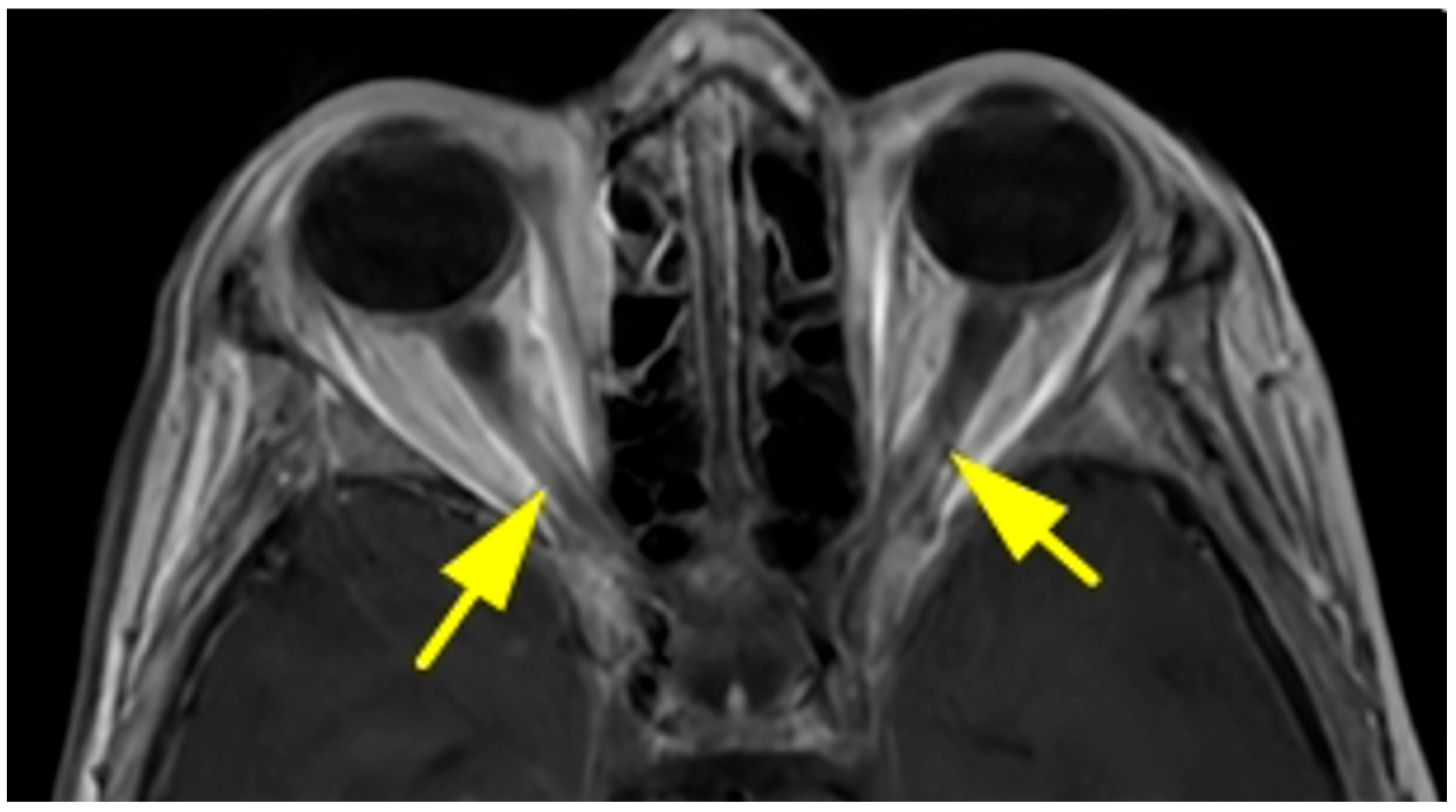
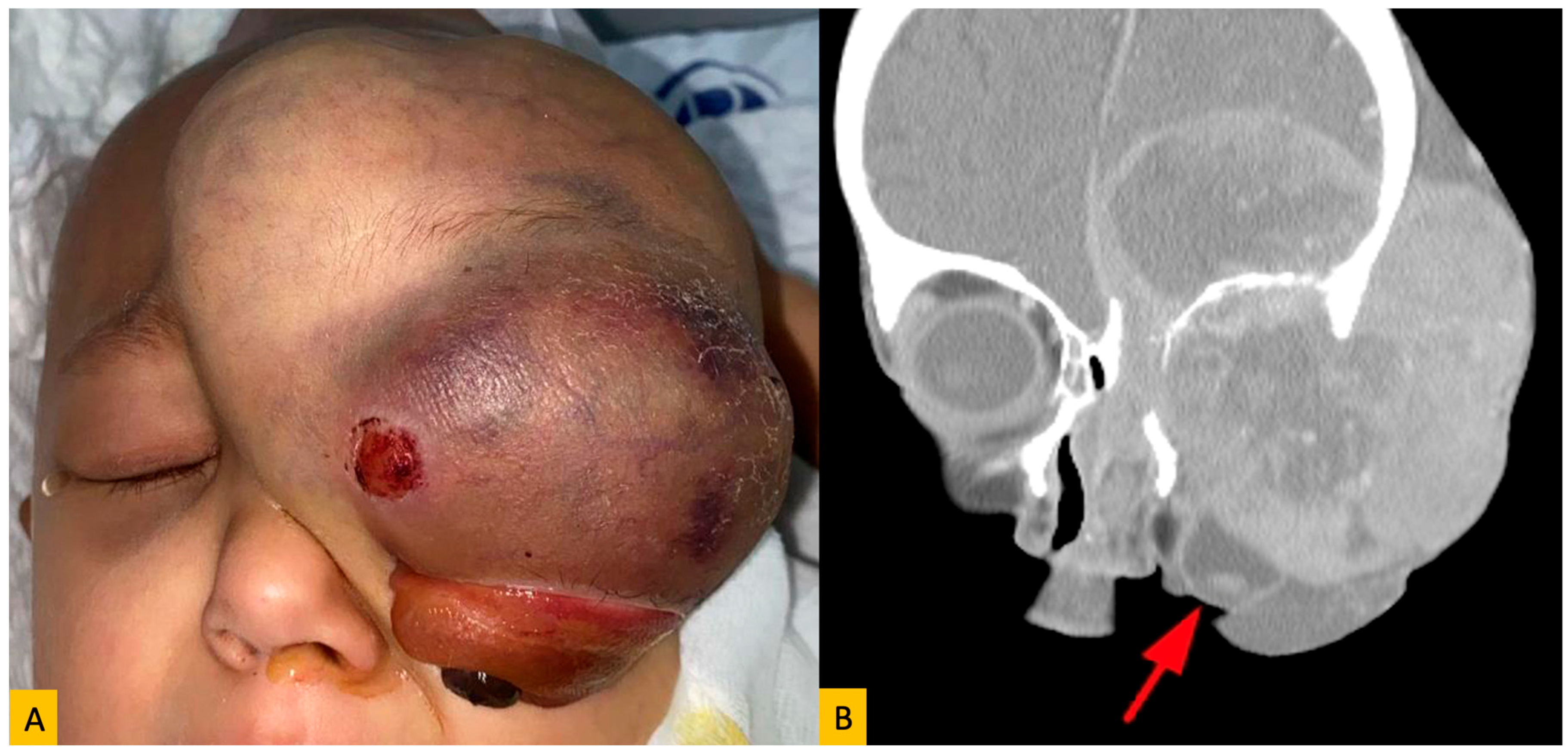

| Primary Site | Tumor Type | Number of Patients | % of Total | Male (n) | Female (n) |
|---|---|---|---|---|---|
| Breast | Carcinoma | 21 | 25.3% | 0 | 21 |
| Prostate Gland | Carcinoma | 3 | 3.6% | 3 | 0 |
| Lung | Carcinoma | 9 | 10.8% | 5 | 4 |
| Skin | Melanoma (n = 4), SCC (n = 2), BCC (n = 1) | 7 | 8.4% | 3 | 4 |
| Adrenal Gland | Neuroblastoma | 16 | 19.2% | 5 | 11 |
| Gastrointestinal Tract | Carcinoma | 3 | 3.6% | 2 | 1 |
| Urinary Tract | Carcinoma | 2 | 2.4% | 2 | 0 |
| Bone | Sarcoma (n = 5), Myeloma (n = 5) | 10 | 12.0% | 6 | 4 |
| Primary Site | 0–19 Years | 20–39 Years | 40–59 Years | 60–79 Years | ≥80 Years |
|---|---|---|---|---|---|
| Breast | 0 | 5 | 9 | 6 | 1 |
| Prostate Gland | 0 | 0 | 1 | 2 | 0 |
| Lung | 0 | 1 | 2 | 6 | 0 |
| Skin | 0 | 0 | 4 | 1 | 2 |
| Adrenal Gland | 16 | 0 | 0 | 0 | 0 |
| Gastrointestinal Tract | 0 | 0 | 1 | 2 | 0 |
| Urinary Tract | 0 | 0 | 1 | 1 | 0 |
| Bone | 2 | 2 | 3 | 3 | 0 |
| Category | Subgroup | Mean Survival (Day) | Median Survival (Day) | p-Value |
|---|---|---|---|---|
| Gender | Female | 400.4 | 127.0 | 0.037 |
| Male | 165.4 | 70.0 | ||
| Age Group | <18 years | 332.0 | 245.0 | 0.473 |
| ≥19 years | 311.1 | 80.0 | ||
| Primary Tumor | Breast | 383.0 | 86.0 | 0.721 |
| Neuroblastoma | 338.0 | 204.0 | ||
| Others | 358.0 | 70.0 |
| Age Group | Mean Time to Metastasis (Year) | Median Time to Metastasis (Year) | p-Value |
|---|---|---|---|
| 0–19 years | 1.03 | 2.0 | 0.664 |
| 20–39 years | 3.40 | 4.0 | |
| 40–59 years | 6.87 | 3.0 | |
| 60–79 years | 5.09 | 7.0 | |
| ≥80 years | 1.66 | 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulas, B.; Ozcan, A.A.; Celikten, F.A.; Kaya, O.; Bayram, E. Prognostic Insights into Orbital Metastases: A Comprehensive Analysis of Clinical Features and Survival Outcomes. Diagnostics 2025, 15, 2542. https://doi.org/10.3390/diagnostics15192542
Ulas B, Ozcan AA, Celikten FA, Kaya O, Bayram E. Prognostic Insights into Orbital Metastases: A Comprehensive Analysis of Clinical Features and Survival Outcomes. Diagnostics. 2025; 15(19):2542. https://doi.org/10.3390/diagnostics15192542
Chicago/Turabian StyleUlas, Burak, Altan Atakan Ozcan, Feyza Alara Celikten, Omer Kaya, and Ertugrul Bayram. 2025. "Prognostic Insights into Orbital Metastases: A Comprehensive Analysis of Clinical Features and Survival Outcomes" Diagnostics 15, no. 19: 2542. https://doi.org/10.3390/diagnostics15192542
APA StyleUlas, B., Ozcan, A. A., Celikten, F. A., Kaya, O., & Bayram, E. (2025). Prognostic Insights into Orbital Metastases: A Comprehensive Analysis of Clinical Features and Survival Outcomes. Diagnostics, 15(19), 2542. https://doi.org/10.3390/diagnostics15192542





