Hyperreflective Dots on SD-OCT: Implications for Predicting Treatment Outcomes in Diabetic Macular Edema
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Examination
2.3. Treatment
2.4. HFs Counting Methods
2.5. Statistical Analysis
3. Results
3.1. Baseline Demographics
3.2. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daruich, A.; Matet, A.; Moulin, A.; Kowalczuk, L.; Nicolas, M.; Sellam, A.; Rothschild, P.R.; Omri, S.; Gélizé, E.; Jonet, L.; et al. Mechanisms of macular edema: Beyond the surface. Prog. Retin. Eye Res. 2018, 63, 20–68. [Google Scholar] [CrossRef]
- Thomas, R.L.; Dunstan, F.D.; Luzio, S.D.; Chowdhury, S.R.; North, R.V.; Hale, S.L.; Gibbins, R.L.; Owens, D.R. Prevalence of diabetic retinopathy within a national diabetic retinopathy screening service. Br. J. Ophthalmol. 2015, 99, 64–68. [Google Scholar] [CrossRef]
- Das, A.; McGuire, P.G.; Rangasamy, S. Diabetic Macular Edema: Pathophysiology and Novel Therapeutic Targets. Ophthalmology 2015, 122, 1375–1394. [Google Scholar] [CrossRef]
- Elnahry, A.G.; Noureldine, A.M.; Abdel-Kader, A.A.; Sorour, O.A.; Ramsey, D.J. Optical Coherence Tomography Angiography Biomarkers Predict Anatomical Response to Bevacizumab in Diabetic Macular Edema. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 395–405. [Google Scholar] [CrossRef]
- Tatsumi, T. Current Treatments for Diabetic Macular Edema. Int. J. Mol. Sci. 2023, 24, 9591. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jansonius, N.M.; Chen, H.; Los, L.I. Hyperreflective Dots on OCT as a Predictor of Treatment Outcome in Diabetic Macular Edema: A Systematic Review. Ophthalmol. Retina 2022, 6, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Okuwobi, I.P.; Ji, Z.; Fan, W.; Yuan, S.; Bekalo, L.; Chen, Q. Automated Quantification of Hyperreflective Foci in SD-OCT With Diabetic Retinopathy. IEEE J. Biomed. Health Inform. 2020, 24, 1125–1136. [Google Scholar] [CrossRef]
- De Benedetto, U.; Sacconi, R.; Pierro, L.; Lattanzio, R.; Bandello, F. Optical coherence tomographic hyperreflective foci in early stages of diabetic retinopathy. Retina 2015, 35, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Waldstein, S.M.; Vogl, W.D.; Bogunovic, H.; Sadeghipour, A.; Riedl, S.; Schmidt-Erfurth, U. Characterization of Drusen and Hyperreflective Foci as Biomarkers for Disease Progression in Age-Related Macular Degeneration Using Artificial Intelligence in Optical Coherence Tomography. JAMA Ophthalmol. 2020, 138, 740–747. [Google Scholar] [CrossRef]
- Duic, C.; Pfau, K.; Keenan, T.D.L.; Wiley, H.; Thavikulwat, A.; Chew, E.Y.; Cukras, C. Hyperreflective Foci in Age-Related Macular Degeneration are Associated with Disease Severity and Functional Impairment. Ophthalmol. Retina 2023, 7, 307–317. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yoon, C.K.; Park, U.C.; Park, K.H.; Lee, E.K. Choroidal Hyperreflective Foci as Biomarkers of Severity in Stargardt Disease. Retina 2025, 45, 774–784. [Google Scholar] [CrossRef]
- Bolz, M.; Schmidt-Erfurth, U.; Deak, G.; Mylonas, G.; Kriechbaum, K.; Scholda, C. Optical coherence tomographic hyperreflective foci: A morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology 2009, 116, 914–920. [Google Scholar] [CrossRef]
- Chen, N.N.; Chen, W.D.; Lai, C.H.; Kuo, C.N.; Chen, C.L.; Huang, J.C.; Wu, P.C.; Wu, P.L.; Chen, C.Y. Optical Coherence Tomographic Patterns as Predictors of Structural Outcome After Intravitreal Ranibizumab in Diabetic Macula Edema. Clin. Ophthalmol. 2020, 14, 4023–4030. [Google Scholar] [CrossRef]
- von Schulthess, E.L.; Maunz, A.; Chakravarthy, U.; Holekamp, N.; Pauleikhoff, D.; Patel, K.; Bachmeier, I.; Yu, S.; Cohen, Y.; Scherb, M.P.; et al. Intraretinal Hyper-Reflective Foci Are Almost Universally Present and Co-Localize With Intraretinal Fluid in Diabetic Macular Edema. Investig. Ophthalmol. Vis. Sci. 2024, 65, 26. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, C.; Yang, Q.; Xie, H.; Zhang, J.; Qiu, Q.; Liu, K.; Luo, D.; Liu, F.; Zhang, J. Imaging Hyperreflective Foci as an Inflammatory Biomarker after Anti-VEGF Treatment in Neovascular Age-Related Macular Degeneration Patients with Optical Coherence Tomography Angiography. BioMed Res. Int. 2021, 2021, 6648191. [Google Scholar] [CrossRef] [PubMed]
- Frizziero, L.; Midena, G.; Danieli, L.; Torresin, T.; Perfetto, A.; Parrozzani, R.; Pilotto, E.; Midena, E. Hyperreflective Retinal Foci (HRF): Definition and Role of an Invaluable OCT Sign. J. Clin. Med. 2025, 14, 3021. [Google Scholar] [CrossRef]
- Vujosevic, S.; Bini, S.; Midena, G.; Berton, M.; Pilotto, E.; Midena, E. Hyperreflective intraretinal spots in diabetics without and with nonproliferative diabetic retinopathy: An in vivo study using spectral domain OCT. J. Diabetes Res. 2013, 2013, 491835. [Google Scholar] [CrossRef] [PubMed]
- Uji, A.; Murakami, T.; Nishijima, K.; Akagi, T.; Horii, T.; Arakawa, N.; Muraoka, Y.; Ellabban, A.A.; Yoshimura, N. Association between hyperreflective foci in the outer retina, status of photoreceptor layer, and visual acuity in diabetic macular edema. Am. J. Ophthalmol. 2012, 153, 710–717.e1. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.R.; Lee, S.Y.; Kim, Y.H.; Byeon, H.E.; Kim, J.H.; Lee, K. Hyperreflective foci in diabetic macular edema with serous retinal detachment: Association with dyslipidemia. Acta Diabetol. 2020, 57, 861–866. [Google Scholar] [CrossRef]
- Hatamnejad, A.; Orr, S.; Dadak, R.; Khanani, A.; Singh, R.; Choudhry, N. Anti-VEGF and steroid combination therapy relative to anti-VEGF mono therapy for the treatment of refractory DME: A systematic review of efficacy and meta-analysis of safety. Acta Ophthalmol. 2024, 102, e204–e214. [Google Scholar] [CrossRef]
- Sharma, D.; Zachary, I.; Jia, H. Mechanisms of Acquired Resistance to Anti-VEGF Therapy for Neovascular Eye Diseases. Investig. Ophthalmol. Vis. Sci. 2023, 64, 28. [Google Scholar] [CrossRef] [PubMed]
- Agostini, H.; Abreu, F.; Baumal, C.R.; Chang, D.S.; Csaky, K.G.; Demetriades, A.M.; Kodjikian, L.; Lim, J.I.; Margaron, P.; Monés, J.M.; et al. Faricimab for neovascular age-related macular degeneration and diabetic macular edema: From preclinical studies to phase 3 outcomes. Graefe’s Arch. Clin. Exp. Ophthalmol. = Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2024, 262, 3437–3451. [Google Scholar] [CrossRef] [PubMed]
- Chatziralli, I. Ranibizumab for the treatment of diabetic retinopathy. Expert Opin. Biol. Ther. 2021, 21, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Luo, D.; Qiu, Q.; Xu, G.T.; Zhang, J. Hyperreflective Foci in Diabetic Macular Edema with Subretinal Fluid: Association with Visual Outcomes after Anti-VEGF Treatment. Ophthalmic Res. 2023, 66, 39–47. [Google Scholar] [CrossRef]
- Al-Latayfeh, M.; Abdel Rahman, M.; Shatnawi, R. Outcome of Single Dexamethasone Implant Injection in the Treatment of Persistent Diabetic Macular Edema After Anti-VEGF Treatment: Real-Life Data from a Tertiary Hospital in Jordan. Clin. Ophthalmol. 2021, 15, 1285–1291. [Google Scholar] [CrossRef]
- Vujosevic, S.; Lupidi, M.; Donati, S.; Astarita, C.; Gallinaro, V.; Pilotto, E. Role of inflammation in diabetic macular edema and neovascular age-related macular degeneration. Surv. Ophthalmol. 2024, 69, 870–881. [Google Scholar] [CrossRef]
- Yao, X.; Zhao, Z.; Zhang, W.; Liu, R.; Ni, T.; Cui, B.; Lei, Y.; Du, J.; Ai, D.; Jiang, H.; et al. Specialized Retinal Endothelial Cells Modulate Blood-Retina Barrier in Diabetic Retinopathy. Diabetes 2024, 73, 225–236. [Google Scholar] [CrossRef]
- Utsumi, T.; Noma, H.; Yasuda, K.; Goto, H.; Shimura, M. Effects of ranibizumab on growth factors and mediators of inflammation in the aqueous humor of patients with diabetic macular edema. Graefe’s Arch. Clin. Exp. Ophthalmol. = Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2021, 259, 2597–2603. [Google Scholar] [CrossRef]
- Taloni, A.; Coco, G.; Rastelli, D.; Buffon, G.; Scorcia, V.; Giannaccare, G. Safety and Efficacy of Dexamethasone Intravitreal Implant Given Either First-Line or Second-Line in Diabetic Macular Edema. Patient Prefer. Adherence 2023, 17, 3307–3329. [Google Scholar] [CrossRef]
- Liu, S.; Wang, D.; Chen, F.; Zhang, X. Hyperreflective foci in OCT image as a biomarker of poor prognosis in diabetic macular edema patients treating with Conbercept in China. BMC Ophthalmol. 2019, 19, 157. [Google Scholar] [CrossRef]
- Chatziralli, I.P.; Sergentanis, T.N.; Sivaprasad, S. Hyperreflective foci as an independent visual outcome predictor in macular edema due to retinal vascular diseases treated with intravitreal dexamethasone or ranibizumab. Retina 2016, 36, 2319–2328. [Google Scholar] [CrossRef]
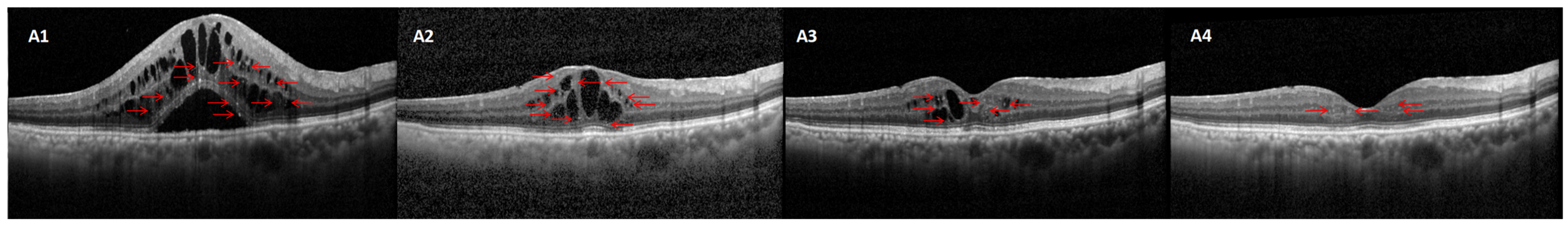
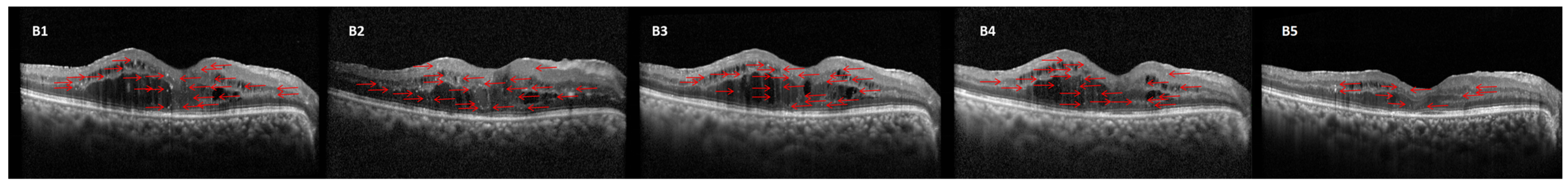
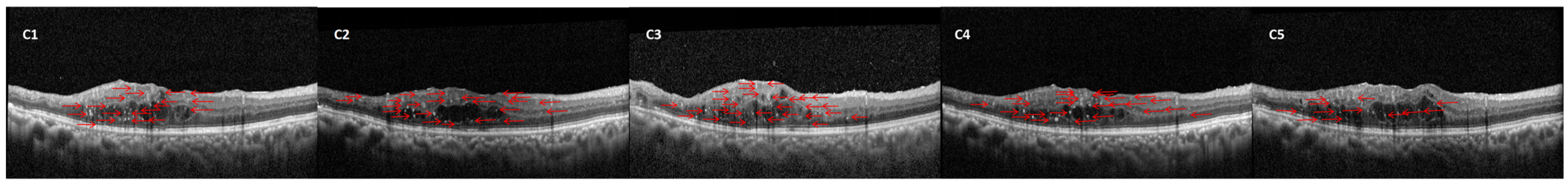
| Characteristics | Responder Group (n = 73) | Non-Responder Group (n = 39) | p Value |
|---|---|---|---|
| Eyes/patients | 73/52 | 39/26 | - |
| Age (year), mean ± SD | 59.27 ± 7.16 | 55.63 ± 7.73 | 0.776 |
| Male/female | 28/24 | 15/11 | 0.747 |
| BMI (kg/m2), mean ± SD | 24.88 ± 1.97 | 25.01 ± 1.73 | 0.706 |
| Systemic profile: | |||
| HbA1c (%), mean ± SD | 6.66 ± 1.98 | 7.13 ± 1.87 | 0.273 |
| Duration of DM (y), median (IQR) | 8.35 (4.00, 11.50) | 7.50 (4.50, 10.00) | 0.234 |
| Hypertension, n (%) | 20 (38.5) | 11 (42.3) | 0.744 |
| Ocular profile, n (%) | |||
| History of laser | 12 (16.4) | 7 (17.9) | 0.839 |
| pseudophakic | 9 (12.3) | 6 (15.4) | 0.651 |
| DRIL, n (%) | 2 (2.7) | 2 (5.1) | 0.516 |
| ELM/EZ disruption, n (%) | 3 (4.1) | 2 (5.1) | 0.804 |
| LogMAR BCVA, mean ± SD | 0.54 ± 0.73 | 0.52 ± 0.61 | 0.827 |
| IOP (mmHg), mean ± SD | 15.30 ± 3.47 | 14.83 ± 3.55 | 0.709 |
| CMT (mm), mean ± SD | 456.53 ± 109.73 | 468.99 ± 127.10 | 0.614 |
| Characteristics | Responder Group (n = 26) | Non-Responder Group (n = 13) | p Value |
|---|---|---|---|
| Eyes/patients | 26/17 | 13/9 | - |
| Age (year), mean ± SD | 57.25 ± 7.36 | 54.58 ± 9.93 | 0.598 |
| Male/female | 10/7 | 5/4 | 0.873 |
| BMI (kg/m2), mean ± SD | 24.99 ± 2.73 | 25.11 ± 1.63 | 0.698 |
| Systemic profile: | |||
| HbA1c (%), mean ± SD | 7.08 ± 1.17 | 7.19 ± 1.56 | 0.673 |
| Duration of DM (y), median (IQR) | 7.35 (4.25, 10.50) | 7.80 (4.35, 11.00) | 0.304 |
| Hypertension, n (%) | 7 (41.2) | 4 (44.4) | 0.873 |
| Ocular profile, n (%) | |||
| History of laser | 5 (19.2) | 2 (15.4) | 0.768 |
| pseudophakic | 4 (15.4) | 2 (15.4) | 1.000 |
| DRIL, n (%) | 1 (3.8) | 1 (7.7) | 0.608 |
| ELM/EZ disruption, n (%) | 1 (3.8) | 1 (7.7) | 0.608 |
| LogMAR BCVA, mean ± SD | 0.53 ± 0.39 | 0.51 ± 0.67 | 0.772 |
| IOP (mmHg), mean ± SD | 14.60 ± 4.71 | 15.03 ± 2.59 | 0.609 |
| CMT (mm), mean ± SD | 472.53 ± 112.03 | 464.57 ± 137.83 | 0.511 |
| BCVA (LogMAR), Mean ± SD | Responder Group (n = 73) | Non-Responder Group (n = 39) | p Value |
|---|---|---|---|
| Baseline | 0.54 ± 0.73 | 0.52 ± 0.61 | 0.827 |
| 1st injection | 0.44 ± 0.42 | 0.50 ± 0.50 | 0.044 * |
| 2nd injection | 0.39 ± 0.37 | 0.49 ± 0.48 | 0.031 * |
| 3rd injection | 0.35 ± 0.40 | 0.47 ± 0.38 | 0.027 * |
| CMT (μm), Mean ± SD | Responder Group (n = 73) | Non-Responder Group (n = 39) | p Value |
|---|---|---|---|
| Baseline | 456.53 ± 109.73 | 468.99 ± 127.10 | 0.614 |
| 1st injection | 337.77 ± 93.18 | 440.95 ± 99.01 | 0.027 * |
| 2nd injection | 290.69 ± 66.38 | 433.18 ± 78.66 | 0.016 * |
| 3rd injection | 235.47 ± 49.13 | 427.45 ± 52.91 | 0.008 * |
| BCVA (LogMAR), Mean ± SD | Responder Group (n = 73) | Non-Responder Group (n = 39) | p Value |
|---|---|---|---|
| Baseline | 0.53 ± 0.39 | 0.51 ± 0.67 | 0.772 |
| 1 month | 0.37 ± 0.41 | 0.43 ± 0.88 | 0.039 * |
| CMT (μm), Mean ± SD | Responder Group (n = 73) | Non-Responder Group (n = 39) | p Value |
|---|---|---|---|
| Baseline | 472.53 ± 112.03 | 464.57 ± 137.83 | 0.747 |
| 1 month | 255.99 ± 69.83 | 397.48 ± 82.77 | 0.014 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, M.; Sun, A.; Zhang, H. Hyperreflective Dots on SD-OCT: Implications for Predicting Treatment Outcomes in Diabetic Macular Edema. Diagnostics 2025, 15, 2539. https://doi.org/10.3390/diagnostics15192539
Li S, Li M, Sun A, Zhang H. Hyperreflective Dots on SD-OCT: Implications for Predicting Treatment Outcomes in Diabetic Macular Edema. Diagnostics. 2025; 15(19):2539. https://doi.org/10.3390/diagnostics15192539
Chicago/Turabian StyleLi, Siying, Muzi Li, Aimin Sun, and Hongwei Zhang. 2025. "Hyperreflective Dots on SD-OCT: Implications for Predicting Treatment Outcomes in Diabetic Macular Edema" Diagnostics 15, no. 19: 2539. https://doi.org/10.3390/diagnostics15192539
APA StyleLi, S., Li, M., Sun, A., & Zhang, H. (2025). Hyperreflective Dots on SD-OCT: Implications for Predicting Treatment Outcomes in Diabetic Macular Edema. Diagnostics, 15(19), 2539. https://doi.org/10.3390/diagnostics15192539





