A Mutational Landscape in Acute Myeloid Leukemia: Overview and Prognostic Impacts
Abstract
1. Introduction
| Mutations | Incidence Rate in AML | Functional Group | |
|---|---|---|---|
| Pediatric | Adult | ||
| NPM1 [1,8] | 8–10% | 30–35% | Nucleophosmin |
| FLT3-ITD [14] | 15% | 20–35% | Signaling/Kinase Pathway |
| DNMT3A [1] | 2.1% | 20% | DNA Methylation |
| MECOM [15,16] | <1% | 4% | Transcription Factor |
| ASXL1 [17,18] | 10.81% | 14.4–19.1% | Chromatin modification |
| TP53 [1] | 2.1% | 10% | Transcription Factor |
| CEBPA [1] | 18% | ~10% | Transcription Factor |
| KMT2A [15] | 10–15% | 3% | Chromatin modification |
| RUNX1 [1] | 2.8% | 10–15% | Transcription Factor |
| TET2 [19] | 6% | 7.6% | DNA Methylation |
| GATA2 [1] | 2.6% | 5% | Transcription Factor |
| BCOR [20,21] | 1.7% | 3.8–5% | Transcriptional Corepressor |
2. Contemporary Diagnostic Modalities in AML
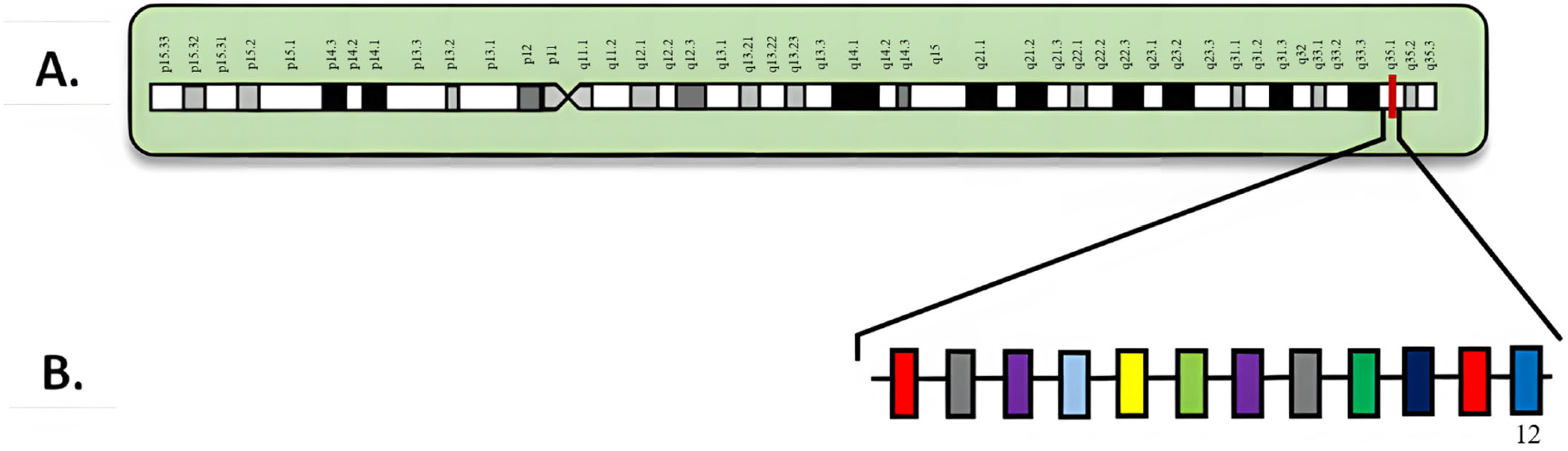
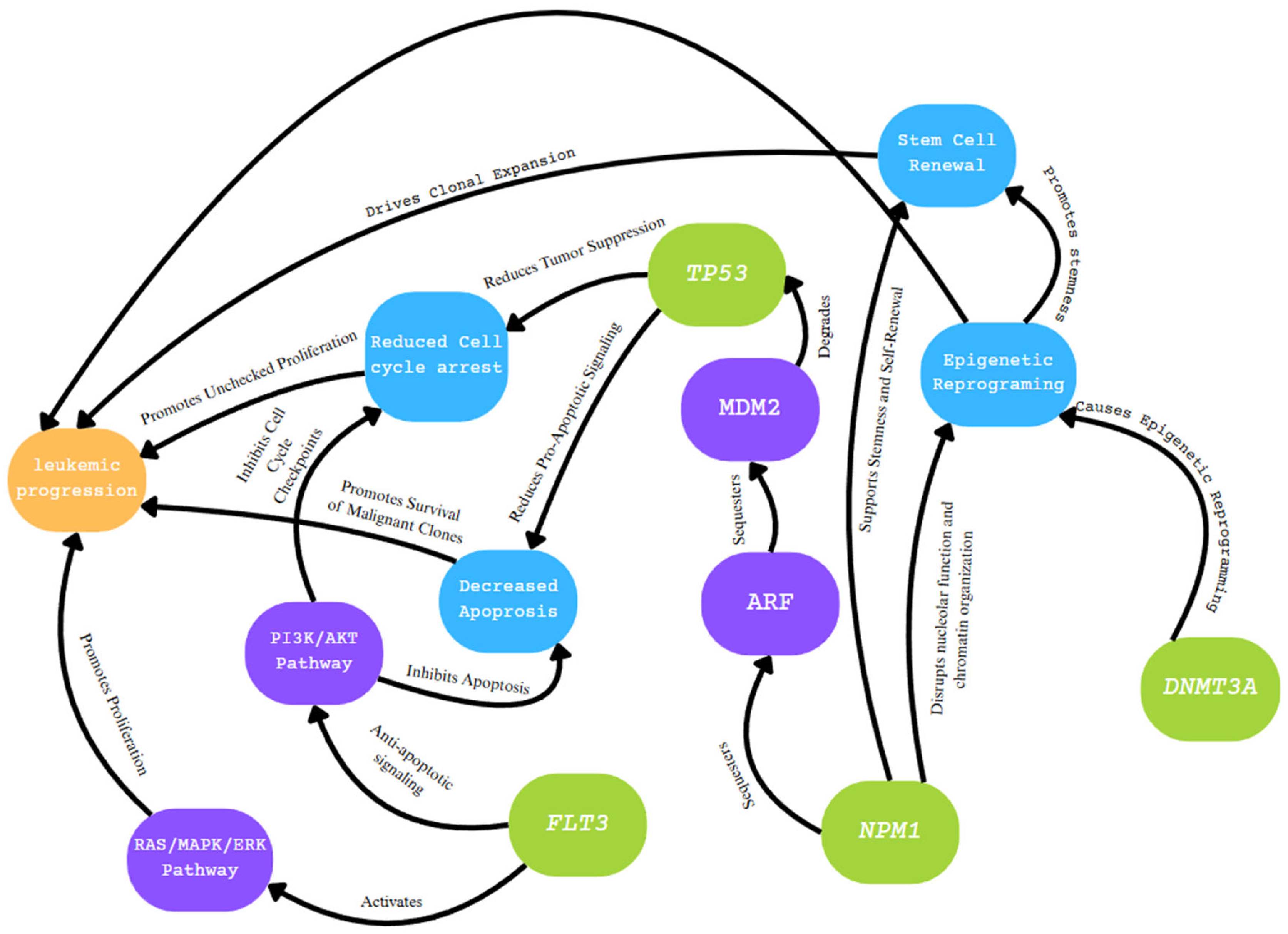
3. NPM1
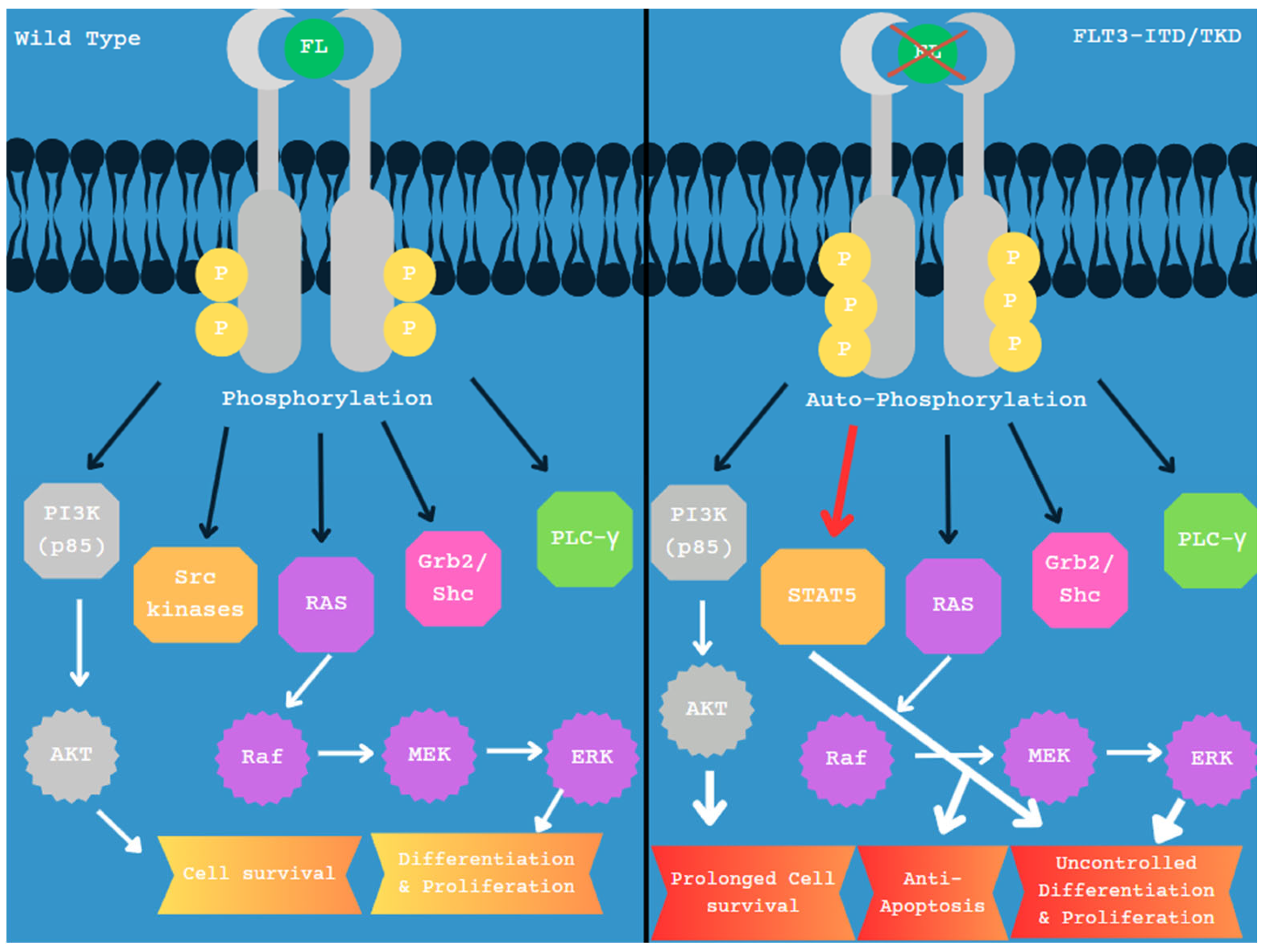
4. FLT3
5. DNMT3A
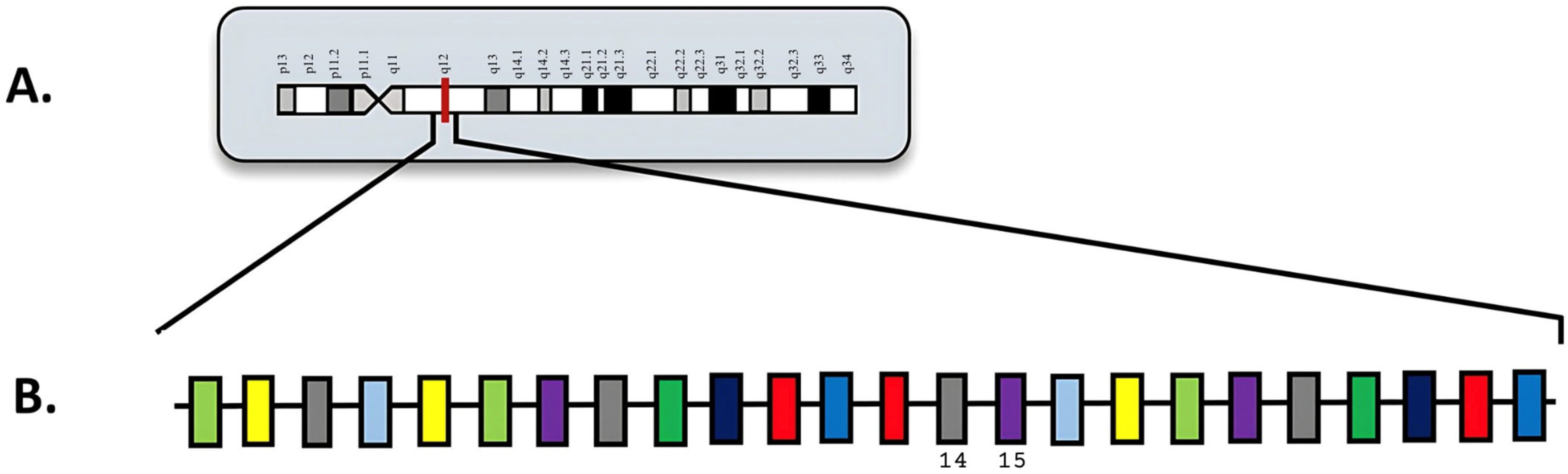
6. MECOM
7. ASXL1
8. TP53
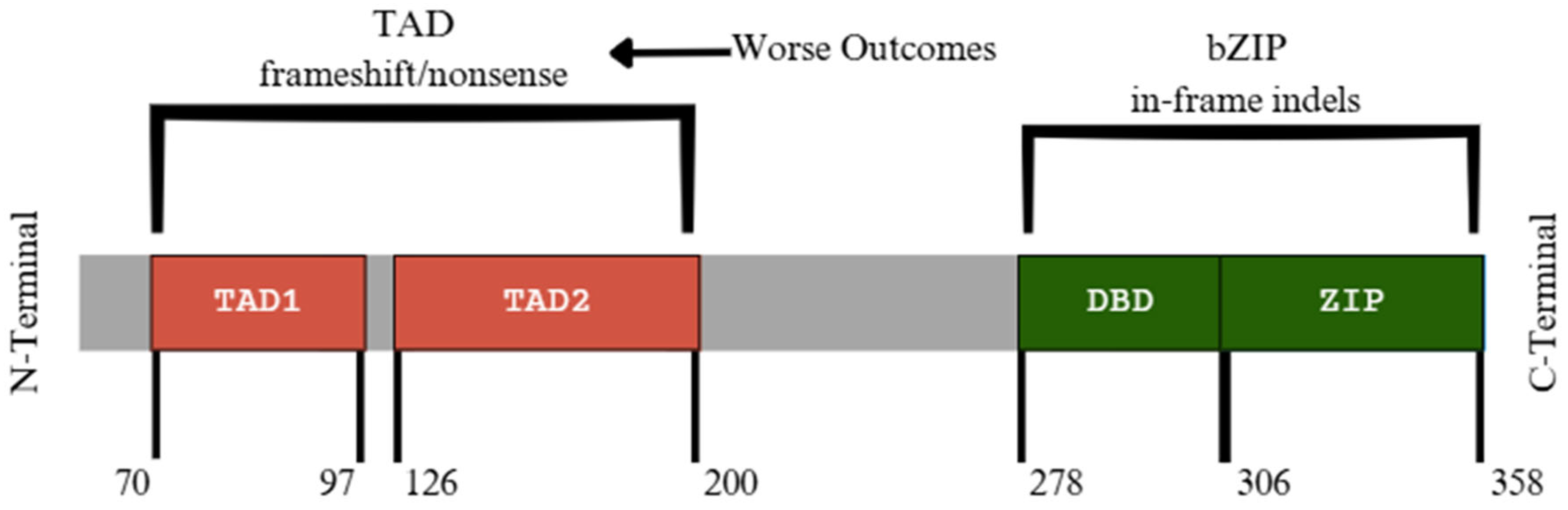
9. CEBPA
10. Therapeutic Approaches and Treatment Limitations in AML
11. Future Directions of AML Research
12. Clinical Implications and Changing Approaches to Patient Care
13. Discussions and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makkar, H.; Majhi, R.K.; Goel, H.; Gupta, A.K.; Chopra, A.; Tanwar, P.; Seth, R. Acute Myeloid Leukemia: Novel Mutations and Their Clinical Implications. Am. J. Blood Res. 2023, 13, 12–27. [Google Scholar]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 Recommendations from an International Expert Panel on Behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; DiNardo, C.D.; Appelbaum, F.; Craddock, C.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; Larson, R.A.; et al. Genetic Risk Classification for Adults with AML Receiving Less-Intensive Therapies: The 2024 ELN Recommendations. Blood 2024, 144, 2169–2173. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, J.; Zwaan, C.; van den Heuvel-Eibrink, M. Pediatric AML: From Biology to Clinical Management. J. Clin. Med. 2015, 4, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Bolouri, H.; Farrar, J.E.; Triche, T.; Ries, R.E.; Lim, E.L.; Alonzo, T.A.; Ma, Y.; Moore, R.; Mungall, A.J.; Marra, M.A.; et al. The Molecular Landscape of Pediatric Acute Myeloid Leukemia Reveals Recurrent Structural Alterations and Age-Specific Mutational Interactions. Nat. Med. 2017, 24, 103–112. [Google Scholar] [CrossRef]
- Aung, M.M.K.; Mills, M.L.; Bittencourt-Silvestre, J.; Keeshan, K. Insights into the Molecular Profiles of Adult and Paediatric Acute Myeloid Leukaemia. Mol. Oncol. 2021, 15, 2253–2272. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Cortes, J.E. Mutations in AML: Prognostic and Therapeutic Implications. Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 348–355. [Google Scholar] [CrossRef]
- Ranieri, R.; Pianigiani, G.; Sciabolacci, S.; Perriello, V.M.; Marra, A.; Cardinali, V.; Pierangeli, S.; Milano, F.; Gionfriddo, I.; Brunetti, L.; et al. Current Status and Future Perspectives in Targeted Therapy of NPM1-Mutated AML. Leukemia 2022, 36, 2351–2367. [Google Scholar] [CrossRef]
- Falini, B.; Brunetti, L.; Sportoletti, P.; Martelli, M.P. NPM1-Mutated Acute Myeloid Leukemia: From Bench to Bedside. Blood 2020, 136, 1707–1721. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S. What Is next Generation Sequencing? Arch. Dis. Child. - Educ. Pract. Ed. 2013, 98, 236–238. [Google Scholar] [CrossRef]
- Levy, B.; Baughn, L.B.; Akkari, Y.; Chartrand, S.; LaBarge, B.; Claxton, D.; Lennon, P.A.; Cujar, C.; Kolhe, R.; Kroeger, K.; et al. Optical Genome Mapping in Acute Myeloid Leukemia: A Multicenter Evaluation. Blood Adv. 2023, 7, 1297–1307. [Google Scholar] [CrossRef]
- Bionano Optical Genome Mapping. Available online: https://www.mdanderson.org/research/research-resources/core-facilities/advanced-technology-genomics-core/services-and-fees/bionano-optical-genome-mapping.html (accessed on 2 April 2025).
- Han Yu, P.; Yan Zhang, Z.; Yuan Kang, Y.; Huang, P.; Yang, C.; Naranmandura, H. Acute Myeloid Leukemia with T(8;21) Translocation: Molecular Pathogenesis, Potential Therapeutics and Future Directions. Biochem. Pharmacol. 2025, 233, 116774. [Google Scholar] [CrossRef]
- Qiu, K.-Y.; Liao, X.-Y.; Liu, Y.; Huang, K.; Li, Y.; Fang, J.-P.; Zhou, D.-H. Poor Outcome of Pediatric Patients with Acute Myeloid Leukemia Harboring High FLT3/ITD Allelic Ratios. Nat. Commun. 2022, 13, 3679. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wang, W.; Yang, S.; El Achi, H.; Fang, H.; Nahmod, K.A.; Toruner, G.A.; Xu, J.; Thakral, B.; Ayoub, E.; et al. 3q26.2/MECOM Rearrangements by Pericentric Inv(3): Diagnostic Challenges and Clinicopathologic Features. Cancers 2023, 15, 458. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.A.; Kutny, M.A.; Alonzo, T.A.; Gerbing, R.B.; Joaquin, J.; Raimondi, S.C.; Gamis, A.S.; Meshinchi, S. Leukemic Mutations in the Methylation-Associated Genes DNMT3A and IDH2 Are Rare Events in Pediatric AML: A Report from the Children’s Oncology Group. Pediatr. Blood Cancer 2011, 57, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Chen, K.; Tian, X.; Zou, R.; Feng, X.; Li, C.; Li, J.; Zheng, M.; Mai, H.; Yang, L.; et al. Clinical Characteristics and Prognosis Analysis of Patients with de Novo ASXL1-Mutated AML Treated with the C-HUNAN-AML-15 Protocol: A Multicenter Study by the South China Pediatric AML Collaborative Group. Cancer Med. 2023, 12, 13182–13192. [Google Scholar] [CrossRef]
- Lipilkin, P.V.; Kulaeva, E.D.; Mashkina, E.V. Prognostic Value of ASXL1 Mutations in Acute Myeloid Leukemia: A Meta-Analysis. Leuk. Res. 2022, 120, 106910. [Google Scholar] [CrossRef]
- Yao, Y.; Lin, X.; Wang, C.; Gu, Y.; Jin, J.; Zhu, Y.; Wang, H. Identification of a Novel NPM1 Mutation in Acute Myeloid Leukemia. Exp. Hematol. Oncol. 2023, 12, 87. [Google Scholar] [CrossRef]
- de Rooij, J.D.E.; van den Heuvel-Eibrink, M.M.; Hermkens, M.C.H.; Verboon, L.J.; Arentsen-Peters, S.T.C.J.M.; Fornerod, M.; Baruchel, A.; Stary, J.; Reinhardt, D.; de Haas, V.; et al. BCOR and BCORL1 Mutations in Pediatric Acute Myeloid Leukemia. Haematologica 2015, 100, e194–e195. [Google Scholar] [CrossRef]
- Sportoletti, P.; Sorcini, D.; Falini, B. BCOR Gene Alterations in Hematologic Diseases. Blood 2021, 138, 2455–2468. [Google Scholar] [CrossRef]
- Chaudhary, S.; Chaudhary, P.; Ahmad, F.; Arora, N. Acute Myeloid Leukemia and Next-Generation Sequencing Panels for Diagnosis: A Comprehensive Review. J. Pediatr. Hematol./Oncol. 2024, 46, 125–137. [Google Scholar] [CrossRef]
- Leisch, M.; Jansko, B.; Zaborsky, N.; Greil, R.; Pleyer, L. Next Generation Sequencing in AML-on the Way to Becoming a New Standard for Treatment Initiation and/or Modulation? Cancers 2019, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Illumina AmpliSeq for Illumina Myeloid Panel. Available online: https://www.illumina.com/products/by-type/sequencing-kits/library-prep-kits/ampliseq-myeloid-panel.html? (accessed on 14 May 2025).
- QIAGEN QIAseq Targeted DNA Human Myeloid Neoplasms Panel|GeneGlobe. Available online: https://geneglobe.qiagen.com/us/product-groups/qiaseq-targeted-dna-panels/DHS-003Z? (accessed on 14 May 2025).
- Quest Diagnostics LeukoVantage Myeloid Neoplasm Mutation Panels|Quest Diagnostics. Available online: https://www.questdiagnostics.com/healthcare-professionals/clinical-education-center/faq/faq208? (accessed on 14 May 2025).
- OGT SureSeq MyPanel Custom AML NGS Panel|OGT. Available online: https://www.ogt.com/products/product-search/sureseq-mypanel-custom-aml-next-generation-sequencing-ngs-panel/ (accessed on 15 May 2025).
- Zhao, M.; Medeiros, L.J.; Wang, W.; Tang, G.; Jung, H.S.; Sfamenos, S.M.; Fang, H.; Toruner, G.A.; Hu, S.; Yin, C.C.; et al. Newly Designed Breakapart FISH Probe Helps to Identify Cases with True MECOM Rearrangement in Myeloid Malignancies. Cancer Genet. 2022, 262–263, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen, I.; Rajendran, Y.; Acharya, A.; Öhman, S.; Arvio, M.; Paetau, R.; Siren, A.; Avela, K.; Granvik, J.; Leal, S.M.; et al. Optical Genome Mapping Unveils Hidden Structural Variants in Neurodevelopmental Disorders. Sci. Rep. 2024, 14, 11239. [Google Scholar] [CrossRef] [PubMed]
- Polonis, K.; Blommel, J.H.; Hughes, A.E.O.; Spencer, D.; Thompson, J.A.; Schroeder, M.C. Innovations in Short-Read Sequencing Technologies and Their Applications to Clinical Genomics. Clin. Chem. 2025, 71, 97–108. [Google Scholar] [CrossRef]
- Ran, W.-J.; Xu, L.-P.; Zhang, X.-H.; Chang, Y.-J.; Mo, X.-D.; Sun, Y.-Q.; Huang, X.-J.; Wang, Y. Clinical Effects of RUNX1 Mutations on the Outcomes of Patients with Acute Myeloid Leukemia Treated with Allogeneic Hematopoietic Stem-Cell Transplantation. Curr. Oncol. 2025, 32, 294. [Google Scholar] [CrossRef]
- Smith, A.C.; Tsui, H.; Usta, S.; Capo-Chichi, J.-M. What the VAF? A Guide to the Interpretation of Variant Allele Fraction, Percent Mosaicism, and Copy Number in Cancer. Mol. Cytogenet. 2025, 18, 13. [Google Scholar] [CrossRef]
- Zarka, J.; Short, N.J.; Kanagal-Shamanna, R.; Issa, G.C. Nucleophosmin 1 Mutations in Acute Myeloid Leukemia. Genes 2020, 11, 649. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- McGowan-Jordan, J.; Hastings, R.J.; Moore, S. ISCN 2020: An International System for Human Cytogenomic Nomenclature (2020). Reprint of: Cytogenetic and Genome Research 2020, Vol. 160, No. 7-8; S Karger AG: Basel, Switzerland, 2020; pp. 17–27. [Google Scholar]
- Jentzsch, M.; Grimm, J.; Bill, M.; Goldmann, K.; Schulz, J.; Niederwieser, D.; Platzbecker, U.; Schwind, S. Outcomes of Older Patients with NPM1 Mutated and FLT3-ITD Negative Acute Myeloid Leukemia Receiving Allogeneic Transplantation. HemaSphere 2020, 4, e326. [Google Scholar] [CrossRef]
- Wang, R.; Xu, P.; Chang, L.-L.; Zhang, S.-Z.; Zhu, H.-H. Targeted Therapy in NPM1-Mutated AML: Knowns and Unknowns. Front. Oncol. 2022, 12, 972606. [Google Scholar] [CrossRef]
- Récher, C. Transplant in AML: Just Follow the NPM1 Guide! Blood 2024, 143, 1881–1882. [Google Scholar] [CrossRef]
- Issa, G.C.; Bidikian, A.; Venugopal, S.; Konopleva, M.; DiNardo, C.D.; Kadia, T.M.; Borthakur, G.; Jabbour, E.; Pemmaraju, N.; Yilmaz, M.; et al. Clinical Outcomes Associated with NPM1 Mutations in Patients with Relapsed or Refractory AML. Blood Adv. 2023, 7, 933–942. [Google Scholar] [CrossRef]
- Xu, L.-H.; Fang, J.-P.; Liu, Y.-C.; Jones, A.I.; Chai, L. Nucleophosmin Mutations Confer an Independent Favorable Prognostic Impact in 869 Pediatric Patients with Acute Myeloid Leukemia. Blood Cancer J. 2020, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, V.; Khan, A.M.; Justin, J.; Reddy, S.N.; Aly, M.M.; Kewan, T.; Bahaj, W.; Gurnari, C.; Visconte, V.; Carr, D.; et al. Comprehensive Age-Stratified Impact of NPM1 Mutation in Acute Myeloid Leukemia: A Real-World Experience. Cancers 2025, 17, 1020. [Google Scholar] [CrossRef] [PubMed]
- Prata, P.H.; Bally, C.; Prebet, T.; Recher, C.; Venton, G.; Thomas, X.; Raffoux, E.; Pigneux, A.; Cluzeau, T.; Desoutter, J.; et al. NPM1 Mutation Is Not Associated with Prolonged Complete Remission in Acute Myeloid Leukemia Patients Treated with Hypomethylating Agents. Haematologica 2018, 103, e455–e457. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Ushijima, Y.; Kiyoi, H. Recent Advances in AML with Mutated NPM1. Int. J. Hematol. 2024, 120, 556–565. [Google Scholar] [CrossRef]
- Oñate, G.; Bataller, A.; Garrido, A.; Hoyos, M.; Arnan, M.; Vives, S.; Coll, R.; Tormo, M.; Sampol, A.; Escoda, L.; et al. Prognostic Impact of DNMT3A Mutation in Acute Myeloid Leukemia with Mutated NPM1. Blood Adv. 2022, 6, 882–890. [Google Scholar] [CrossRef]
- Borlenghi, E.; Cattaneo, C.; Bertoli, D.; Cerqui, E.; Archetti, S.; Passi, A.; Oberti, M.; Zollner, T.; Giupponi, C.; Pagani, C.; et al. Prognostic Relevance of NPM1 and FLT3 Mutations in Acute Myeloid Leukaemia, Longterm Follow-up-A Single Center Experience. Cancers 2022, 14, 4716. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Shimony, S.; Stahl, M.; Stone, R.M. Acute Myeloid Leukemia: 2025 Update on Diagnosis, Risk-Stratification, and Management. Am. J. Hematol. 2025, 100, 860–891. [Google Scholar] [CrossRef]
- Greiner, J.; Mohamed, E.; Fletcher, D.M.; Schuler, P.J.; Schrezenmeier, H.; Götz, M.; Guinn, B. Immunotherapeutic Potential of Mutated NPM1 for the Treatment of Acute Myeloid Leukemia. Cancers 2024, 16, 3443. [Google Scholar] [CrossRef]
- Candoni, A.; Coppola, G. A 2024 Update on Menin Inhibitors. A New Class of Target Agents against KMT2A-Rearranged and NPM1-Mutated Acute Myeloid Leukemia. Hematol. Rep. 2024, 16, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Shimosato, Y.; Yamamoto, K.; Jia, Y.; Zhang, W.; Shiba, N.; Hayashi, Y.; Ito, S.; Kitamura, T.; Goyama, S. NPM1-Fusion Proteins Promote Myeloid Leukemogenesis through XPO1-Dependent HOX Activation. Leukemia 2024, 39, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.; Uddin, M.H.; Dhillon, V.; Aguilar, J.J.; Aboukameel, A.; Khan, H.; Bannoura, S.; Yang, J.; Dyson, G.; Buck, S.; et al. Combined Use of Ziftomenib and Selinexor Is Effective in NPM1 Mutant Acute Myeloid Leukemia. Blood 2024, 144, 2768. [Google Scholar] [CrossRef]
- Grafone, T.; Palmisano, M.; Nicci, C.; Storti, S. An Overview on the Role of FLT3-Tyrosine Kinase Receptor in Acute Myeloid Leukemia: Biology and Treatment. Oncol. Rev. 2012, 6, e8. [Google Scholar] [CrossRef]
- Takahashi, S. Downstream Molecular Pathways of FLT3 in the Pathogenesis of Acute Myeloid Leukemia: Biology and Therapeutic Implications. J. Hematol. Oncol. 2011, 4, 13. [Google Scholar] [CrossRef]
- Schlenk, R.F.; Kayser, S.; Bullinger, L.; Kobbe, G.; Casper, J.; Ringhoffer, M.; Held, G.; Brossart, P.; Lübbert, M.; Salih, H.R.; et al. Differential Impact of Allelic Ratio and Insertion Site in FLT3-ITD-Positive AML with Respect to Allogeneic Transplantation. Blood 2014, 124, 3441–3449. [Google Scholar] [CrossRef]
- Bacher, U.; Haferlach, C.; Kern, W.; Haferlach, T.; Schnittger, S. Prognostic Relevance of FLT3-TKD Mutations in AML: The Combination Matters--an Analysis of 3082 Patients. Blood 2008, 111, 2527–2537. [Google Scholar] [CrossRef]
- Shimony, S.; Fell, G.; Chen, E.C.; Tsai, H.K.; Wadleigh, M.; Winer, E.S.; Garcia, J.S.; Luskin, M.R.; Stahl, M.; Neuberg, D.S.; et al. FLT3-ITD Does Not Predict Inferior Prognosis in Acute Myeloid Leukemia Patients Aged ≥60 Years. Blood Adv. 2023, 7, 5354–5358. [Google Scholar] [CrossRef]
- Konopleva, M.; Thirman, M.J.; Pratz, K.W.; Garcia, J.S.; Recher, C.; Pullarkat, V.; Kantarjian, H.M.; DiNardo, C.D.; Dail, M.; Duan, Y.; et al. Impact of FLT3 Mutation on Outcomes after Venetoclax and Azacitidine for Patients with Treatment-Naïve Acute Myeloid Leukemia. Clin. Cancer Res. 2022, 28, 2744–2752. [Google Scholar] [CrossRef]
- Döhner, H.; Dolnik, A.; Tang, L.; Seymour, J.F.; Minden, M.D.; Stone, R.M.; del Castillo, T.B.; Al-Ali, H.K.; Santini, V.; Vyas, P.; et al. Cytogenetics and Gene Mutations Influence Survival in Older Patients with Acute Myeloid Leukemia Treated with Azacitidine or Conventional Care. Leukemia 2018, 32, 2546–2557. [Google Scholar] [CrossRef]
- Rataj, J.; Gorecki, L.; Muthna, D.; Sorf, A.; Krystof, V.; Klener, P.; Ceckova, M.; Rezacova, M.; Korabecny, J. Targeting FMS-like Tyrosine Kinase 3 (FLT3) in Acute Myeloid Leukemia: Novel Molecular Approaches and Therapeutic Challenges. Biomed. Pharmacother. 2024, 182, 117788. [Google Scholar] [CrossRef]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Khattab, A.M.T.; Ghaffar, A.A.A.A.; El-Sewefy, D.A.; ElSakhawy, Y.N.; Salem, R.M.; Omar, H.S.A. Characteristics of DNMT3A Mutation in Acute Myeloid Leukemia and Its Prognostic Implication. Egypt. J. Med. Hum. Genet. 2024, 25, 17–26. [Google Scholar] [CrossRef]
- Park, D.J.; Kwon, A.; Cho, B.-S.; Kim, H.-J.; Hwang, K.-A.; Kim, M.; Kim, Y. Characteristics of DNMT3A Mutations in Acute Myeloid Leukemia. Blood Res. 2020, 55, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.F.; Lima, A.S.; Piqué-Borràs, M.-R.; Silveira, D.R.; Coelho-Silva, J.L.; Pereira-Martins, D.A.; Weinhäuser, I.; Franca-Neto, P.L.; Quek, L.; Corby, A.; et al. Co-Occurrence of DNMT3A, NPM1, FLT3 Mutations Identifies a Subset of Acute Myeloid Leukemia with Adverse Prognosis. Blood 2020, 135, 870–875. [Google Scholar] [CrossRef]
- Chaudry, S.F.; Chevassut, T.J.T. Epigenetic Guardian: A Review of the DNA Methyltransferase DNMT3A in Acute Myeloid Leukaemia and Clonal Haematopoiesis. Biomed Res. Int. 2017, 2017, 5473197. [Google Scholar] [CrossRef]
- Wakita, S.; Marumo, A.; Morita, K.; Kako, S.; Toya, T.; Najima, Y.; Doki, N.; Kanda, J.; Kuroda, J.; Mori, S.; et al. Mutational Analysis of DNMT3A Improves the Prognostic Stratification of Patients with Acute Myeloid Leukemia. Cancer Sci. 2023, 114, 1297–1308. [Google Scholar] [CrossRef]
- Buscarlet, M.; Provost, S.; Zada, Y.F.; Barhdadi, A.; Bourgoin, V.; Lépine, G.; Mollica, L.; Szuber, N.; Dubé, M.-P.; Busque, L. DNMT3A and TET2 Dominate Clonal Hematopoiesis and Demonstrate Benign Phenotypes and Different Genetic Predispositions. Blood 2017, 130, 753–762. [Google Scholar] [CrossRef]
- Weeks, L.D.; Ebert, B.L. Causes and Consequences of Clonal Hematopoiesis. Blood 2023, 142, 2235–2246. [Google Scholar] [CrossRef]
- Murphy, T.; Zou, J.; Arruda, A.; Wang, T.T.; Zhao, Z.; Zheng, Y.; Gupta, V.; Maze, D.; McNamara, C.; Minden, M.D.; et al. Exclusion of Persistent Mutations in Splicing Factor Genes and Isocitrate Dehydrogenase 2 Improves the Prognostic Power of Molecular Measurable Residual Disease Assessment in Acute Myeloid Leukemia. Haematologica 2023, 109, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Freeman, S.D.; Ossenkoppele, G.J.; Buccisano, F.; Hourigan, C.S.; Ngai, L.L.; Tettero, J.M.; Bachas, C.; Baer, C.; Béné, M.-C.; et al. 2021 Update on MRD in Acute Myeloid Leukemia: A Consensus Document from the European LeukemiaNet MRD Working Party. Blood 2021, 138, 2753–2767. [Google Scholar] [CrossRef] [PubMed]
- Iyama, S.; Chi, S.; Idogawa, M.; Ikezoe, T.; Fukushima, K.; Utsu, Y.; Kanda, J.; Yoshimoto, G.; Kobayashi, T.; Hosono, N.; et al. Prognostic Impact of TET2 Mutations in Patients with Acute Myeloid Leukemia: HM-SCREEN-Japan 01 and 02 Study. Ann. Hematol. 2025, 104, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Chantana, P.; Chantiya, C.; Weerapat, O.; Smith, K.; Ekarat, R.; Thanawat, R.; Wasithep, L.; Supawee, S.; Pimjai, N.; Teeraya, P.; et al. Clinical Characteristics and Outcomes of Myeloid Neoplasms with MECOM Rearrangement: Results from a Nationwide Multicenter Study. Blood 2023, 142, 4213. [Google Scholar] [CrossRef]
- Liu, K.; Tirado, C.A. MECOM: A Very Interesting Gene Involved Also in Lymphoid Malignancies. J. Assoc. Genet. Technol. 2019, 45, 109–114. [Google Scholar]
- Schnittger, S.; Eder, C.; Jeromin, S.; Alpermann, T.; Fasan, A.; Grossmann, V.; Kohlmann, A.; Illig, T.; Klopp, N.; Wichmann, H.-E.; et al. ASXL1 Exon 12 Mutations Are Frequent in AML with Intermediate Risk Karyotype and Are Independently Associated with an Adverse Outcome. Leukemia 2012, 27, 82–91. [Google Scholar] [CrossRef]
- Abdel-Wahab, O.; Adli, M.; LaFave, L.M.; Gao, J.; Hricik, T.; Shih, A.H.; Pandey, S.; Patel, J.P.; Chung, Y.R.; Koche, R.; et al. ASXL1 Mutations Promote Myeloid Transformation through Loss of PRC2-Mediated Gene Repression. Cancer Cell 2012, 22, 180–193. [Google Scholar] [CrossRef]
- Yang, L.; Wei, X.; Gong, Y. Prognosis and Risk Factors for ASXL1 Mutations in Patients with Newly Diagnosed Acute Myeloid Leukemia and Myelodysplastic Syndrome. Cancer Med. 2024, 13, e6871. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, S.; Ren, Q.; Wang, Y.; Jiang, Z. Prognostic Value of ASXL1 Mutations in Patients with Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Meta-Analysis. Asia Pac. J. Clin. Oncol. 2023, 19, e183–e194. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Su, W.; Dou, Z.; Zhao, D.; Jin, X.; Lei, H.; Wang, J.; Xie, X.; Cheng, B.; et al. Mutant P53 in Cancer: From Molecular Mechanism to Therapeutic Modulation. Cell Death Dis. 2022, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Nong, T.; Mehra, S.; Taylor, J. Common Driver Mutations in AML: Biological Impact, Clinical Considerations, and Treatment Strategies. Cells 2024, 13, 1392. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.-Y. TP53 Mutation in Acute Myeloid Leukemia: An Old Foe Revisited. Cancers 2023, 15, 4816. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Maiti, A.; Loghavi, S.; Pourebrahim, R.; Kadia, T.M.; Rausch, C.R.; Furudate, K.; Daver, N.G.; Alvarado, Y.; Ohanian, M.; et al. Outcomes of TP53-Mutant Acute Myeloid Leukemia with Decitabine and Venetoclax. Cancer 2021, 127, 3772–3781. [Google Scholar] [CrossRef]
- Grob, T.; Al Hinai, A.S.A.; Sanders, M.A.; Kavelaars, F.G.; Rijken, M.; Gradowska, P.L.; Biemond, B.J.; Breems, D.A.; Maertens, J.; van Marwijk Kooy, M.; et al. Molecular Characterization of Mutant TP53 Acute Myeloid Leukemia and High-Risk Myelodysplastic Syndrome. Blood 2022, 139, 2347–2354. [Google Scholar] [CrossRef]
- Prochazka, K.T.; Pregartner, G.; Rücker, F.G.; Heitzer, E.; Pabst, G.; Wölfler, A.; Zebisch, A.; Berghold, A.; Döhner, K.; Sill, H. Clinical Implications of Subclonal TP53 Mutations in Acute Myeloid Leukemia. Haematologica 2018, 104, 516–523. [Google Scholar] [CrossRef]
- Yan, B.; Claxton, D.; Huang, S.; Qiu, Y. AML Chemoresistance: The Role of Mutant TP53 Subclonal Expansion and Therapy Strategy. Exp. Hematol. 2020, 87, 13–19. [Google Scholar] [CrossRef]
- Su, L.; Shi, Y.-Y.; Liu, Z.-Y.; Gao, S.-J. Acute Myeloid Leukemia with CEBPA Mutations: Current Progress and Future Directions. Front. Oncol. 2022, 12, 806137. [Google Scholar] [CrossRef]
- Georgi, J.-A.; Stasik, S.; Kramer, M.; Meggendorfer, M.; Röllig, C.; Haferlach, T.; Valk, P.; Linch, D.; Herold, T.; Duployez, N.; et al. Prognostic Impact of CEBPA Mutational Subgroups in Adult AML. Leukemia 2024, 38, 281–290. [Google Scholar] [CrossRef]
- Tian, T.V.; Graf, T. CEBPA (CCAAT/Enhancer Binding Protein (C/EBP), Alpha). Available online: https://atlasgeneticsoncology.org/gene/40050/cebpa-%28ccaat-enhancer-binding-protein-%28c-ebp%29-alpha%29 (accessed on 15 May 2025).
- Wakita, S.; Sakaguchi, M.; Oh, I.; Kako, S.; Toya, T.; Najima, Y.; Doki, N.; Kanda, J.; Kuroda, J.; Mori, S.; et al. Prognostic Impact of CEBPA bZIP Domain Mutation in Acute Myeloid Leukemia. Blood Adv. 2022, 6, 238–247. [Google Scholar] [CrossRef]
- Dillon, R.; Potter, N.; Freeman, S.; Russell, N. How We Use Molecular Minimal Residual Disease (MRD) Testing in Acute Myeloid Leukaemia (AML). Br. J. Haematol. 2020, 193, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Othus, M.; Rodríguez-Arbolí, E.; Orvain, C.; Milano, F.; Sandmaier, B.M.; Davis, C.; Basom, R.; Appelbaum, F.R.; Walter, R.B. Measurable Residual Disease as Predictor of Post-Day +100 Relapses Following Allografting in Adult AML. Blood Adv. 2024, 9, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Aitken, M.J.L.; Ravandi, F.; Patel, K.P.; Short, N.J. Prognostic and Therapeutic Implications of Measurable Residual Disease in Acute Myeloid Leukemia. J. Hematol. Oncol. 2021, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Löwenberg, B. Sense and Nonsense of High-Dose Cytarabine for Acute Myeloid Leukemia. Blood 2013, 121, 26–28. [Google Scholar] [CrossRef]
- Wei, A.H.; Döhner, H.; Pocock, C.; Montesinos, P.; Afanasyev, B.; Dombret, H.; Ravandi, F.; Sayar, H.; Jang, J.-H.; Porkka, K.; et al. Oral Azacitidine Maintenance Therapy for Acute Myeloid Leukemia in First Remission. N. Engl. J. Med. 2020, 383, 2526–2537. [Google Scholar] [CrossRef]
- Zawacka, J.E. P53 Biology and Reactivation for Improved Therapy in MDS and AML. Biomark. Res. 2024, 12, 34. [Google Scholar] [CrossRef]
- Lim, J.J.; Othus, M.; Shaw, C.M.; Russell, K.; Halpern, A.B.; Appelbaum, J.S.; Hendrie, P.; Walter, R.B.; Estey, E.H.; Percival, M.-E.M. Time Independent Factors That Predict Relapse in Adults with Acute Myeloid Leukemia. Blood Cancer J. 2024, 14, 5. [Google Scholar] [CrossRef]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef]
- Romer-Seibert, J.S.; Meyer, S.E. Genetic Heterogeneity and Clonal Evolution in Acute Myeloid Leukemia. Curr. Opin. Hematol. 2021, 28, 64–70. [Google Scholar] [CrossRef]
- Abaza, Y.; McMahon, C.; Garcia, J.S. Advancements and Challenges in the Treatment of AML. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e438662. [Google Scholar] [CrossRef]
- Wang, E.S.; Baron, J. Management of Toxicities Associated with Targeted Therapies for Acute Myeloid Leukemia: When to Push through and When to Stop. Hematology 2020, 2020, 57–66. [Google Scholar] [CrossRef]
- Roboz, G.J.; DiNardo, C.D.; Stein, E.M.; de Botton, S.; Mims, A.S.; Prince, G.T.; Altman, J.K.; Arellano, M.L.; Donnellan, W.; Erba, H.P.; et al. Ivosidenib Induces Deep Durable Remissions in Patients with Newly Diagnosed IDH1-Mutant Acute Myeloid Leukemia. Blood 2020, 135, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Schlenk, R.F.; Weber, D.; Fiedler, W.; Salih, H.R.; Wulf, G.; Salwender, H.; Schroeder, T.; Kindler, T.; Lübbert, M.; Wolf, D.; et al. Midostaurin Added to Chemotherapy and Continued Single-Agent Maintenance Therapy in Acute Myeloid Leukemia with FLT3-ITD. Blood 2019, 133, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.M.; Fischer, T.; Paquette, R.; Schiller, G.; Schiffer, C.A.; Ehninger, G.; Cortes, J.; Kantarjian, H.M.; DeAngelo, D.J.; Huntsman-Labed, A.; et al. Phase IB Study of the FLT3 Kinase Inhibitor Midostaurin with Chemotherapy in Younger Newly Diagnosed Adult Patients with Acute Myeloid Leukemia. Leukemia 2012, 26, 2061–2068. [Google Scholar] [CrossRef]
- Pulte, E.D.; Norsworthy, K.J.; Wang, Y.; Xu, Q.; Qosa, H.; Gudi, R.; Przepiorka, D.; Fu, W.; Okusanya, O.O.; Goldberg, K.B.; et al. FDA Approval Summary: Gilteritinib for Relapsed or Refractory Acute Myeloid Leukemia with a FLT3 Mutation. Clin. Cancer Res. 2021, 27, 3515–3521. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.G.X.; Iacobucci, I.; Shah, S.; Mitchell, A.; Wong, G.; Bansal, S.; Chen, D.; Gao, Q.; Kim, H.; Kennedy, J.A.; et al. Single-Cell Transcriptional Atlas of Human Hematopoiesis Reveals Genetic and Hierarchy-Based Determinants of Aberrant AML Differentiation. Blood Cancer Discov. 2025, 6, 307–324. [Google Scholar] [CrossRef]
- Wu, X.; Yang, X.; Dai, Y.; Zhao, Z.; Zhu, J.; Guo, H.; Yang, R. Single-Cell Sequencing to Multi-Omics: Technologies and Applications. Biomark. Res. 2024, 12, 110. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, J.; Lin, Y.; Liu, T.; Zhao, Y.; Zhao, H. ScMODAL: A General Deep Learning Framework for Comprehensive Single-Cell Multi-Omics Data Alignment with Feature Links. Nat. Commun. 2025, 16, 4994. [Google Scholar] [CrossRef]
- Jeong, Y.; Ronen, J.; Kopp, W.; Lutsik, P.; Akalin, A. ScMaui: A Widely Applicable Deep Learning Framework for Single-Cell Multiomics Integration in the Presence of Batch Effects and Missing Data. BMC Bioinform. 2024, 25, 257. [Google Scholar] [CrossRef]
- Ma, L.; Liu, J.; Sun, W.; Zhao, C.; Yu, L. ScMFG: A Single-Cell Multi-Omics Integration Method Based on Feature Grouping. BMC Genom. 2025, 26, 132. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Zhang, Z.; Shen, H.; Tang, X.; Wu, D.; Bao, X.; Xu, G.; Chen, S. Application of Omics in the Diagnosis, Prognosis, and Treatment of Acute Myeloid Leukemia. Biomark. Res. 2024, 12, 60. [Google Scholar] [CrossRef]
- Maurer, K.; Raths, F.; Gouin, K.; Park, C.Y.; Lawson, M.; Koh, J.; Fabani, M.; Livak, K.J.; Ritz, J.; Soiffer, R.J.; et al. Deconvolving Immunologic Networks of the AML Marrow Microenvironment with Spatial Multi-Omic Profiling. Blood 2024, 144, 928. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Duffy, M.P.; Ahn, K.J.; Sussman, J.H.; Pang, M.; Smith, D.; Duncan, G.; Zhang, I.; Huang, J.; Lin, Y.; et al. Mapping the Cellular Biogeography of Human Bone Marrow Niches Using Single-Cell Transcriptomics and Proteomic Imaging. Cell 2024, 187, 3120–3140. [Google Scholar] [CrossRef] [PubMed]
- Replogle, J.M.; Saunders, R.A.; Pogson, A.N.; Hussmann, J.A.; Lenail, A.; Guna, A.; Mascibroda, L.; Wagner, E.J.; Adelman, K.; Lithwick-Yanai, G.; et al. Mapping Information-Rich Genotype-Phenotype Landscapes with Genome-Scale Perturb-Seq. Cell 2022, 185, 2559–2575. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, R.; Zhou, C.; Rajaram, S.; Tai, J.W.; Kim, S.; Tanaka, K.; Tiwari, C.; Gao, G.; Carleton, M.; Sworder, B.J.; et al. Abstract 3252: Circulating Tumor DNA (CtDNA) Enables Enhanced Measurable Residual Disease (MRD) Detection and Non-Invasive Clonal Profiling in Acute Myeloid Leukemia (AML). Cancer Res. 2025, 85, 3252. [Google Scholar] [CrossRef]
- Ilié, M.; Hofman, V.; Bontoux, C.; Heeke, S.; Lespinet-Fabre, V.; Bordone, O.; Lassalle, S.; Lalvée, S.; Tanga, V.; Allegra, M.; et al. Setting up an Ultra-Fast Next-Generation Sequencing Approach as Reflex Testing at Diagnosis of Non-Squamous Non-Small Cell Lung Cancer; Experience of a Single Center (LPCE, Nice, France). Cancers 2022, 14, 2258. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Liu, Y.; Li, Y. A Review of Common Immunotherapy and Nano Immunotherapy for Acute Myeloid Leukemia. Front. Immunol. 2025, 16, 1505247. [Google Scholar] [CrossRef]
- DeAngelo, D.J.; Jonas, B.A.; Liesveld, J.L.; Bixby, D.L.; Advani, A.S.; Marlton, P.; Magnani, J.L.; Thackray, H.M.; Feldman, E.J.; O’Dwyer, M.E.; et al. Phase 1/2 Study of Uproleselan Added to Chemotherapy in Patients with Relapsed or Refractory Acute Myeloid Leukemia. Blood 2022, 139, 1135–1146. [Google Scholar] [CrossRef]
- World Health Organization. Advancing Equity and Resilience in a Turbulent World a Global Health Strategy for 2025–2028 Fourteenth General Programme of Work; World Health Organization: Geneva, Switzerland, 2025. [Google Scholar]
- Lee, H.; Han, J.H.; Kim, J.K.; Yoo, J.; Yoon, J.-H.; Cho, B.S.; Kim, H.-J.; Lim, J.; Jekarl, D.W.; Kim, Y. Machine Learning Predicts 30-Day Outcome among Acute Myeloid Leukemia Patients: A Single-Center, Retrospective, Cohort Study. J. Clin. Med. 2023, 12, 5940. [Google Scholar] [CrossRef]
- Nazari, E.; Farzin, A.H.; Aghemiri, M.; Avan, A.; Tara, M.; Tabesh, H. Deep Learning for Acute Myeloid Leukemia Diagnosis. J. Med. Life 2020, 13, 382–387. [Google Scholar] [CrossRef]

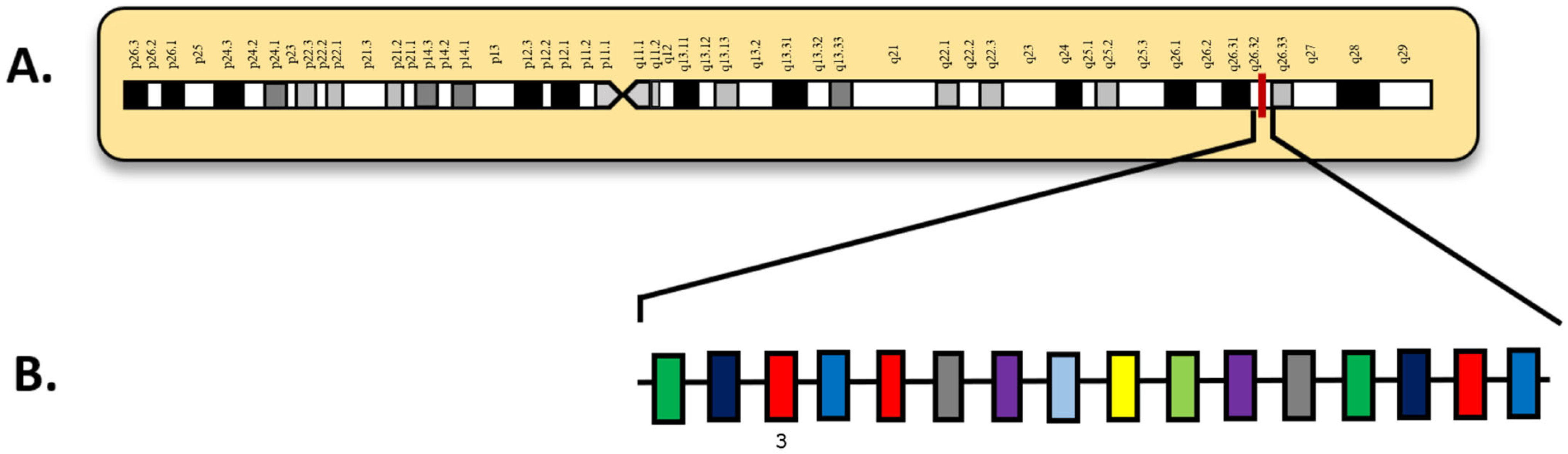
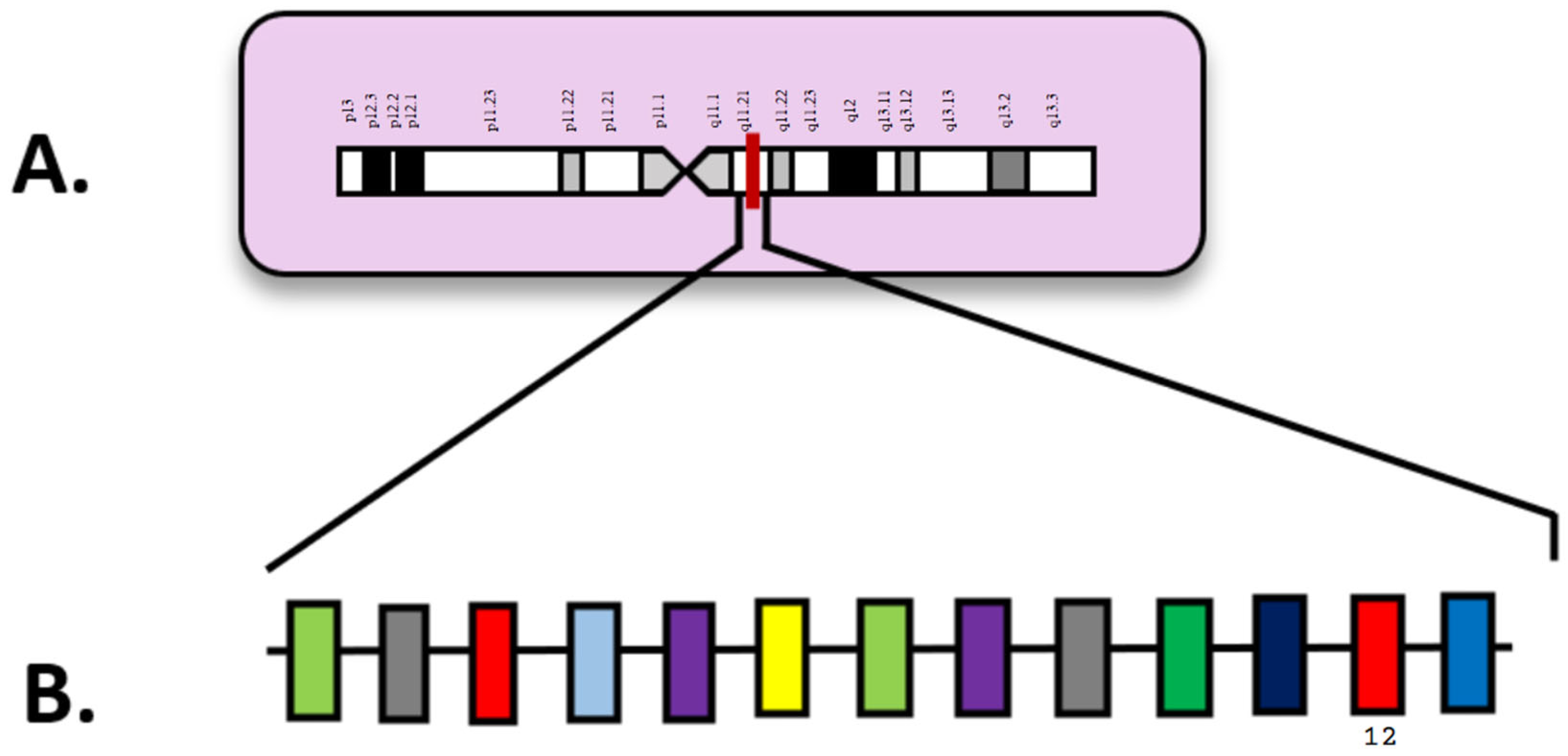
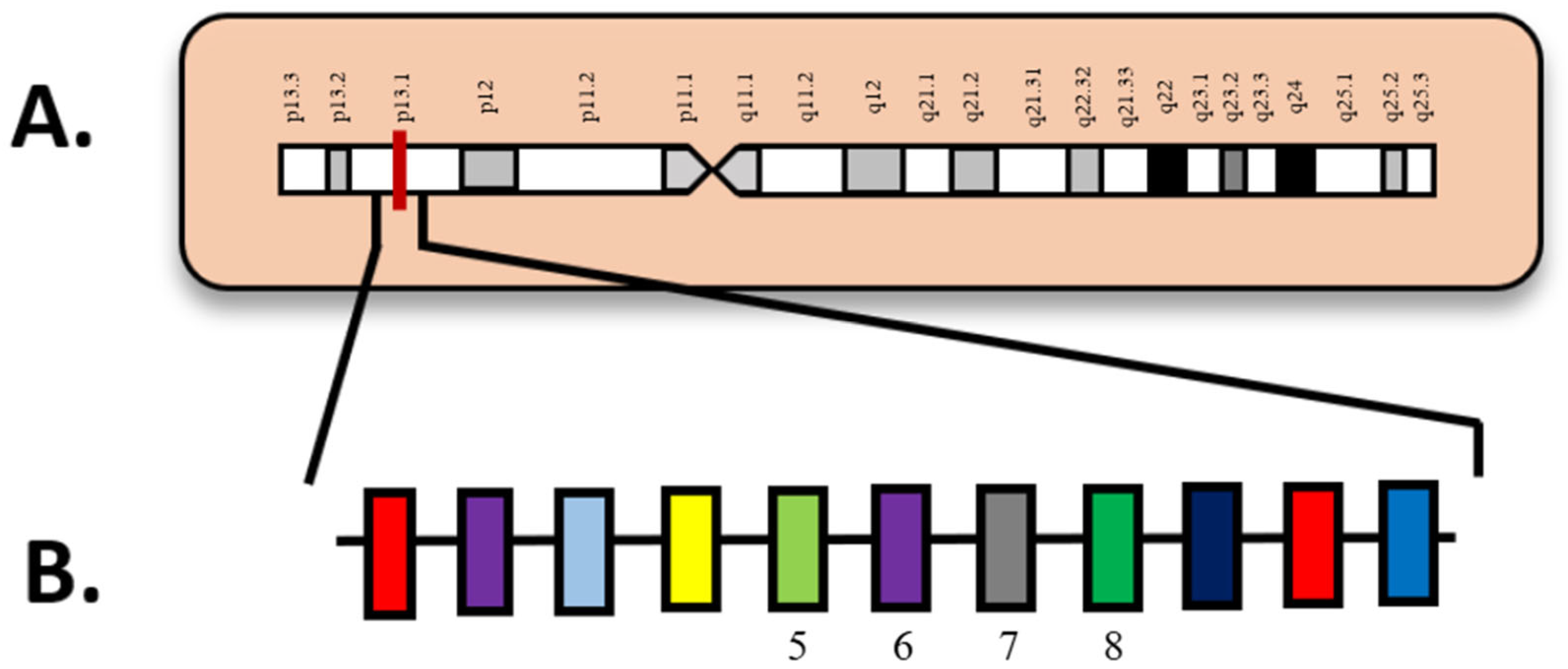
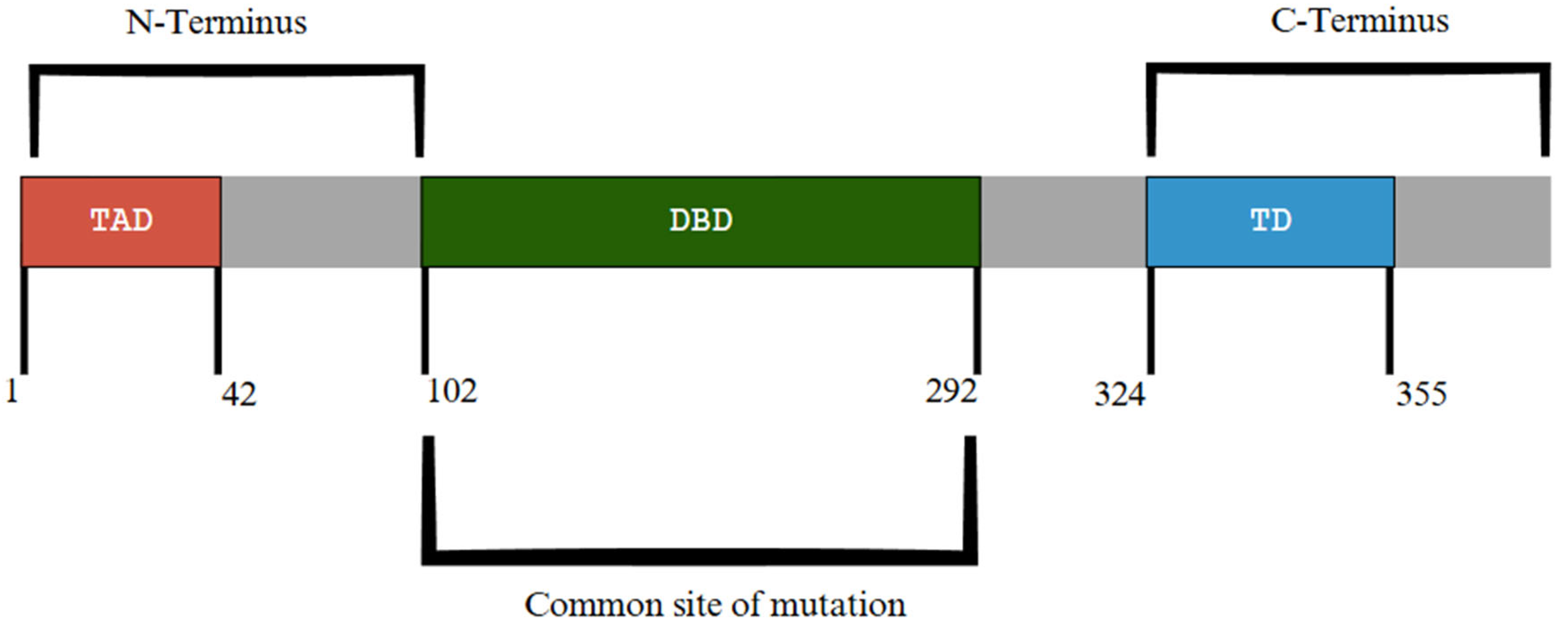
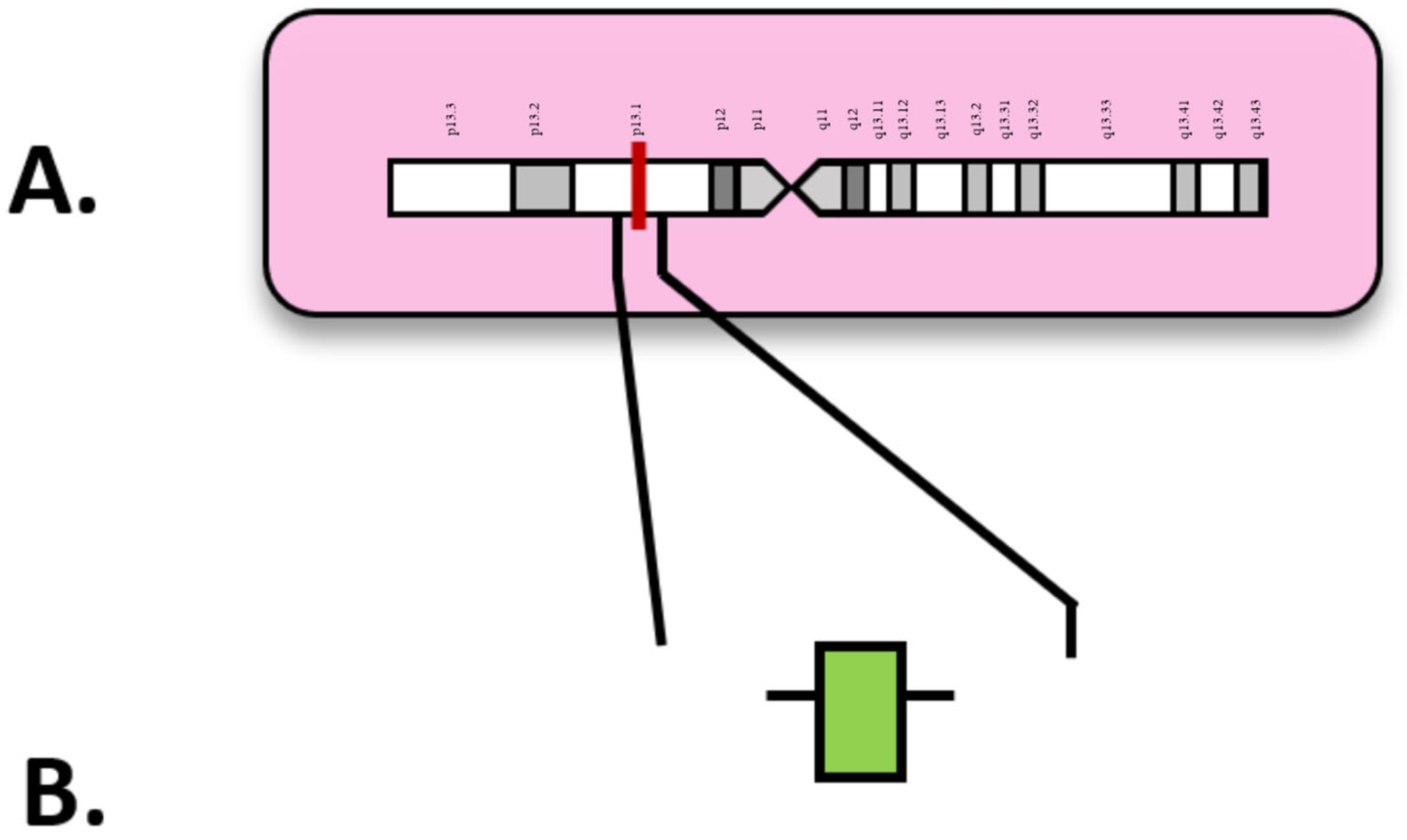
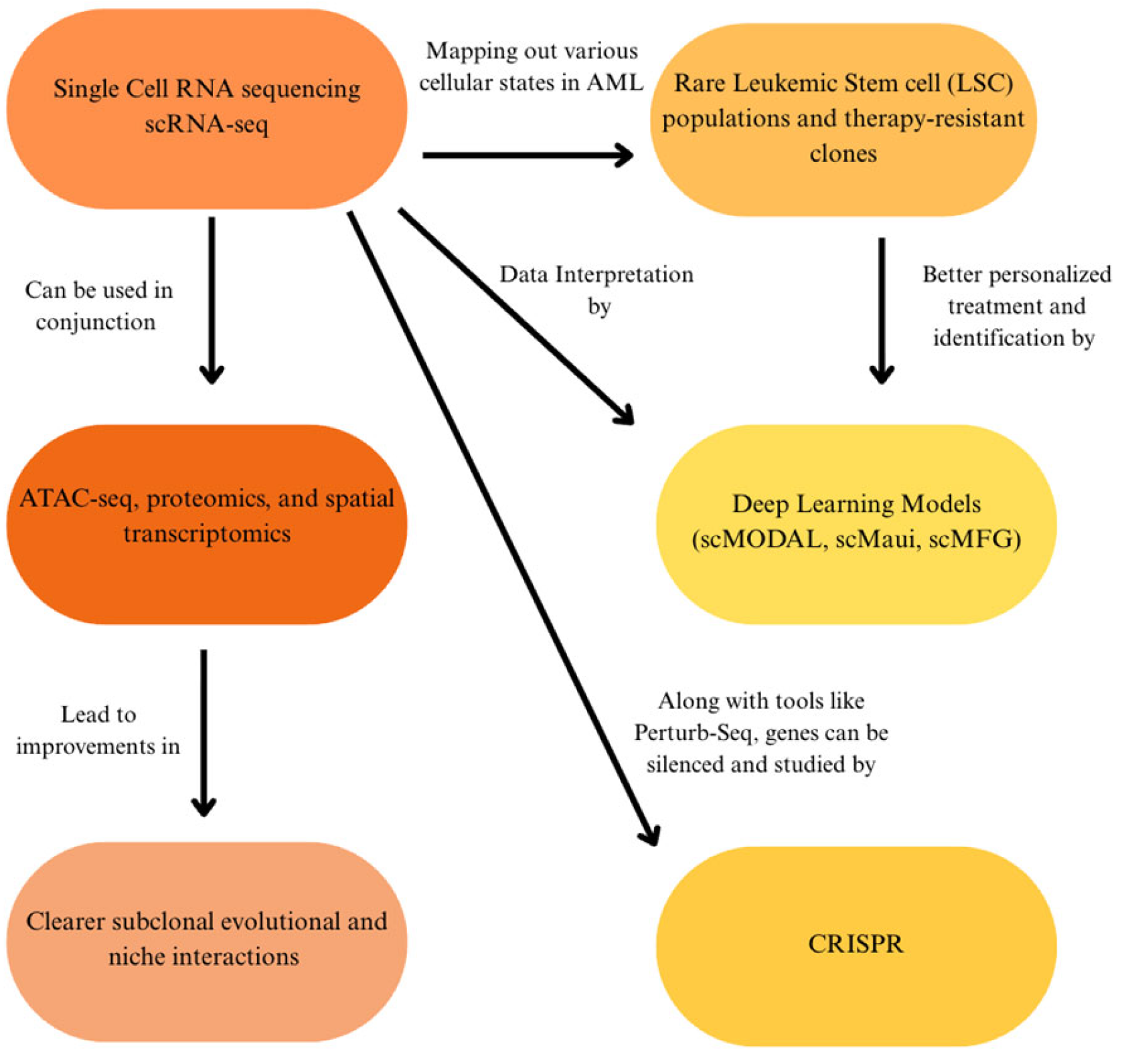
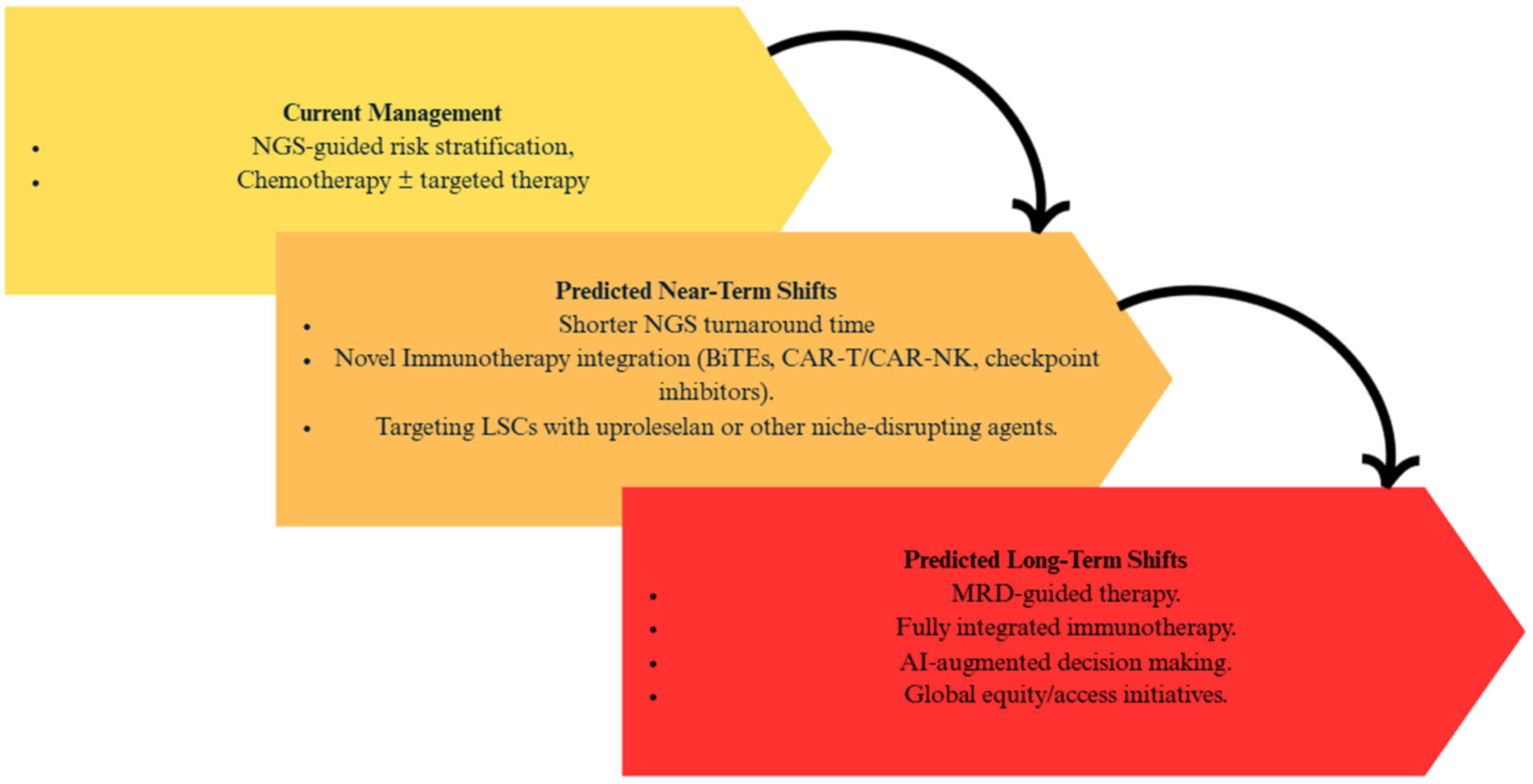
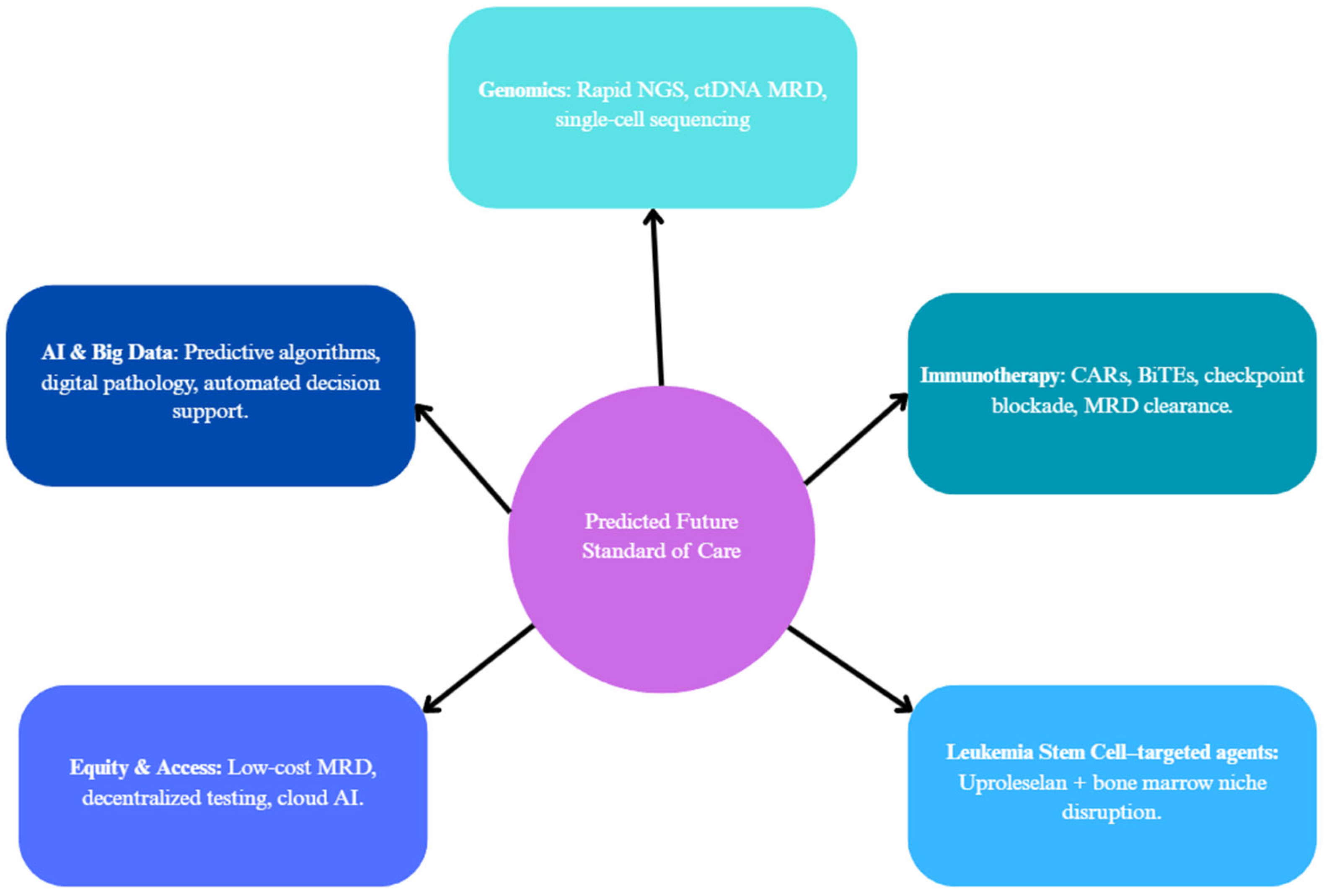
| Attribute | NGS (Short-Read Targeted Panels/WES/WGS) | FISH | Optical Genome Mapping (OGM) |
|---|---|---|---|
| Primary targets | SNVs, small indels; some CNVs/SVs depending on design | Known rearrangements, aneuploidies, and copy number at targeted loci | Large/complex SVs (translocations, inversions, insertions, deletions, CNVs); repeat expansions; genome-wide |
| Sensitivity | High for SNVs/indels; VAF often ≥1–5% for panels; SV/CNV sensitivity depends on assay/bioinformatics [22,23,24,25,26,27,28,29,30] | High for targeted loci if probe exists; detects mosaicism to ~5–10% depending on lab [28] | Genome-wide SV detection; claims down to ~5% VAF for mosaic/heterogeneous samples (assay-dependent) [11,12] |
| Specificity | High with orthogonal confirmation; false positives from artifacts/low-complexity regions; panel design matters [22,23,24,25,26,27,28,29,30] | High for predefined targets; interpretation can be confounded by signal overlap/signal noise [28] | High for large SVs; specificity impacted by complex duplications/repeats; orthogonal confirmation recommended [11,12] |
| Resolution | Single-base for SNVs/indels; exon-level to gene-level for CNVs/SVs depending on depth/algorithm [22,23,24,25,26,27,28,29,30] | Probe-level (typically 100–200 kb around target; break-apart or fusion probes) [28] | Kilobase-scale mapping of long molecules; resolves structure/orientation and partners for many SVs [11,12] |
| Prior knowledge needed | Not for broad panels/WES/WGS; targeted panels predefine genes/hotspots | Yes—probe must be designed for suspected locus/rearrangement | No (genome-wide), unbiased for SV discovery |
| Advantages | Detects recurrent AML SNVs/indels (e.g., NPM1, DNMT3A, FLT3); scalable; actionable variant interpretation frameworks exist [22,23,24,25,26,27,28,29,30] | Rapid, cost-effective for confirming known rearrangements (e.g., MECOM, RUNX1); single-cell resolution | Single assay for all major SV classes; clarifies complex/cryptic rearrangements and fusions; complements NGS [11,12] |
| Limitations | Short reads miss/under-resolve complex SVs, repeats; panel bias and variable exon coverage; SV calling requires optimized pipelines [22,23,24,25,26,27,28,29,30] | Limited scope (only where probes exist); cannot genome-wide screen; lower structural detail | Limited for small variants (SNVs/indels); VAF cutoffs need clinical context; orthogonal confirmation often needed [11,12,31] |
| Typical VAF/abundance limits | Panels often call ≥1–5% VAF for SNVs (lab-dependent); SV/CNV thresholds vary [22,23,24,25,26,27,28,29,30] | Mosaic detection depends on probe/assay (~5–10% typical) [28] | Reported ~5% for SVs in cancer samples; interpret with disease-specific rules (e.g., TP53 in ICC) [12,31] |
| Best use cases in AML | Mutational profiling for diagnosis/risk (e.g., NPM1, FLT3-ITD/TKD, DNMT3A, ASXL1, TP53); MRD with specialized assays | Rapid confirmation of specific rearrangements (e.g., MECOM break-apart), aneuploidies, and hallmark fusions | Discovery/refinement of karyotype-scale abnormalities, cryptic/complex rearrangements, fusion partners; complement to NGS |
| Turnaround (typical) | Days (panels) to 1–2 weeks (WES/WGS); lab-specific | 24–72 h for targeted probes | Days to ~1 week; lab-specific |
| Notes on validation | Confirm key calls (esp. low VAF or SVs) with orthogonal method (ddPCR, Sanger, FISH) | Use with orthogonal methods for breakpoint/partner resolution | Confirm clinically actionable SVs with NGS/FISH or PCR, per lab policy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Hassan, F.; Tirado, C.A. A Mutational Landscape in Acute Myeloid Leukemia: Overview and Prognostic Impacts. Diagnostics 2025, 15, 2537. https://doi.org/10.3390/diagnostics15192537
Chen J, Hassan F, Tirado CA. A Mutational Landscape in Acute Myeloid Leukemia: Overview and Prognostic Impacts. Diagnostics. 2025; 15(19):2537. https://doi.org/10.3390/diagnostics15192537
Chicago/Turabian StyleChen, Jeff, Fares Hassan, and Carlos A. Tirado. 2025. "A Mutational Landscape in Acute Myeloid Leukemia: Overview and Prognostic Impacts" Diagnostics 15, no. 19: 2537. https://doi.org/10.3390/diagnostics15192537
APA StyleChen, J., Hassan, F., & Tirado, C. A. (2025). A Mutational Landscape in Acute Myeloid Leukemia: Overview and Prognostic Impacts. Diagnostics, 15(19), 2537. https://doi.org/10.3390/diagnostics15192537






