Synchronous Pulmonary and Cecal High-Grade Neuroendocrine Carcinomas Presenting as Hepatic Metastases: A Diagnostic Challenges and Literature Review
Abstract
1. Introduction
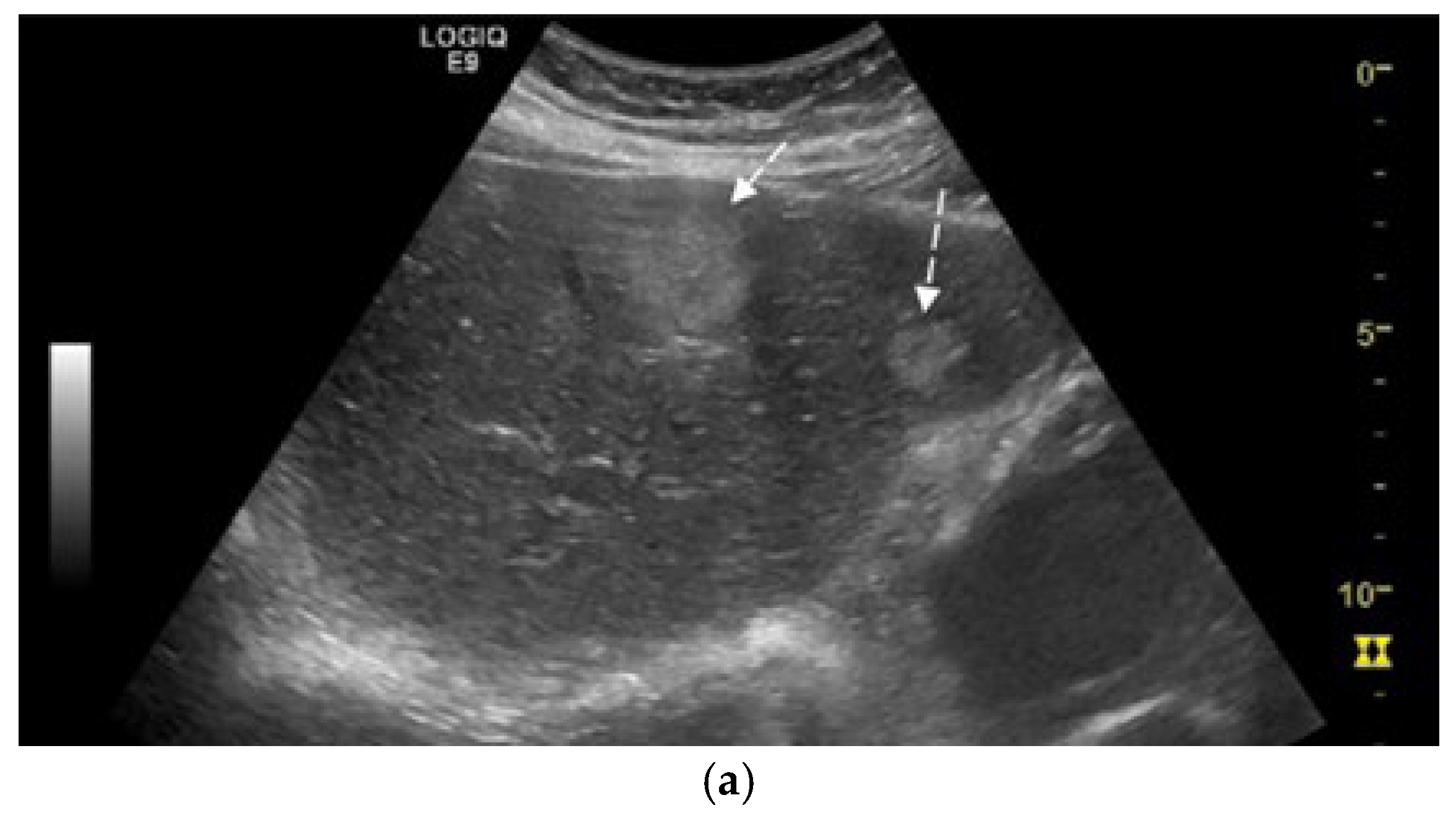
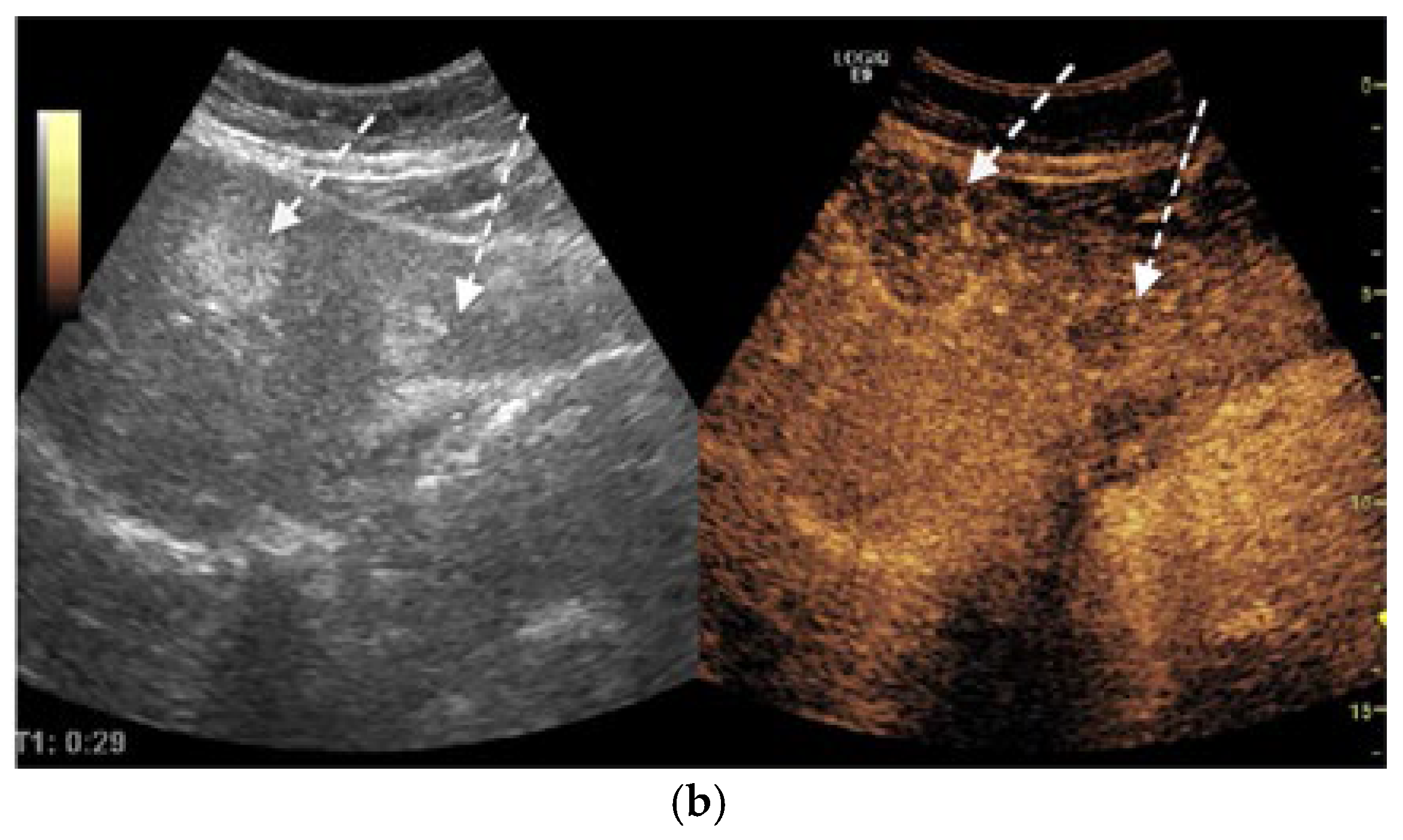
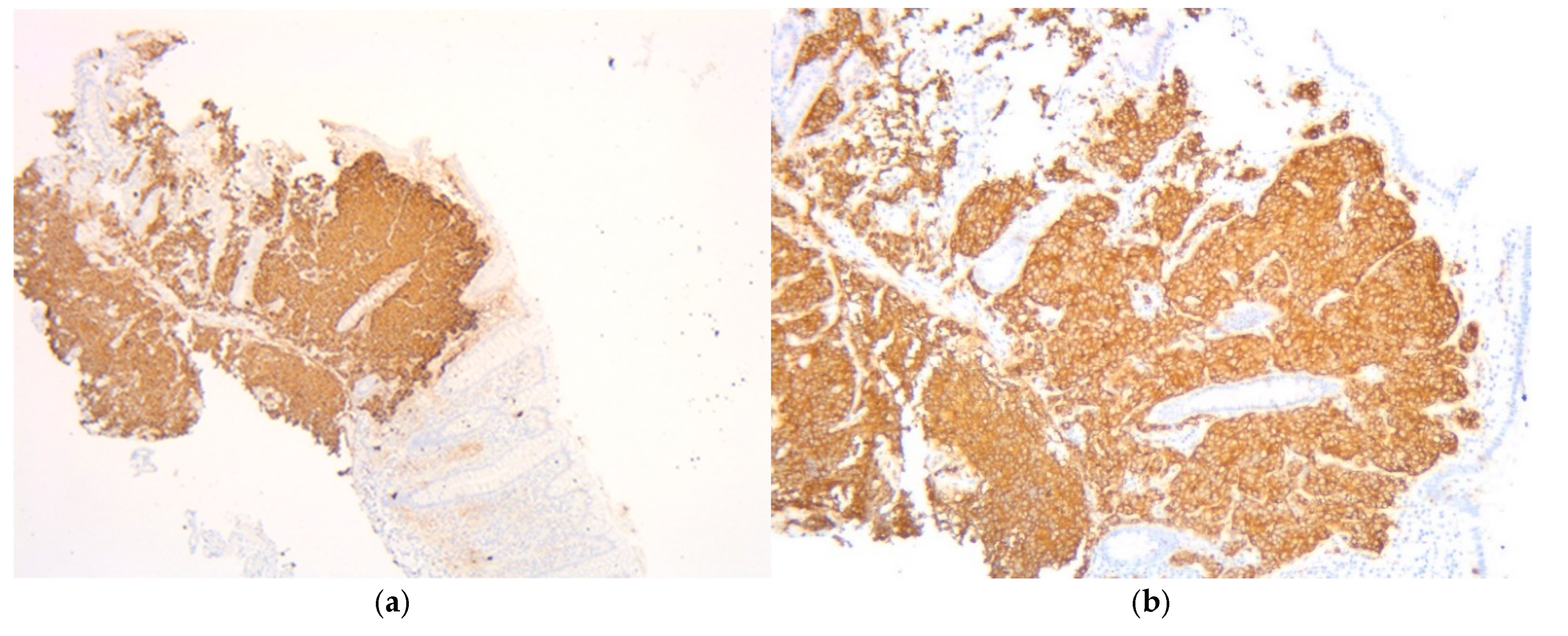
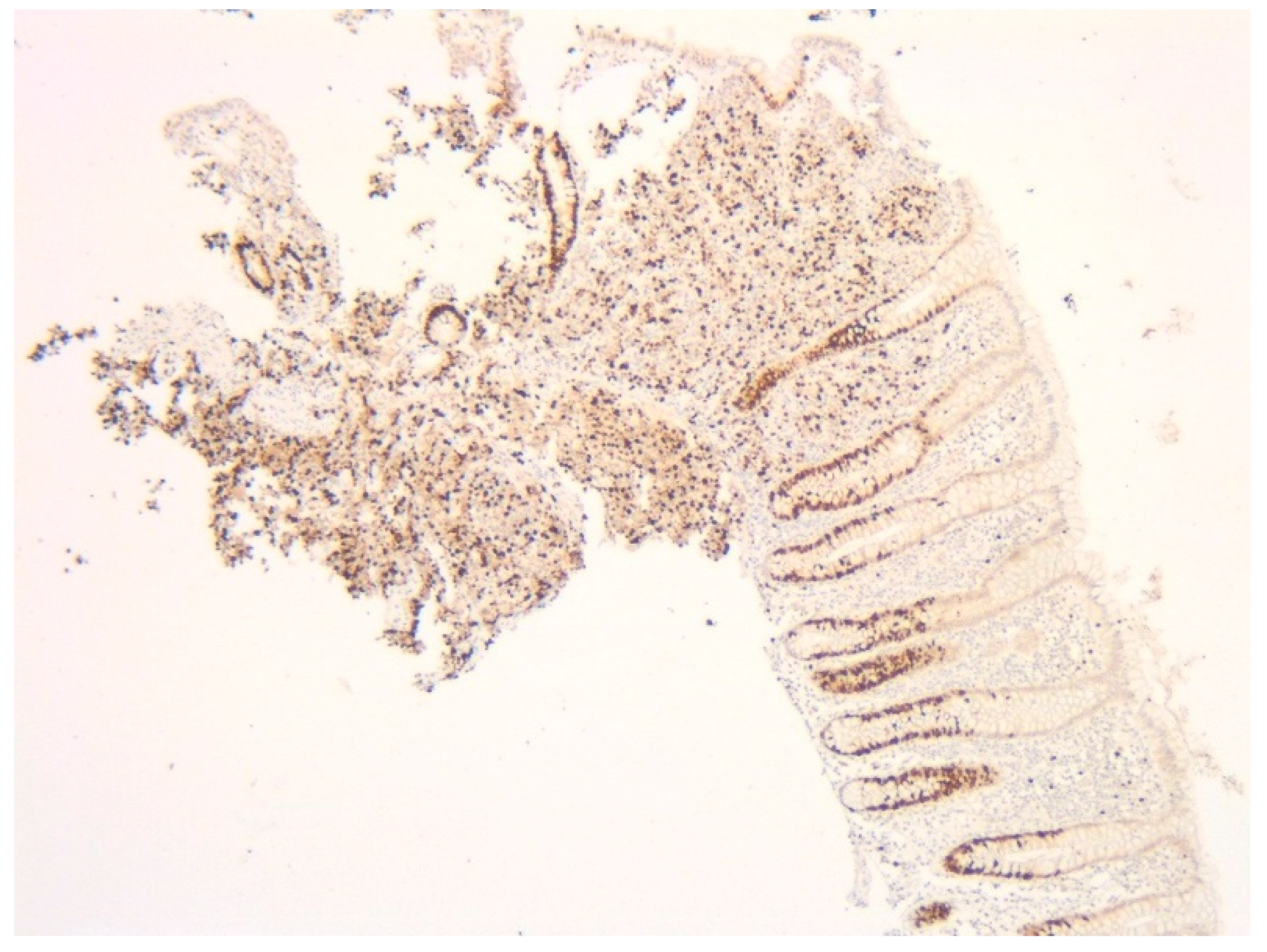
2. Case Presentation
3. Literature Review
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NENs | Neuroendocrine neoplasms |
| NETs | Neuroendocrine tumors |
| NECs | Neuroendocrine carcinomas |
| SVR | Sustained virologic response |
| APF | Alpha-fetoprotein |
| CA19-9 | Carbohydrate antigen 19-9 |
| CEA | Carcinoembryonic antigen |
| CEUS | Contrast-enhanced ultrasonography |
| CT | Computed tomography |
| EGD | Esophagogastroduodenoscopy |
| LGE | Lower gastrointestinal endoscopy |
| IHC | Immunohistochemical tests |
| HPF | High power fields |
| BCL-2 | B-cell lymphoma 2 protein |
| HCC | Hepatocellular carcinoma |
| PET | Positron emission tomography |
| PHNETS | Primary hepatic neuroendocrine tumors |
| CgA | Chromogranin A |
| 5-HIAA | 5-hydroxyindoleacetic acid |
| VIP | Vasoactive intestinal peptide |
| PP | Pancreatic polypeptide |
| NSE | Neuron-specific enolase |
| INSM 1 | Insulinoma-associated protein 1 |
| TTF-1 | Thyroid transcription factor-1 |
| ISL1 | Insulin gene enhancer protein-1 |
| NESP 55 | Neuroendocrine secretory protein-55 |
| BM | Bone metastases |
References
- Saxena, A.; Chua, T.C.; Sarkar, A.; Chu, F.; Liauw, W.; Zhao, J.; Morris, D.L. Progression and survival results after radical hepatic metastasectomy of indolent advanced neuroendocrine neoplasms (NENs) supports an aggressive surgical approach. Surgery 2011, 149, 209–220. [Google Scholar] [CrossRef]
- Ahmed, M. Gastrointestinal neuroendocrine tumors. World J. Gastrointest. Oncol. 2020, 12, 791–807. [Google Scholar] [CrossRef]
- Maggard, M.A.; O’Connell, J.B.; Ko, C.Y. Updated population-based review of carcinoid tumors. Ann. Surg. 2004, 240, 117–122. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Tsoukalas, N.; Galanopoulos, M.; Tolia, M.; Kiakou, M.; Nakos, G.; Papakostidi, A.; Koumakis, G. Rectal neuroendocrine tumor with uncommon metastatic spread: A case report and review of literature. World J. Gastrointest. Oncol. 2016, 8, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.; Gustafsson, B.I.; Chan, A.; Svejda, B.; Kidd, M.; Modlin, I.M. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol. Metab. Clin. North Am. 2011, 40, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, T.F.; Hakami, R.A.; AlShammari, S.; Zayed, M.A.; AlSohaibani, M.O.; Bin Traiki, T. A perforated colonic neuroendocrine tumor with liver metastasis: A case report and literature review. Am. J. Case Rep. 2019, 20, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Atwal, D.; Joshi, K.P.; Jeffus, S.; Ntambi, J.; Mahmoud, F. Well-differentiated neuroendocrine tumor—A low b-grade tumor’s aggressive course and dismal outcome: A case report. Perm. J. 2017, 21, 16–193. [Google Scholar] [CrossRef]
- Sarmiento, J.M.; Que, F.G. Hepatic surgery for metastases from neuroendocrine tumors. Surg. Oncol. Clin. N. Am. 2003, 12, 231–242. [Google Scholar] [CrossRef]
- Calin, M.L.; Sadiq, A.; Arevalo, G.; Fuentes, R.; Flanders, V.L.; Gupta, N.; Nasri, B.; Singh, K. The first case report of robotic multivisceral resection for synchronous liver metastasis from pancreatic neuroendocrine tumor: A case report and literature review. J. Laparoendosc. Adv. Surg. Tech. 2016, 26, 818–824. [Google Scholar] [CrossRef]
- Hamada, Y.; Tameda, M.; Nakagawa, H. Metachronous liver metastasis during long-term follow-up after endoscopic submucosal dissection of a small rectal neuroendocrine neoplasm. Intern. Med. 2025, 64, 2323–2327. [Google Scholar] [CrossRef]
- Wyffels, K.; De Wilde, C.; Van Huysse, J.; De Wilde, V. Malignant behavior of a well-differentiated digestive neuroendocrine tumor with peritoneal carcinomatosis: A case report. Exp. Ther. Med. 2023, 25, 283. [Google Scholar] [CrossRef]
- Aijaz, P.; Niazi, M.A.; Chela, H.K.; Zahid, K.; Daglilar, E. Epigastric pain and dysphagia in a 36-year-old man due to primary esophageal small cell carcinoma. Am. J. Case Rep. 2024, 25, e943392. [Google Scholar] [CrossRef]
- Hsiao, T.H.; Wu, C.C.; Tseng, H.H.; Chen, J.H. Synchronous but separate neuroendocrine tumor and high-grade dysplasia/adenoma of the gall bladder: A case report. World J. Clin. Cases 2022, 10, 2322–2329. [Google Scholar] [CrossRef]
- Morikawa, K.; Igarashi, T.; Misumi, S.; Fukuda, T.; Ojiri, H.; Matsudaira, H.; Shiba, H.; Sato, S. A case of pseudocystic liver metastases from an atypical lung carcinoid tumor. Radiol. Case Rep. 2019, 14, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, X.; Lu, X.; Yuan, Z.; Yang, Y.; Qiu, C.; Li, H. Primary hepatic neuroendocrine tumor: Two cases report with literature review. Front. Oncol. 2023, 13, 1225583. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Yu, Q. Twenty years of gastrointestinal neuroendocrine tumors in the United States: Incidence and survival outcomes from 2000 to 2020. Gastroenterology 2024, 166, S956. [Google Scholar] [CrossRef]
- Martinez Ponce, J.P.; Ubysz, O.; Vanhecke, T. Carcinoid heart disease: A rare complication of metastatic neuroendocrine tumor. Cureus 2025, 17, e78148. [Google Scholar] [CrossRef]
- Shukla, A.; Kalayarasan, R.; Gnanasekaran, S.; Pottakkat, B. Appraisal of gastric stump carcinoma and current state of affairs. World J. Clin. Cases 2023, 11, 2864–2873. [Google Scholar] [CrossRef]
- Boehm, S.A.; Moenig, S.P.; Wolfgarten, E.E.; Wickenhauser, C.; Wolters, U.; Hoelscher, A.H. Synchronous nonfunctioning neuroendocrine carcinoma of the pancreas and appendix. J. Clin. Gastroenterol. 2003, 36, 452–453. [Google Scholar] [CrossRef]
- Tsunenari, T.; Aosasa, S.; Ogata, S.; Hoshikawa, M.; Nishikawa, M.; Noro, T.; Shinto, E.; Tsujimoto, H.; Ueno, H.; Hamabe, F.; et al. Synchronous neuroendocrine tumors in both the pancreas and ileum: A case report. Int. J. Surg. Case Rep. 2016, 22, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Zhang, Q.; Li, Y.; Han, F. Synchronous multiple carcinoma with small intestine and pulmonary neuroendocrine involvement: A case report. Medicine 2017, 96, e8623. [Google Scholar] [CrossRef] [PubMed]
- Bruera, G.; Giuliani, A.; Romano, L.; Chiominto, A.; Di Sibio, A.; Mastropietro, S.; Cosenza, P.; Ricevuto, E.; Schietroma, M.; Carlei, F.; et al. Poorly differentiated neuroendocrine rectal carcinoma with uncommon immunohistochemical features and clinical presentation with a subcutaneous metastasis, treated with first-line intensive triplet chemotherapy plus bevacizumab FIr-B/FOx regimen: An experience of multidisciplinary management in clinical practice. BMC Cancer 2019, 19, 960. [Google Scholar] [CrossRef]
- Song, I.H.; Lee, Y.S.; Sun, D.-I.; Hong, Y.-K.; Lee, K.-Y. Metachronous double primary neuroendocrine tumors in larynx and lung: A case report. J. Int. Med. Res. 2020, 48, 300060520962928. [Google Scholar] [CrossRef]
- Omori, S.; Harada, N.; Toshima, T.; Takeishi, K.; Itoh, S.; Ikegami, T.; Yoshizumi, T.; Mori, M. Multiple liver metastases originating from synchronous double cancer of neuroendocrine tumor and rectal cancer: A case report. Surg. Case Rep. 2020, 6, 36. [Google Scholar] [CrossRef]
- Cai, H.-J.; Wang, H.; Cao, N.; Huang, B.; Kong, F.-L.; Lu, L.-R.; Huang, Y.-Y.; Wang, W. Calcitonin-negative neuroendocrine tumor of the thyroid with metastasis to liver—Rare presentation of an unusual tumor: A case report and review of literature. World J. Clin. Cases 2020, 8, 179–187. [Google Scholar] [CrossRef]
- Rooper, L.M.; Bishop, J.A.; Westra, W.H. INSM1 is a sensitive and specific marker of neuroendocrine differentiation. Am. J. Surg. Pathol. 2017, 41, 1561–1569. [Google Scholar] [CrossRef]
- Pellegrino, F.; Granata, V.; Fusco, R.; Grassi, F.; Tafuto, S.; Perrucci, L.; Tralli, G.; Scaglione, M. Diagnostic management of gastroenteropancreatic neuroendocrine neoplasms: Technique optimization and tips and tricks for radiologists. Tomography 2023, 9, 217–246. [Google Scholar] [CrossRef]
- Liau, J.Y.; Tsai, J.H.; Jeng, Y.M.; Kuo, K.T.; Huang, H.Y.; Liang, C.W.; Yang, C.Y. The diagnostic utility of PAX8 for neuroendocrine tumors: An immunohistochemical reappraisal. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 57–63. [Google Scholar] [CrossRef]
- Werling, R.W.; Yaziji, H.; Bacchi, C.E.; Gown, A.M. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: An immunohistochemical survey of 476 primary and metastatic carcinomas. Am. J. Surg. Pathol. 2003, 27, 303–310. [Google Scholar] [CrossRef]
- Bellizzi, A.M. SATB2 in neuroendocrine neoplasms: Strong expression is restricted to well-differentiated tumours of lower gastrointestinal tract origin and is most frequent in Merkel cell carcinoma among poorly differentiated carcinomas. Histopathology 2020, 76, 251–264. [Google Scholar] [CrossRef]
- El-Haddad, G.; Strosberg, J. Radioembolization for metastatic neuroendocrine tumors. Dig. Dis. Interv. 2021, 5, 103–112. [Google Scholar] [CrossRef]
- Warsinggih; Liliyanto; Prihantono; Ariani, G.D.W.; Faruk, M. Colorectal neuroendocrine tumors: A case series. Int. J. Surg. Case Rep. 2020, 72, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Waisberg, D.R.; Fava, A.S.; Martins, L.C.; Matos, L.L.; Franco, M.I.F.; Waisberg, J. Colonic carcinoid tumors: A clinicopathologic study of 23 patients from a single institution. Arq. Gastroenterol. 2014, 46, 288–293. [Google Scholar] [CrossRef]
- Rinke, A.; Ambrosini, V.; Dromain, C.; Garcia-Carbonero, R.; Haji, A.; Koumarianou, A.; van Dijkum, E.N.; O’Toole, D.; Rindi, G.; Scoazec, J.; et al. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for colorectal neuroendocrine tumours. J. Neuroendocrinol. 2023, 35, e13309. [Google Scholar] [CrossRef]
- Pu, X.; Xu, T.; Ge, C.; He, Y.; Yang, X.; Chang, P. A case of durvalumab-treated double primary cancers of the colon and lung. Ann. Palliat. Med. 2020, 9, 3614–3622. [Google Scholar] [CrossRef]
- Maekawa, A.; Kudo, A.; Kishino, M.; Murase, Y.; Watanabe, S.; Ishikawa, Y.; Ueda, H.; Akahoshi, K.; Ogawa, K.; Ono, H.; et al. Hormonal tumor mapping for liver metastases of gastroenteropancreatic neuroendocrine neoplasms: A novel therapeutic strategy. J. Cancer Res. Clin. Oncol. 2021, 147, 2429–2440. [Google Scholar] [CrossRef]
- Sakuma, Y.; Yasuda, Y.; Sata, N.; Hosoya, Y.; Shimizu, A.; Fujii, H.; Matsubara, D.; Fukushima, N.; Miki, A.; Maeno, M.; et al. Pancreatic neuroendocrine tumor with metastasis to the spleen: A case report. BMC Cancer 2017, 17, 37. [Google Scholar] [CrossRef]
- Lucandri, G.; Fiori, G.; Lucchese, S.; Pende, V.; Farina, M.; Giordano, M.; Santoro, E. Extended surgical resection for non-functioning duodenal neuroendocrine tumor. J. Surg. Case Rep. 2022, 2022, rjac391. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Benevento, E.; De Cicco, F.; Fazzalari, B.; Guadagno, E.; Hasballa, I.; Tarsitano, M.G.; Isidori, A.M.; Colao, A.; Faggiano, A. Neuroendocrine neoplasms in the context of inherited tumor syndromes: A reappraisal focused on targeted therapies. J. Endocrinol. Investig. 2023, 46, 213–234. [Google Scholar] [CrossRef]
- Xue, J.; Li, X.; Qu, N.; Wu, Z.; Wang, G.; Chenhuang, Z.; Cao, X. A case report of diagnosis of liver metastasis of medullary thyroid carcinoma by multimodal ultrasound. Radiol. Case Rep. 2023, 18, 2279–2281. [Google Scholar] [CrossRef]
- Mahajan, S.; Shaha, A.; Grewal, R.K. Incidental detection of medullary thyroid carcinoma by 68Ga-DOTATATE PET/CT in a patient with neuroendocrine tumor liver metastases. Clin. Nucl. Med. 2017, 43, 206–208. [Google Scholar] [CrossRef]
- Modica, R.; Benevento, E.; Liccardi, A.; Cannavale, G.; Minotta, R.; DI Iasi, G.; Colao, A. Recent advances and future challenges in the diagnosis of neuroendocrine neoplasms. Minerva Endocrinol. 2024, 49, 158–174. [Google Scholar] [CrossRef]
- Modica, R.; Benevento, E.; Altieri, B.; Minotta, R.; Liccardi, A.; Cannavale, G.; Di Iasi, G.; Colao, A. Role of bone metastases in lung neuroendocrine neoplasms: Clinical presentation, treatment and impact on prognosis. Int. J. Mol. Sci. 2024, 25, 8957. [Google Scholar] [CrossRef]


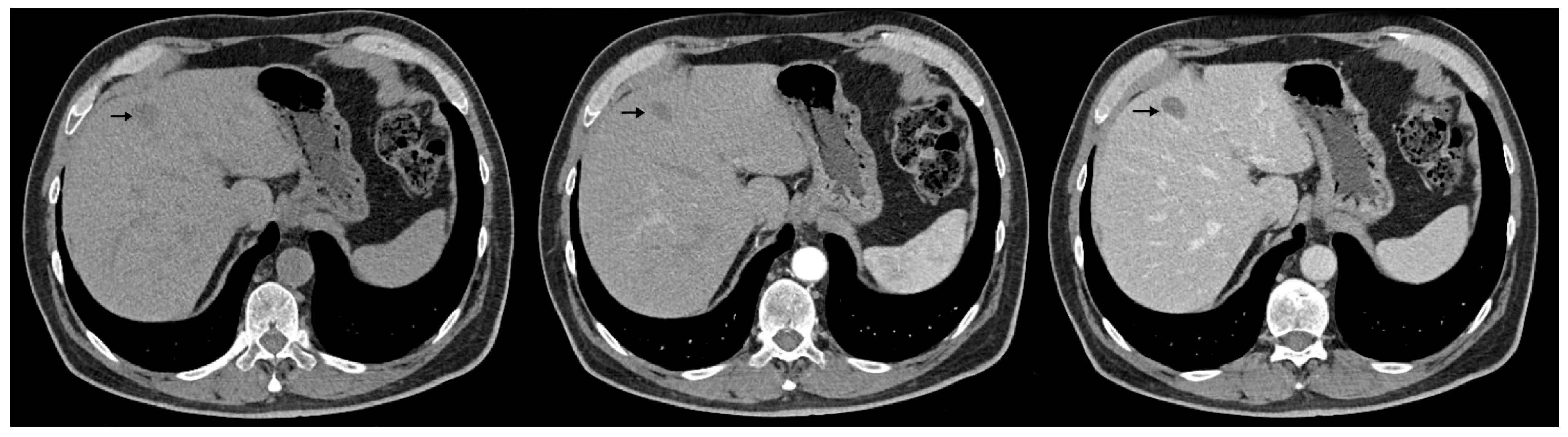
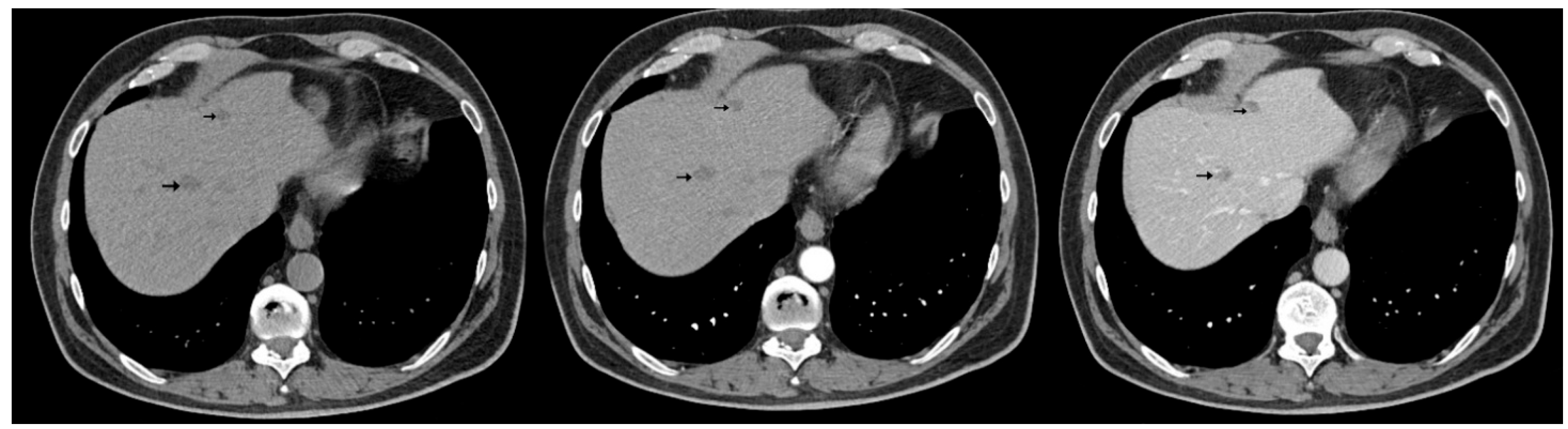
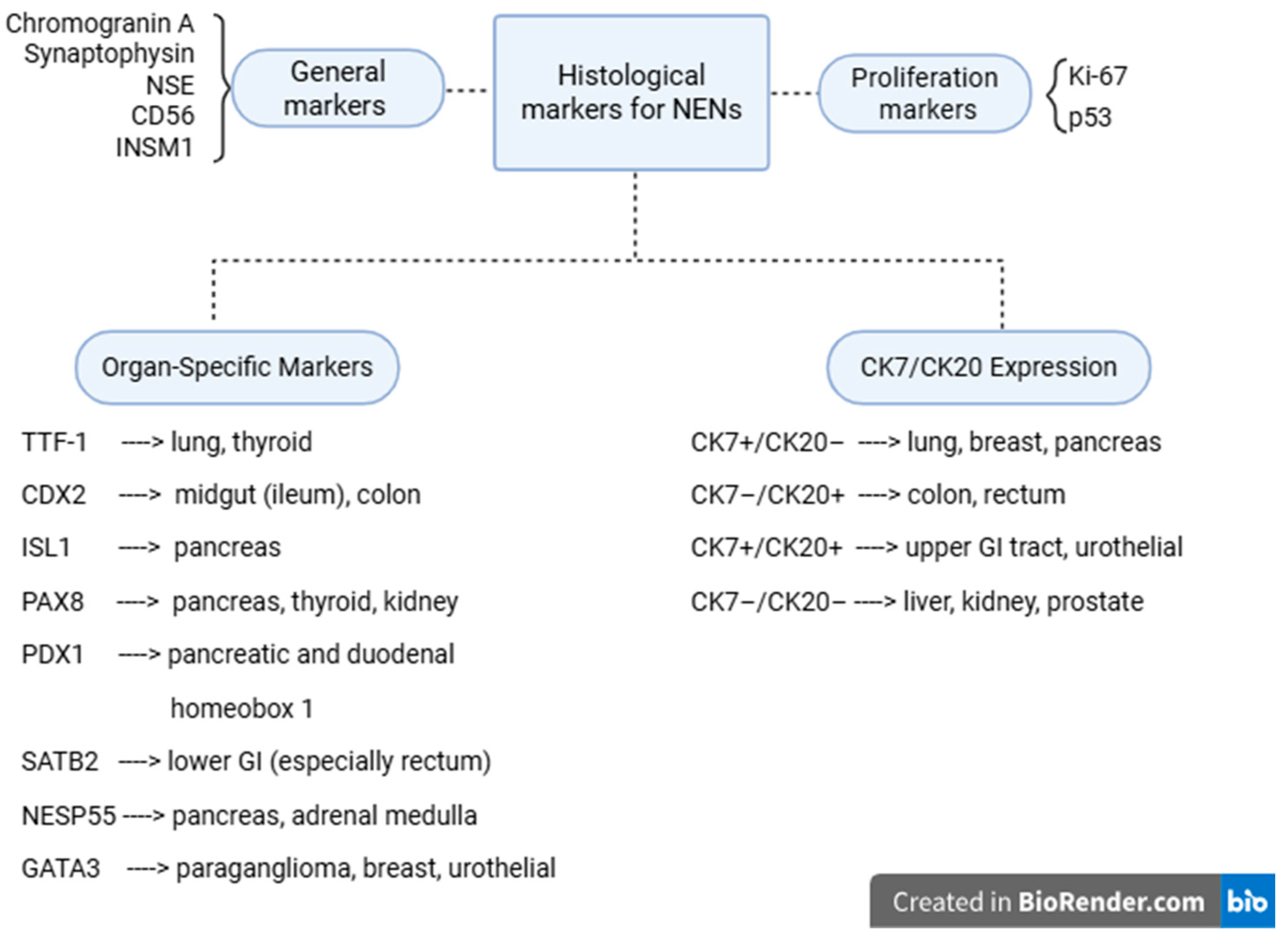
| Reported Cases | Localization | Synchronous (S)/ Metachronous (M) | Liver Metastases |
|---|---|---|---|
| Boehm S. et al. (2003) [21] | Pancreas and Appenidix | S | absent |
| Tsunenari et al. (2016) [22] | Pancreas and Ileum | S | absent |
| Shan et al. (2017) [23] | Small intestine and Lung | S | absent |
| Bruera et al. (2019) [24] | Rectum and Skin | S | absent |
| Song In Hye et al. (2020) [25] | Larynx and Lung | M | absent |
| Omori Sachie et al. (2020) [26] | Rectum and Mesentery | S | present |
| Hamada Y et al. (2025) [12] | Lung and Rectum | M | present |
| Our case | Lung and Cecum | S | present |
| Therapy/ Approach | Indication | Mechanism/ Strategy | Clinical Notes/ References |
|---|---|---|---|
| Surgical resection | Localized/ resectable disease | Complete excision of primary and/or metastatic lesions | Only curative option |
| Cytoreductive surgery | Extensive but resectable disease | Resection of ≥90% of tumor | Improve survival and control hormonal symptoms [10] |
| Hepatectomy | Resectable liver metastases | Segmental liver resection, often with primary tumor removal | Robotic-assisted surgery for minimally invasive procedures [11] |
| Transarterial embolization and chemoembolization | Unresectable hypervascular liver metastases | Embolization ± localized chemotherapy delivery via hepatic artery | Effective in cytoreduction; supports multimodal therapy [17,38] |
| Radiofrequency ablation | Small, isolated liver lesions | Thermal destruction of tumor tissue using needle electrode | Used when surgery is Contraindicated [39] |
| Somatostatin analogs (SSAs) | Functional, well-differentiated (G1/G2), receptor-positive tumors | Symptom control and antiproliferative action via somatostatin receptor binding | Octreotide, lanreotide—used in systemic or adjuvant treatment [9,39] |
| Chemotherapy/ Targeted therapy | High-grade (G3), poorly differentiated/ rapidly progressing | Systemic cytotoxic agents and/or targeted molecular therapy | Platinum-based regimens or everolimus/sunitinib [17,40] |
| Hormonal Tumor Mapping | Functional tumors with multiple lesions | Identifies dominant hormone-secreting lesion via selective arterial secretagogue injection | Enables targeted, less extensive surgery [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sârbu, G.E.; Jucan, A.E.; Mihai, C.V.; Atodiresei, C.; Ene, M.; Ungureanu, C.; Mihai, I.R.; Nedelciuc, O.; Dranga, M.; Cijevschi Prelipcean, C.; et al. Synchronous Pulmonary and Cecal High-Grade Neuroendocrine Carcinomas Presenting as Hepatic Metastases: A Diagnostic Challenges and Literature Review. Diagnostics 2025, 15, 2535. https://doi.org/10.3390/diagnostics15192535
Sârbu GE, Jucan AE, Mihai CV, Atodiresei C, Ene M, Ungureanu C, Mihai IR, Nedelciuc O, Dranga M, Cijevschi Prelipcean C, et al. Synchronous Pulmonary and Cecal High-Grade Neuroendocrine Carcinomas Presenting as Hepatic Metastases: A Diagnostic Challenges and Literature Review. Diagnostics. 2025; 15(19):2535. https://doi.org/10.3390/diagnostics15192535
Chicago/Turabian StyleSârbu, Georgiana Elena, Alina Ecaterina Jucan, Claudiu Vasile Mihai, Carmen Atodiresei, Madalina Ene, Carmen Ungureanu, Ioana Ruxandra Mihai, Otilia Nedelciuc, Mihaela Dranga, Cristina Cijevschi Prelipcean, and et al. 2025. "Synchronous Pulmonary and Cecal High-Grade Neuroendocrine Carcinomas Presenting as Hepatic Metastases: A Diagnostic Challenges and Literature Review" Diagnostics 15, no. 19: 2535. https://doi.org/10.3390/diagnostics15192535
APA StyleSârbu, G. E., Jucan, A. E., Mihai, C. V., Atodiresei, C., Ene, M., Ungureanu, C., Mihai, I. R., Nedelciuc, O., Dranga, M., Cijevschi Prelipcean, C., & Mihai, C. (2025). Synchronous Pulmonary and Cecal High-Grade Neuroendocrine Carcinomas Presenting as Hepatic Metastases: A Diagnostic Challenges and Literature Review. Diagnostics, 15(19), 2535. https://doi.org/10.3390/diagnostics15192535






